Perspectives on Psoriasiform Adverse Events from Immune Checkpoint Inhibitors: Lessons Learned from Our Practice
Abstract
1. Introduction
2. Case Reports
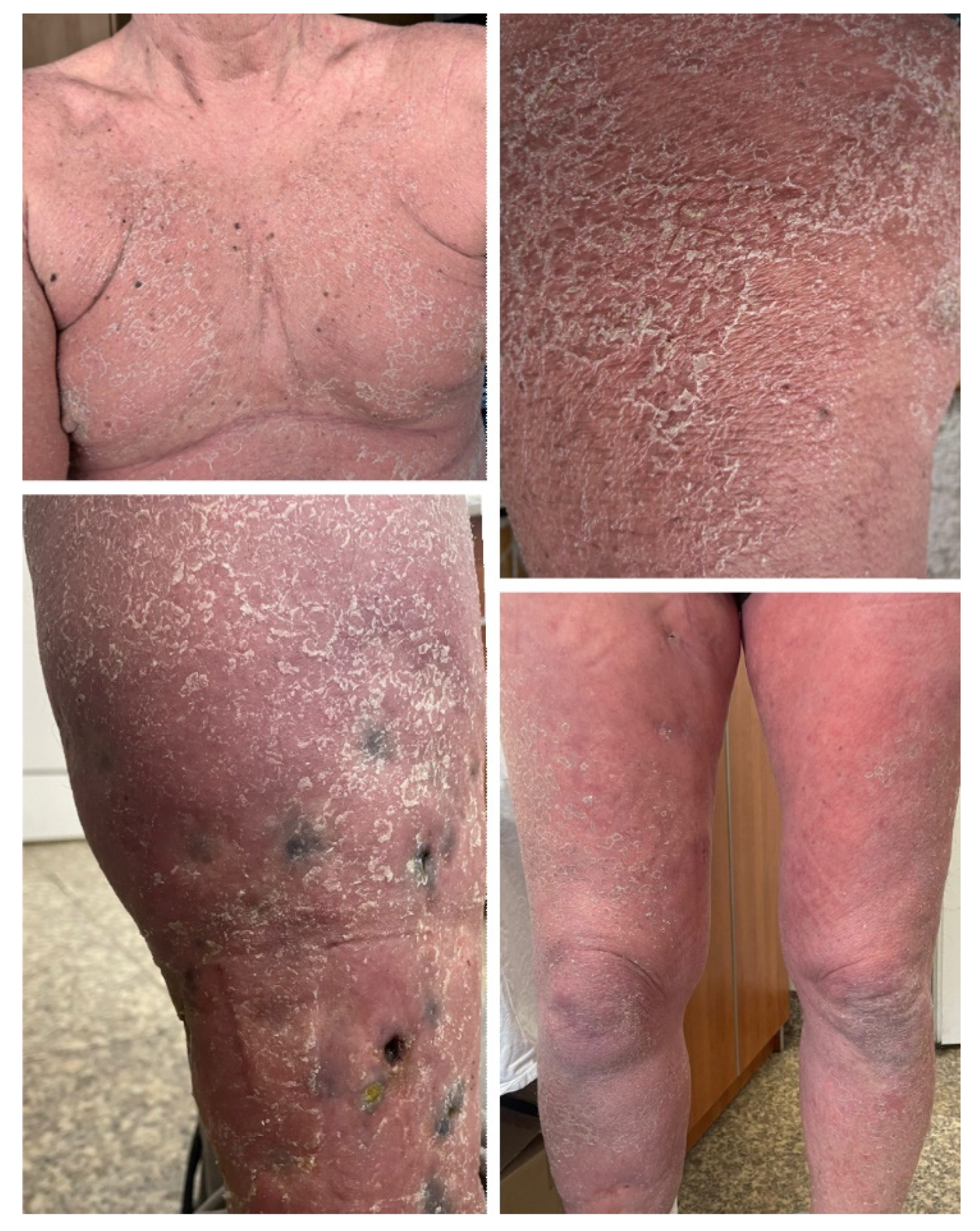
2.1. Case 1
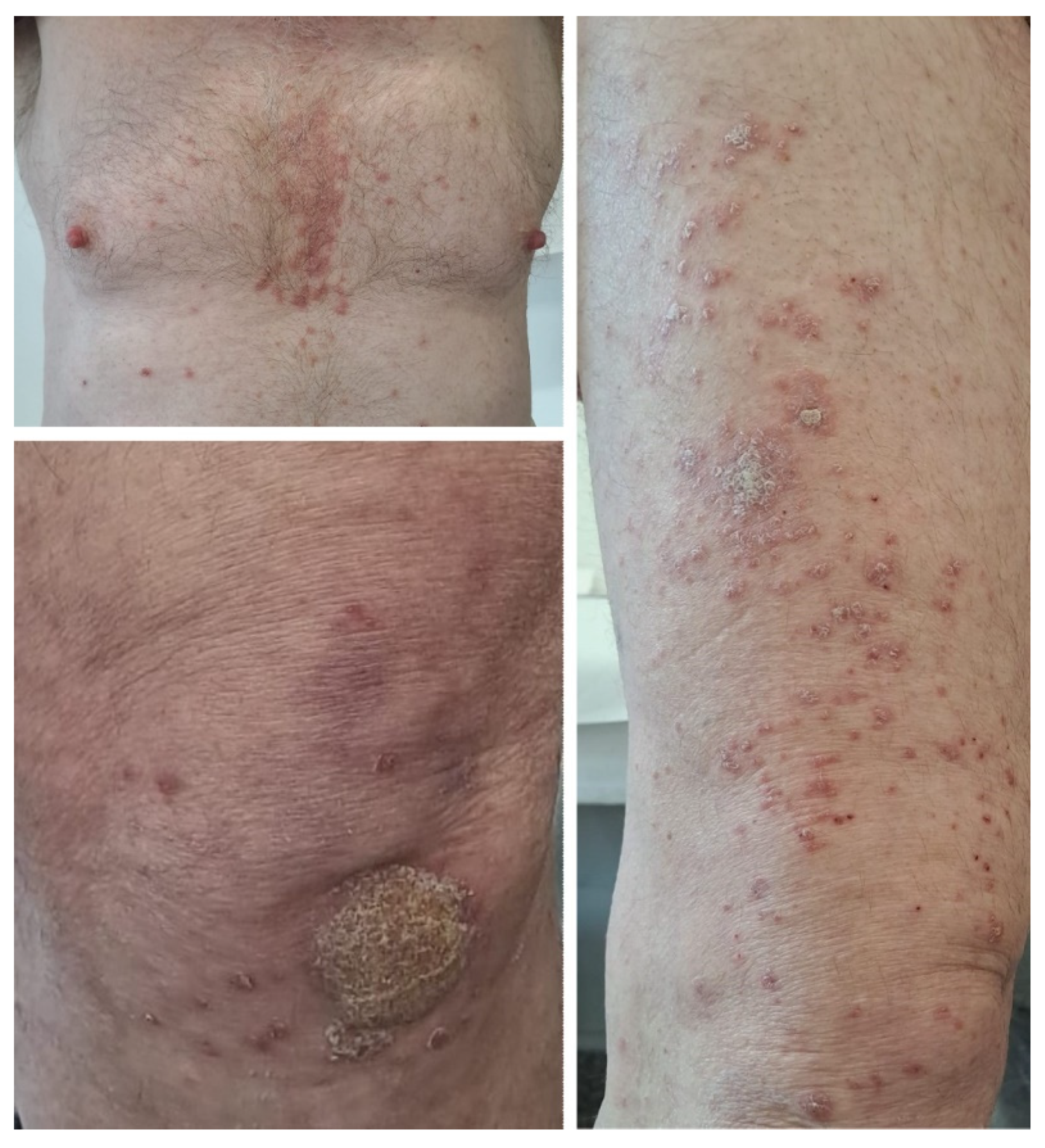
2.2. Case 2
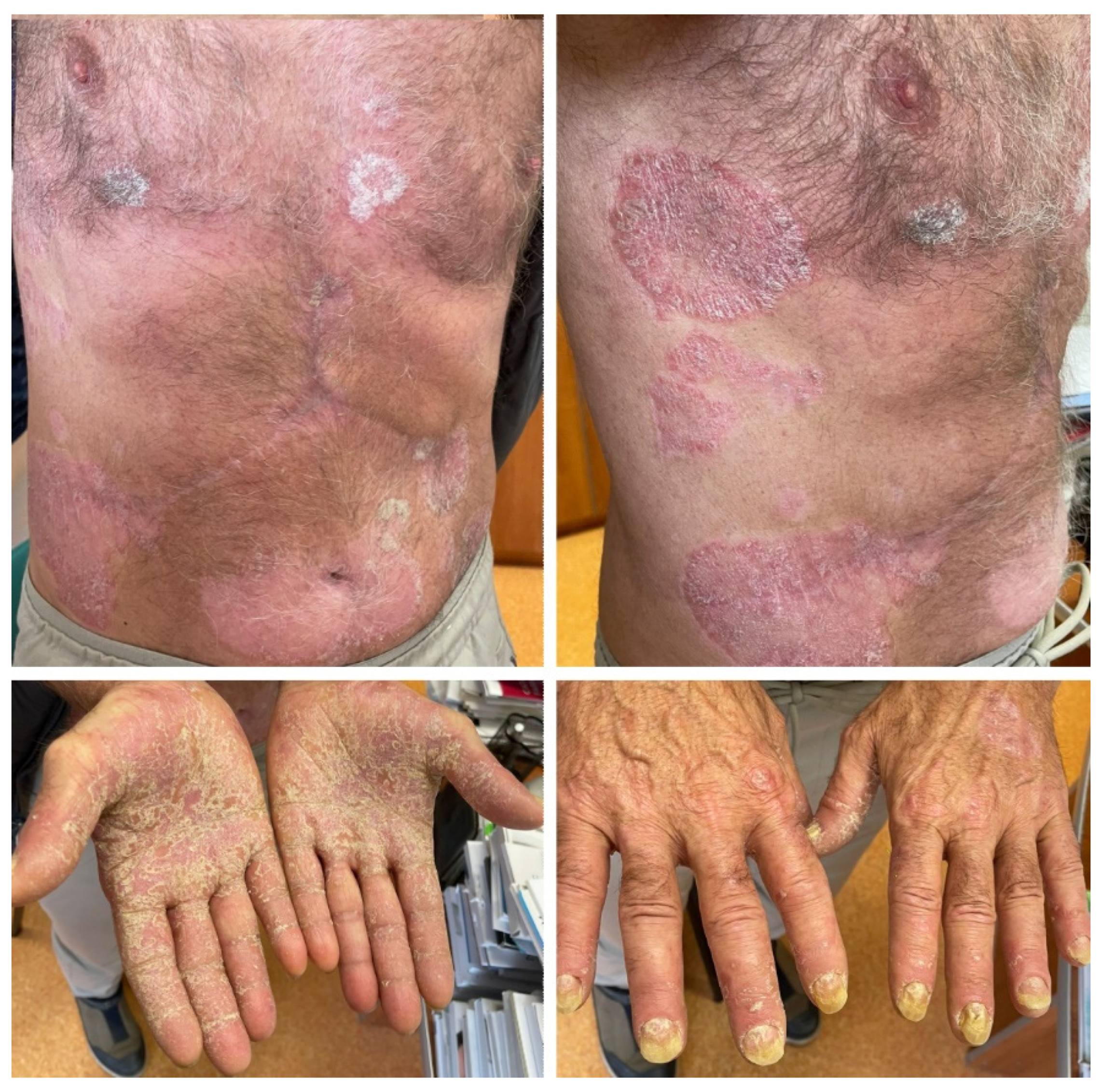
2.3. Case 3
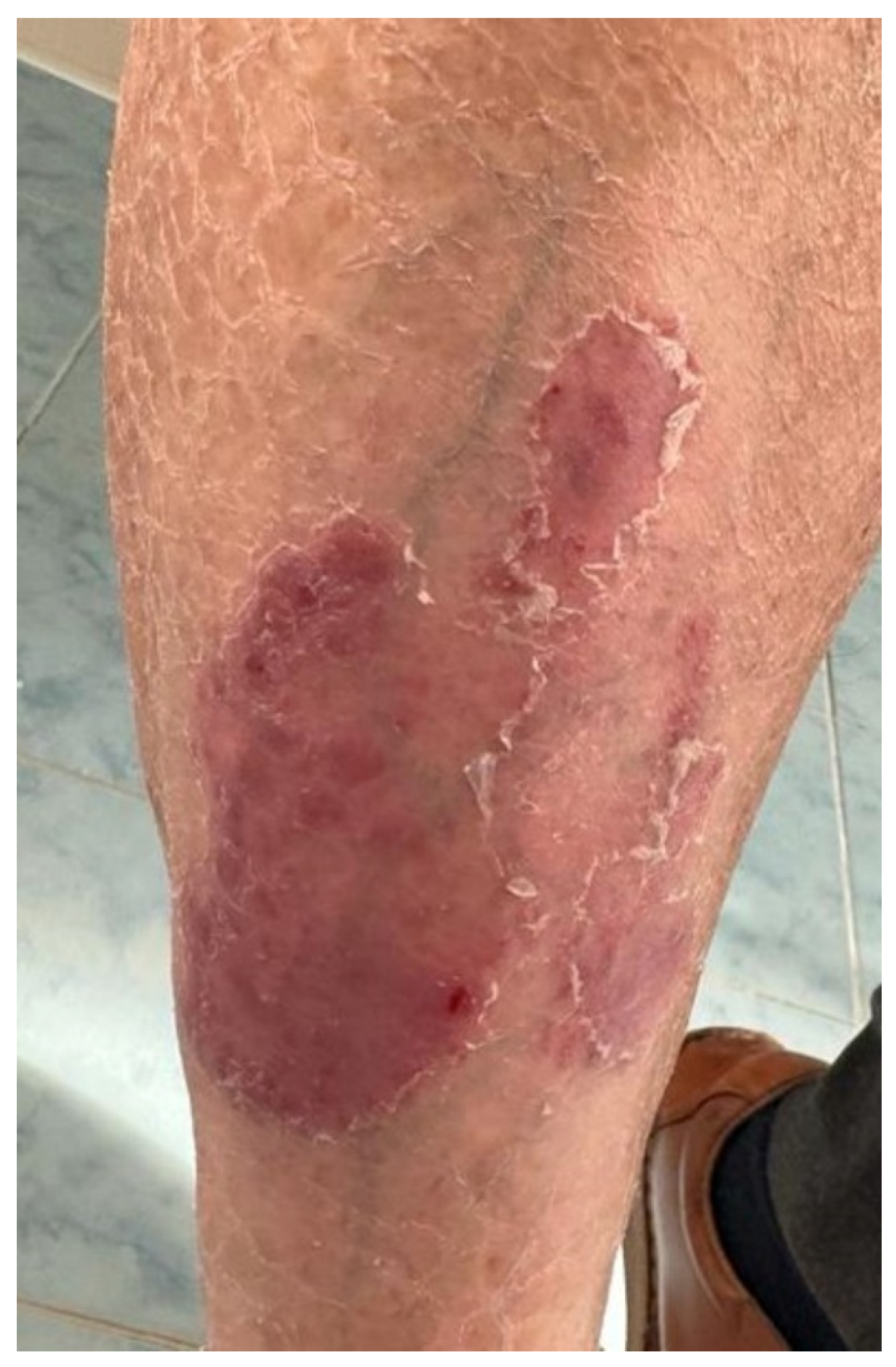
2.4. Case 4
2.5. Case 5

| Neoplasm | ICI | Age | Gender | Personal History of Psoriasis | Family History of Psoriasis | Time/Median Time until Psoriasis Onset | Type of Lesions | Psoriasis Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Metastatic melanoma | Pembrolizumab first, then nivolumab + ipilimumab, then nivolumab monotherapy | 60 | Male | No | No | 4 days after initiation of combination therapy | Erythrodermic psoriasis | Topical and systemic corticosteroids, acitretin 25 mg daily, keratolytic agents, emollients | Complete resolution of skin lesions, and treatment was continued. Patient died due to progressive metastatic disease |
| Case 2 | Advanced urothelial cancer | Avelumab | 74 | Male | Yes, limited to elbows and knees | No | After third dose | Widespread plaque psoriasis | Acitretin 20 mg daily, topical corticoids, keratolytic agents | Lesions slowly improved, then stabilized, treatment was continued |
| Case 3 | Hepatocellular carcinoma | Atezolizumab | 66 | Male | Yes, mild disease | No | 5 months after initiation of immunotherapy | Generalized psoriasis with severe palmo–plantar involvment | Acitretin 20 mg daily, topical corticoids, keratolytic agents, emollients | Skin lesions showed slow improvement, treatment was continued |
| Case 4 | Lung cancer | Pembrolizumab | 61 | Male | No | No | 1 year after initiation of pembrolizumab | Plaque psoriasis | Topical corticoids, vitamin D analogs, keratolytic agents | Immunotherapy was continued, with rare flares of psoriasis lesions |
| Case 5 | Metastatic melanoma | Nivolumab | 63 | Female | No | No | 12 months after initiation of nivolumab | Plaque psoriasis on elbows and knees | Topical corticoids, vitamin D analogs | Immunotherapy was continued |
3. Review of the Literature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Brom, V.C.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of Immune Checkpoint Molecules on Macrophages in Cancer, Infection, and Autoimmune Pathologies. Front. Immunol. 2022, 13, 837645. [Google Scholar] [CrossRef]
- Dutta, S.; Ganguly, A.; Chatterjee, K.; Spada, S.; Mukherjee, S. Targets of Immune Escape Mechanisms in Cancer: Basis for Development and Evolution of Cancer Immune Checkpoint Inhibitors. Biology 2023, 12, 218. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, eaax0182. [Google Scholar] [CrossRef]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2021, 25, 59–76. [Google Scholar] [CrossRef]
- Panariello, L.; Fattore, D.; Annunziata, M.C.; Piantedosi, F.; Gilli, M.; Fabbrocini, G. Bullous pemphigoid and nivolumab: Dermatologic management to support and continue oncologic therapy. Eur. J. Cancer 2018, 103, 284–286. [Google Scholar] [CrossRef]
- Plachouri, K.M.; Vryzaki, E.; Georgiou, S. Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Summarized Overview. Curr. Drug Saf. 2019, 14, 14–20. [Google Scholar] [CrossRef]
- Pintova, S.; Sidhu, H.; Friedlander, P.A.; Holcombe, R.F. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 2013, 23, 498–501. [Google Scholar] [CrossRef]
- Joseph, R.W.; Cappel, M.; Goedjen, B.; Gordon, M.; Kirsch, B.; Gilstrap, C.; Bagaria, S.; Jambusaria-Pahlajani, A. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol. Res. 2015, 3, 18–22. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; ESMO Guidelines Committee. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Kochi, Y.; Miyachi, H.; Tagashira, R.; Koga, H.; Ishii, N.; Sugiura, K.; Ikeda, J.I.; Matsue, H.; Inozume, T. Simultaneous development of generalized pustular psoriasis and pemphigoid with multiple autoantibodies in a complete responder of pembrolizumab for lung cancer. J. Dermatol. 2023, 50, 1343–1346. [Google Scholar] [CrossRef]
- Belzer, A.; Mortlock, R.D.; Pach, J.; Cohen, J.M.; Leventhal, J.S. The effect of baseline eczema or psoriasis on the morphology of cutaneous immune-related adverse events due to immune checkpoint inhibitor therapy. J. Am. Acad. Dermatol. 2023, 88, 1198–1200. [Google Scholar] [CrossRef]
- Hansen, I.; Heidrich, I.; Abeck, F.; Kött, J.; Booken, N.; Gebhardt, C.; Schneider, S.W. Successful treatment of PD-1 inhibitor-induced psoriasis with infliximab. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e621–e623. [Google Scholar] [CrossRef]
- L’Orphelin, J.M.; Cassecuel, J.; Kandolf, L.; Harwood, C.A.; Tookey, P.; Junejo, M.H.; Hogan, S.; Lebbé, C.; Appalla, Z.; Kränke, T.M.; et al. Cutaneous manifestations induced by check point inhibitors in 120 melanoma patients—The European MelSkinTox study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1606–1615. [Google Scholar] [CrossRef]
- Gargiulo, L.; Ibba, L.; Valenti, M.; Costanzo, A.; Narcisi, A. Pembrolizumab-induced plaque psoriasis successfully treated with risankizumab in a patient with stage IV cutaneous melanoma. Melanoma Res. 2023, 33, 152–154. [Google Scholar] [CrossRef]
- Hussain, K.; Kanji, A.; Zaheri, S.; Malek, D.; Terlizzo, M.; Weir, J.; Turajlic, S.; Fearfield, L. Checkpoint inhibitor therapy and psoriasis: A case series. Clin. Exp. Dermatol. 2023, 48, 254–256. [Google Scholar] [CrossRef]
- Gleason, L.; Hunter, E.; Cohen, A.; Suriano, J.; Nikbakht, N. Atezolizumab-induced psoriasiform drug eruption successfully treated with ixekizumab: A case report and literature review. Dermatol. Online J. 2023, 29, 9. [Google Scholar] [CrossRef]
- Dorman, K.; Burkhard-Meier, A.; Di Gioia, D.; Kunz, W.G.; Knösel, T.; Holch, J.W.; Lindner, L.H. Treatment of metastatic alveolar soft part sarcoma with axitinib and pembrolizumab in an 80-year-old patient with a history of autoimmune disorders. Anti-Cancer Drugs 2023, 34, 311–316. [Google Scholar] [CrossRef]
- Nikolaou, V.A.; Apalla, Z.; Carrera, C.; Fattore, D.; Sollena, P.; Riganti, J.; Segura, S.; Freites-Martinez, A.; Lallas, K.; Romano, M.C.; et al. Clinical associations and classification of immune checkpoint inhibitor-induced cutaneous toxicities: A multicentre study from the European Academy of Dermatology and Venereology Task Force of Dermatology for Cancer Patients. Br. J. Dermatol. 2022, 187, 962–969. [Google Scholar] [CrossRef]
- Lim, J.H.; Lo, Y. A case of de novo psoriasis secondary to atezolizumab in a patient with hepatocellular carcinoma. Kaohsiung J. Med. Sci. 2022, 38, 1135–1136. [Google Scholar] [CrossRef]
- Jfri, A.; Leung, B.; Said, J.T.; Semenov, Y.; LeBoeuf, N.R. Prevalence of inverse psoriasis subtype with immune checkpoint inhibitors. Immunother. Adv. 2022, 2, ltac016. [Google Scholar] [CrossRef]
- Kase, M.; Fujita, Y.; Ota, A.; Shimizu, S.; Itoi-Ochi, S.; Sano, S. Loss of epidermal Langerhans cells in psoriasiform lesions of de novo induced or worsened pre-existing psoriasis following uses of immune checkpoint inhibitors. J. Dermatol. 2022, 49, 916–920. [Google Scholar] [CrossRef]
- Ma, V.T.; Lao, C.D.; Fecher, L.A.; Schiopu, E. Successful use of secukinumab in two melanoma patients with immune checkpoint inhibitor-induced inflammatory arthropathy. Immunotherapy 2022, 14, 593–598. [Google Scholar] [CrossRef]
- Mohta, A.; Arora, A.; Jain, S.K. Atezolizumab induced de novo rupoid psoriasis: A rare cutaneous manifestation of immune checkpoint inhibitors. J. Cosmet. Dermatol. 2022, 21, 2005–2008. [Google Scholar] [CrossRef]
- Seervai, R.N.H.; Heberton, M.; Cho, W.C.; Gill, P.; Murphy, M.B.; Aung, P.P.; Nagarajan, P.; Torres-Cabala, C.A.; Patel, A.B.; Ruiz-Bañobre, J.; et al. Severe de novo pustular psoriasiform immune-related adverse event associated with nivolumab treatment for metastatic esophageal adenocarcinoma. J. Cutan. Pathol. 2022, 49, 472–481. [Google Scholar] [CrossRef]
- Wong, P.Y.; How, S.H.; Ismail, I.; Hassan, R. Single dose of atezolizumab plus chemotherapy in active psoriasis with advanced non-small cell lung cancer. J. Oncol. Pharm. Pract. 2022, 28, 471–474. [Google Scholar] [CrossRef]
- Tang, K.; Seo, J.; Tiu, B.C.; Le, T.K.; Pahalyants, V.; Raval, N.S.; Ugwu-Dike, P.O.; Zubiri, L.; Naranbhai, V.; Carrington, M.; et al. Association of Cutaneous Immune-Related Adverse Events with Increased Survival in Patients Treated with Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Therapy. JAMA Dermatol. 2022, 158, 189–193. [Google Scholar] [CrossRef]
- Tirpack, A.; Chan, K.K. Pembrolizumab-Induced Phenotypic Switch in Psoriatic Arthritis. J. Clin. Rheumatol. 2021, 27, S683–S684. [Google Scholar] [CrossRef]
- Çelik, U.; Aydemir, E.H.; Engin, B.; Oba, M.Ç.; Yılmaz, M.; Meşe, Ş.G. Dermatological side effects of immunotherapy drugs and targeted cancer therapies: Importance of dermatology and oncology collaboration. J. Oncol. Pharm. Pract. 2021, 27, 1853–1860. [Google Scholar] [CrossRef]
- Onishi, Y.; Arakawa, Y.; Tamagawa-Mineoka, R.; Ohshita, A.; Masuda, K.; Katoh, N. Occurrence of palmoplantar pustulosis during atezolizumab therapy for non-small cell lung cancer. J. Dermatol. 2021, 48, e570–e571. [Google Scholar] [CrossRef]
- Furuta, H.; Kato, S.; Masago, K.; Hida, T. Palmoplantar Pustulosis Caused by Immune-Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, e829–e832. [Google Scholar] [CrossRef] [PubMed]
- Halle, B.R.; Betof Warner, A.; Zaman, F.Y.; Haydon, A.; Bhave, P.; Dewan, A.K.; Ye, F.; Irlmeier, R.; Mehta, P.; Kurtansky, N.R.; et al. Immune checkpoint inhibitors in patients with pre-existing psoriasis: Safety and efficacy. J. Immunother. Cancer 2021, 9, e003066. [Google Scholar] [CrossRef]
- D’Erme, A.M.; Fidanzi, C.; Janowska, A.; Allegrini, G.; Barbara, C.; Cupini, S.; Viacava, P.; Bagnoni, G. Psoriasis caused by pembrolizumab treatment in advanced melanoma: A positive prognostic side effect? Dermatol. Ther. 2021, 34, e15050. [Google Scholar] [CrossRef]
- Calvo, V.; Fernández, M.A.; Collazo-Lorduy, A.; Franco, F.; Núñez, B.; Provencio, M. Use of immune checkpoint inhibitors in patients with solid tumors and pre-existing autoimmune or inflammatory disease: Real-world data. Lung Cancer Manag. 2021, 10, LMT51. [Google Scholar] [CrossRef]
- Killion, L.; Beatty, P.; Byrne, N.; Mahon, J.M.; Salim, A.; Connolly, M.; Tobin, A.M. Nivolumab Induced Psoriasis Successfully Treated with Acitretin. J. Drugs Dermatol. 2021, 20, 911. [Google Scholar] [CrossRef]
- Jatwani, K.; Kaur, H.; Chugh, K.; Jatwani, S. Nivolumab-Induced Psoriatic Arthritis in a Patient with Advanced Small Cell Lung Cancer. J. Clin. Rheumatol. 2021, 27, e162–e163. [Google Scholar] [CrossRef]
- Mullangi, S.; Ponnam, S.; Lekkala, M.R.; Koya, S. A Case of De Novo Psoriasis Secondary to Nivolumab in a Patient with Metastatic Renal Cell Carcinoma. Cureus 2021, 13, e15703. [Google Scholar] [CrossRef]
- Brown, L.J.; Weppler, A.; Bhave, P.; Allayous, C.; Patrinely, J.R., Jr.; Ott, P.; Sandhu, S.; Haydon, A.; Lebbe, C.; Johnson, D.B.; et al. Combination anti-PD1 and ipilimumab therapy in patients with advanced melanoma and pre-existing autoimmune disorders. J. Immunother. Cancer 2021, 9, e002121. [Google Scholar] [CrossRef]
- Thompson, L.L.; Krasnow, N.A.; Chang, M.S.; Yoon, J.; Li, E.B.; Polyakov, N.J.; Molina, G.E.; Said, J.T.; Huang, K.; Kuchroo, J.R.; et al. Patterns of Cutaneous and Noncutaneous Immune-Related Adverse Events among Patients with Advanced Cancer. JAMA Dermatol. 2021, 157, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.; Sibaud, V.; Fattore, D.; Sollena, P.; Ortiz-Brugués, A.; Giacchero, D.; Romano, M.C.; Riganti, J.; Lallas, K.; Peris, K.; et al. Immune checkpoint-mediated psoriasis: A multicenter European study of 115 patients from the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) group. J. Am. Acad. Dermatol. 2021, 84, 1310–1320. [Google Scholar] [CrossRef]
- Gonzalez-Mazón, I.; Sánchez-Bilbao, L.; Martín-Varillas, J.L.; García-Castaño, A.; Delgado-Ruiz, M.; Bernat Piña, I.; Hernández, J.L.; Castañeda, S.; Llorca, J.; González-Gay, M.A.; et al. Immune-related adverse events in patients with solid-organ tumours treated with immunotherapy: A 3-year study of 102 cases from a single centre. Clin. Exp. Rheumatol. 2021, 39, 612–620. [Google Scholar] [CrossRef]
- Mayor Ibarguren, A.; Enrique, E.A.; Diana, P.L.; Ana, C.; Pedro, H.P. Apremilast for immune checkpoint inhibitor-induced psoriasis: A case series. JAAD Case Rep. 2021, 13, 84–89. [Google Scholar] [CrossRef]
- Glinos, G.D.; Fisher, W.S.; Morr, C.S.; Seminario-Vidal, L. Nivolumab-induced psoriasis successfully treated with risankizumab-rzaa in a patient with stage III melanoma. JAAD Case Rep. 2021, 11, 74–77. [Google Scholar] [CrossRef]
- Cutroneo, P.; Ingrasciotta, Y.; Isgrò, V.; Rullo, E.V.; Berretta, M.; Fiorica, F.; Trifirò, G.; Guarneri, C. Psoriasis and psoriasiform reactions secondary to immune checkpoint inhibitors. Dermatol. Ther. 2021, 34, e14830. [Google Scholar] [CrossRef]
- Foti, C.; Tucci, M.; Stingeni, L.; Hansel, K.; Lospalluti, L.; Frisario, R.; Giuffrida, R.; Romita, P. Successful treatment with apremilast of severe psoriasis exacerbation during nivolumab therapy for metastatic melanoma. Dermatol. Ther. 2021, 34, e14653. [Google Scholar] [CrossRef]
- Umeda, Y.; Hayashi, H.; Sugiyama, S.; Aoyama, Y. Systemic capillary leak syndrome triggered by anti-programmed death 1 checkpoint inhibitor in psoriasis. J. Dermatol. 2020, 47, 1322–1325. [Google Scholar] [CrossRef]
- Siciliano, M.A.; Dastoli, S.; d’Apolito, M.; Staropoli, N.; Tassone, P.; Tagliaferri, P.; Barbieri, V. Pembrolizumab-Induced Psoriasis in Metastatic Melanoma: Activity and Safety of Apremilast, a Case Report. Front. Oncol. 2020, 10, 579445. [Google Scholar] [CrossRef]
- Marti-Marti, I.; Gómez, S.; Riera-Monroig, J.; Carrera, C.; Mascaró, J.M. Rupioid psoriasis induced by pembrolizumab. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 580–582. [Google Scholar]
- Lin, W.H.; Lee, K.Y.; Lee, W.R.; Shih, Y.H. Durvalumab-induced de novo annular psoriasiform drug eruption successfully treated with a combination of narrowband ultraviolet B phototherapy and topical treatment. J. Dermatol. 2020, 47, 1041–1045. [Google Scholar] [CrossRef]
- Corneli, P.; di Meo, N.; Fagotti, S.; Conforti, C.; Farinazzo, E.; Zacchi, A.; Retrosi, C.; Vezzoni, R.; Pizzichetta, M.A.; Zalaudek, I. Inverse psoriasis in patient treated with atezolizumab. Int. J. Dermatol. 2020, 59, e331–e332. [Google Scholar] [CrossRef]
- Huang, P.W.; Chu, C.Y. Pembrolizumab-induced linear psoriasis. Lung Cancer 2020, 146, 378–379. [Google Scholar] [CrossRef]
- Nigro, O.; Pinotti, G.; Gueli, R.; Grigioni, E.; Santis, M.; Ceribelli, A.; Selmi, C. Psoriatic arthritis induced by anti-PD1 and treated with apremilast: A case report and review of the literature. Immunotherapy 2020, 12, 549–554. [Google Scholar] [CrossRef]
- Mao, M.; Shi, M.; Li, T.; Wang, Q.; Wu, L. Atezolizumab-induced psoriasis in a patient with metastatic lung cancer—A case report. Transl. Cancer Res. 2020, 9, 3776–3782. [Google Scholar] [CrossRef]
- Di Altobrando, A.; Bruni, F.; Alessandrini, A.; Starace, M.; Misciali, C.; Piraccini, B.M. Severe de-novo palmoplantar and nail psoriasis complicating Nivolumab treatment for metastatic melanoma. Dermatol. Ther. 2020, 33, e13363. [Google Scholar] [CrossRef]
- Takama, H.; Shibata, T.; Ando, Y.; Yanagishita, T.; Ohshima, Y.; Akiyama, M.; Watanabe, D. Pembrolizumab-induced psoriasis vulgaris successfully treated with apremilast. Eur. J. Dermatol. 2020, 30, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Matsumoto, S.; Takeda, Y.; Sugiyama, H. Systemic Psoriasiform Dermatitis Appeared after the Administration of Pembrolizumab. Intern. Med. 2020, 59, 871–872. [Google Scholar] [CrossRef]
- Guven, D.; Kilickap, S.; Guner, G.; Taban, H.; Dizdar, O. Development of de novo psoriasis during nivolumab therapy in a patient with small cell lung cancer. J. Oncol. Pharm. Pract. 2020, 26, 256–258. [Google Scholar] [CrossRef]
- Politi, A.; Angelos, D.; Mauri, D.; Zarkavelis, G.; Pentheroudakis, G. A case report of psoriasis flare following immunotherapy: Report of an important entity and literature review. SAGE Open Med. Case Rep. 2020, 8, 2050313X19897707. [Google Scholar] [CrossRef] [PubMed]
- Tison, A.; Quéré, G.; Misery, L.; Funck-Brentano, E.; Danlos, F.X.; Routier, E.; Robert, C.; Loriot, Y.; Lambotte, O.; Bonniaud, B.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients with Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019, 71, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Mikita, N.; Ikeda, T.; Inaba, Y.; Kunimoto, K.; Kaminaka, C.; Kanazawa, N.; Yamamoto, Y.; Jinnin, M. Psoriatic arthritis induced by anti-programmed death 1 antibody pembrolizumab. J. Dermatol. 2019, 46, e466–e467. [Google Scholar] [CrossRef]
- Monsour, E.P.; Pothen, J.; Balaraman, R. A Novel Approach to the Treatment of Pembrolizumab-induced Psoriasis Exacerbation: A Case Report. Cureus 2019, 11, e5824. [Google Scholar] [CrossRef]
- Nikolaou, V.; Voudouri, D.; Tsironis, G.; Charpidou, A.; Stamoulis, G.; Triantafyllopoulou, I.; Panoutsopoulou, I.; Xidakis, E.; Bamias, A.; Samantas, E.; et al. Cutaneous toxicities of antineoplastic agents: Data from a large cohort of Greek patients. Support. Care Cancer 2019, 27, 4535–4542. [Google Scholar] [CrossRef]
- Scarfì, F.; Lacava, R.; Patrizi, A.; Tartari, F.; Ravaioli, G.M.; Veronesi, G.; Lambertini, M.; Dika, E. Follicular psoriasis induced by pembrolizumab in a patient with advanced non-small-cell lung cancer. Int. J. Dermatol. 2019, 58, e151–e152. [Google Scholar] [CrossRef]
- Johnson, D.; Patel, A.B.; Uemura, M.I.; Trinh, V.A.; Jackson, N.; Zobniw, C.M.; Tetzlaff, M.T.; Hwu, P.; Curry, J.L.; Diab, A. IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity from Immunotherapy. Cancer Immunol. Res. 2019, 7, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Santos-Juanes, J.; Munguía Calzada, P.; Álvarez Fernández, C. Plaque Psoriasis Flare and Peripheral Edema in a Patient Treated with Atezolizumab. Actas Dermosifiliogr. 2019, 110, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Fattore, D.; Annunziata, M.C.; Panariello, L.; Marasca, C.; Fabbrocini, G. Successful treatment of psoriasis induced by immune checkpoint inhibitors with apremilast. Eur. J. Cancer 2019, 110, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.; Cen, P.; Dinh, B.; Mays, S.R.; Patel, A.B. Dramatic response of nivolumab-associated psoriasiform dermatitis to etoposide. Eur. J. Cancer 2019, 107, 97–99. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Hulstaert, E.; Kruse, V.; Brochez, L. Psoriasis Vulgaris Exacerbation during Treatment with a PD-1 Checkpoint Inhibitor: Case Report and Literature Review. Case Rep. Dermatol. 2018, 10, 190–197. [Google Scholar] [CrossRef]
- Troyanova-Slavkova, S.; Eickenscheidt, L.; Dumann, K.; Kowalzick, L. Initially undetected de novo psoriasis triggered by nivolumab for metastatic base of the tongue carcinoma. Hautarzt 2018, 69, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Parisi, A.; Fargnoli, M.C.; Cannita, K.; Irelli, A.; Porzio, G.; Martinazzo, C.; Ficorella, C. Safe Administration of Ipilimumab, Pembrolizumab, and Nivolumab in a Patient with Metastatic Melanoma, Psoriasis, and a Previous Guillain-Barré Syndrome. Case Rep. Oncol. Med. 2018, 2018, 2783917. [Google Scholar] [CrossRef]
- Lidar, M.; Giat, E.; Garelick, D.; Horowitz, Y.; Amital, H.; Steinberg-Silman, Y.; Schachter, J.; Shapira-Frommer, R.; Markel, G. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun. Rev. 2018, 17, 284–289. [Google Scholar] [CrossRef]
- Danlos, F.X.; Voisin, A.L.; Dyevre, V.; Michot, J.M.; Routier, E.; Taillade, L.; Champiat, S.; Aspeslagh, S.; Haroche, J.; Albiges, L.; et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 2018, 91, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chujo, S.; Asahina, A.; Itoh, Y.; Kobayashi, K.; Sueki, H.; Ishiji, T.; Umezawa, Y.; Nakagawa, H. New onset of psoriasis during nivolumab treatment for lung cancer. J. Dermatol. 2018, 45, e55–e56. [Google Scholar] [CrossRef]
- Voudouri, D.; Nikolaou, V.; Laschos, K.; Charpidou, A.; Soupos, N.; Triantafyllopoulou, I.; Panoutsopoulou, I.; Aravantinos, G.; Syrigos, K.; Stratigos, A. Anti-PD1/PDL1 induced psoriasis. Curr. Probl. Cancer 2017, 41, 407–412. [Google Scholar] [CrossRef]
- Sugiura, Y.; Fujimoto, H.; Yamamoto, M.; Nomura, H.; Hashizume, T.; Kawai, O.; Araki, N.; Kawakami, K.; Sueki, H.; Fusegawa, H.; et al. Psoriasis and Psoriatic Arthritis Induced by Nivolumab in a Patient with Advanced Non-Small-Cell Lung Cancer. Gan To Kagaku Ryoho 2017, 44, 787–789. [Google Scholar] [PubMed]
- Elosua-González, M.; Pampín-Franco, A.; Mazzucchelli-Esteban, R.; Mielgo-Rubio, X.; Rodriguez-Vásquez, X.; García-Zamora, E.; López-Estebaranz, J.L. A case of de novo palmoplantar psoriasis with psoriatic arthritis and autoimmune hypothyroidism after receiving nivolumab therapy. Dermatol. Online J. 2017, 23, 13030/qt12n4m6pm. [Google Scholar] [CrossRef]
- Ruiz-Bañobre, J.; Pérez-Pampín, E.; García-González, J.; Gómez-Caamaño, A.; Barón-Duarte, F.J.; López-López, R.; Vázquez-Rivera, F. Development of psoriatic arthritis during nivolumab therapy for metastatic non-small cell lung cancer, clinical outcome analysis and review of the literature. Lung Cancer 2017, 108, 217–221. [Google Scholar] [CrossRef]
- Okiyama, N.; Tanaka, R. Varied immuno-related adverse events induced by immune-check point inhibitors—Nivolumab-associated psoriasiform dermatitis related with increased serum level of interleukin-6. Nihon Rinsho Meneki Gakkai Kaishi 2017, 40, 95–101. [Google Scholar] [CrossRef]
- Ruiz-Bañobre, J.; Abdulkader, I.; Anido, U.; León, L.; López-López, R.; García-González, J. Development of de novo psoriasis during nivolumab therapy for metastatic renal cell carcinoma: Immunohistochemical analyses and clinical outcome. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2017, 125, 259–263. [Google Scholar] [CrossRef]
- Nonomura, Y.; Otsuka, A.; Ohtsuka, M.; Yamamoto, T.; Dummer, R.; Kabashima, K. ADAMTSL5 is upregulated in melanoma tissues in patients with idiopathic psoriasis vulgaris induced by nivolumab. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e100–e101. [Google Scholar] [CrossRef]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef]
- Murata, S.; Kaneko, S.; Harada, Y.; Aoi, N.; Morita, E. Case of de novo psoriasis possibly triggered by nivolumab. J. Dermatol. 2017, 44, 99–100. [Google Scholar] [CrossRef]
- Schmutz, J.L. Psoriasis and psoriatic arthritis induced by nivolumab Opdivo®. Ann. Dermatol. Venereol. 2016, 143, 881–882. [Google Scholar] [CrossRef]
- Law-Ping-Man, S.; Martin, A.; Briens, E.; Tisseau, L.; Safa, G. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology 2016, 55, 2087–2089. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Otsuka, A.; Miyachi, Y.; Kabashima, K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J. Eur. Acad. Dermatol. Venereol. 2016, 30, e89–e91. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.L.; John, T. Severe Psoriasis Flare After Anti-Programmed Death Ligand 1 (PD-L1) Therapy for Metastatic Non-Small Cell Lung Cancer (NSCLC). J. Immunother. 2016, 39, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Totonchy, M.B.; Ezaldein, H.H.; Ko, C.J.; Choi, J.N. Inverse Psoriasiform Eruption During Pembrolizumab Therapy for Metastatic Melanoma. JAMA Dermatol. 2016, 152, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo-Torralba, A.; Ballester-Sánchez, R.; Pujol-Marco, C.; Botella-Estrada, R. Pembrolizumab: A new Drug That Can Induce Exacerbations of Psoriasis. Actas Dermosifiliogr. 2016, 107, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Ohtsuka, M.; Kikuchi, N.; Yamamoto, T. Exacerbation of Psoriasis During Nivolumab Therapy for Metastatic Melanoma. Acta Derm. Venereol. 2016, 96, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Miura, T.; Mori, T.; Ishikawa, M.; Yamamoto, T. Occurrence of Psoriasiform Eruption During Nivolumab Therapy for Primary Oral Mucosal Melanoma. JAMA Dermatol. 2015, 151, 797–799. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Chan, D.V.; Gibson, H.M.; Aufiero, B.M.; Wilson, A.J.; Hafner, M.S.; Mi, Q.S.; Wong, H.K. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun. 2014, 15, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Naluai, A.T.; Nilsson, S.; Samuelsson, L.; Gudjónsdóttir, A.H.; Ascher, H.; Ek, J.; Hallberg, B.; Kristiansson, B.; Martinsson, T.; Nerman, O.; et al. The CTLA4/CD28 gene region on chromosome 2q33 confers susceptibility to celiac disease in a way possibly distinct from that of type 1 diabetes and other chronic inflammatory disorders. Tissue Antigens 2000, 56, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Dorfman, D.M.; Ma, F.R.; Sullivan, E.L.; Munoz, O.; Wood, C.R.; Greenfield, E.A.; Freeman, G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003, 170, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Philips, E.A.; Garcia-España, A.; Tocheva, A.S.; Ahearn, I.M.; Adam, K.R.; Pan, R.; Mor, A.; Kong, X.P. The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals. J. Biol. Chem. 2020, 295, 4372–4380. [Google Scholar] [CrossRef] [PubMed]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Busselaar, J.; Tian, S.; van Eenennaam, H.; Borst, J. Helpless Priming Sends CD8+ T Cells on the Road to Exhaustion. Front. Immunol. 2020, 11, 592569. [Google Scholar] [CrossRef]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef]
- Beltra, J.C.; Manne, S.; Abdel-Hakeem, M.S.; Kurachi, M.; Giles, J.R.; Chen, Z.; Casella, V.; Ngiow, S.F.; Khan, O.; Huang, Y.J.; et al. Developmental Relationships of Four Exhausted CD8+ T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 2020, 52, 825–841. [Google Scholar] [CrossRef]
- Lin, H.; Wei, S.; Hurt, E.M.; Green, M.D.; Zhao, L.; Vatan, L.; Szeliga, W.; Herbst, R.; Harms, P.W.; Fecher, L.A.; et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Investig. 2018, 128, 805–815. [Google Scholar] [CrossRef]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Wu, D.C.; Cheung, J.; Navarro, A.; Xiong, H.; Cubas, R.; Totpal, K.; Chiu, H.; Wu, Y.; Comps-Agrar, L.; et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 2020, 1, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Probst, H.C.; McCoy, K.; Okazaki, T.; Honjo, T.; van den Broek, M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 2005, 6, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.K.; Wolchok, J.D. Recruit or Reboot? How Does Anti-PD-1 Therapy Change Tumor-Infiltrating Lymphocytes? Cancer Cell 2019, 36, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Busselaar, J.; Bosma, D.M.T.; Ossendorp, F. Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy. Eur. J. Immunol. 2021, 51, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; De Guillebon, E.; Blanc, C.; Roussel, H.; Badoual, C.; Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017, 2, e000213. [Google Scholar] [CrossRef] [PubMed]
- Brewitz, A.; Eickhoff, S.; Dähling, S.; Quast, T.; Bedoui, S.; Kroczek, R.A.; Kurts, C.; Garbi, N.; Barchet, W.; Iannacone, M.; et al. CD8+ T Cells Orchestrate pDC-XCR1+ Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity 2017, 46, 205–219. [Google Scholar] [CrossRef]
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; Lee, J.; Kissick, H.T.; Burger, M.C.; Shan, Q.; Hale, J.S.; Lee, J.; Nasti, T.H.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016, 537, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Buchan, S.L.; Fallatah, M.; Thirdborough, S.M.; Taraban, V.Y.; Rogel, A.; Thomas, L.J.; Penfold, C.A.; He, L.Z.; Curran, M.A.; Keler, T.; et al. PD-1 Blockade and CD27 Stimulation Activate Distinct Transcriptional Programs That Synergize for CD8+ T-Cell-Driven Antitumor Immunity. Clin. Cancer Res. 2018, 24, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.; Klinger, M.; Hou, Y.; Cummings, C.; Ribas, A.; Faham, M.; Fong, L. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 2014, 6, 238ra70. [Google Scholar] [CrossRef]
- van Rooij, N.; van Buuren, M.M.; Philips, D.; Velds, A.; Toebes, M.; Heemskerk, B.; van Dijk, L.J.; Behjati, S.; Hilkmann, H.; El Atmioui, D.; et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 2013, 31, e439–e442. [Google Scholar] [CrossRef]
- Chang, C.H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef]
- Singh, N.; Chandler, P.R.; Seki, Y.; Baban, B.; Takezaki, M.; Kahler, D.J.; Munn, D.H.; Larsen, C.P.; Mellor, A.L.; Iwashima, M. Role of CD28 in fatal autoimmune disorder in scurfy mice. Blood 2007, 110, 1199–1206. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016, 28, 254–263. [Google Scholar] [CrossRef]
- Sanlorenzo, M.; Vujic, I.; Daud, A.; Algazi, A.; Gubens, M.; Luna, S.A.; Lin, K.; Quaglino, P.; Rappersberger, K.; Ortiz-Urda, S. Pembrolizumab Cutaneous Adverse Events and Their Association with Disease Progression. JAMA Dermatol. 2015, 151, 1206–1212. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Bonigen, J.; Raynaud-Donzel, C.; Hureaux, J.; Kramkimel, N.; Blom, A.; Jeudy, G.; Breton, A.L.; Hubiche, T.; Bedane, C.; Legoupil, D.; et al. Anti-PD1-induced psoriasis: A study of 21 patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e254–e257. [Google Scholar] [CrossRef]
- Dulos, J.; Carven, G.J.; van Boxtel, S.J.; Evers, S.; Driessen-Engels, L.J.; Hobo, W.; Gorecka, M.A.; de Haan, A.F.; Mulders, P.; Punt, C.J.; et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 2012, 35, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Je, J.H.; Kim, S.H.; Shin, D.; Kim, T.G.; Kim, D.Y.; Kim, S.M.; Lee, M.G. Programmed death-ligand 1, 2 expressions are decreased in the psoriatic epidermis. Arch. Dermatol. Res. 2015, 307, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Okiyama, N.; Okune, M.; Ishitsuka, Y.; Watanabe, R.; Furuta, J.; Ohtsuka, M.; Otsuka, A.; Maruyama, H.; Fujisawa, Y.; et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab recativity. J. Dermatol. Sci. 2017, 86, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Shmidt, E.; Wetter, D.A.; Ferguson, S.B.; Pittelkow, M.R. Psoriasis and palmoplantar pustulosis associated with tumor necrosis factor-α inhibitors: The Mayo Clinic experience, 1998 to 2010. J. Am. Acad. Dermatol. 2012, 67, e179–e185. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Thi, E.P.; Carpio, V.H.; Bi, Y.; Cole, A.G.; Dorsey, B.D.; Fan, K.; Harasym, T.; Iott, C.L.; Kadhim, S.; et al. Checkpoint inhibition through small molecule-induced internalization of programmed death-ligand 1. Nat. Commun. 2021, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Callejo, A.; Frigola, J.; Iranzo, P.; Carbonell, C.; Diaz, N.; Marmolejo, D.; Assaf, J.D.; Cedrés, S.; Martinez-Marti, A.; Navarro, A.; et al. Interrelations between Patients’ Clinicopathological Characteristics and Their Association with Response to Immunotherapy in a Real-World Cohort of NSCLC Patients. Cancers 2021, 13, 3249. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events with Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-Analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef] [PubMed]
- Rogado, J.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Leví, A.; Arranz, R.; Lorenzo, A.; Gullón, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur. J. Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Chiari, R.; Ricciuti, B.; Metro, G.; Perrone, F.; Tiseo, M.; Bersanelli, M.; Bordi, P.; Santini, D.; Giusti, R.; et al. Correlations between the Immune-related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients. Clin. Lung Cancer 2019, 20, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Shankar, B.; Zhang, J.; Naqash, A.R.; Forde, P.M.; Feliciano, J.L.; Marrone, K.A.; Ettinger, D.S.; Hann, C.L.; Brahmer, J.R.; Ricciuti, B.; et al. Multisystem Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 1952–1956. [Google Scholar] [CrossRef]
- Schadendorf, D.; Wolchok, J.D.; Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Chesney, J.; et al. Efficacy and Safety Outcomes in Patients with Advanced Melanoma Who Discontinued Treatment with Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J. Clin. Oncol. 2017, 35, 3807–3814. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients with Melanoma Treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef]
- Bushue, N.; Wan, Y.J. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Balak, D.M.; Hajdarbegovic, E. Drug-induced psoriasis: Clinical perspectives. Psoriasis 2017, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Miller, W.H., Jr. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N. Engl. J. Med. 2017, 376, 1989–1991. [Google Scholar] [CrossRef]
| Number of patients | Total number of patients—1102 Patients with psoriatic skin lesions—1068 Patients with psoriatic arthritis—31 Patients with unspecified psoriasis/psoriatic arthritis—29 | ||
| Gender distribution | Male—509 Female—184 Unspecified—409 Male/female ratio—2.76 | ||
| Age distribution | Mean age—66.81 Most affected age group—61–70 | ||
| Types of neoplastic disease | Lung cancer (227 cases, 27.8%) Melanoma (82 cases, 10%) Urothelial cancer (22 cases, 2.7%) Head and neck scuamocellular carcinoma (19 cases, 2.3%) Renal cancer (19 cases, 2.3%) Hepatocellular carcinoma (11 cases, 1.3%) Digestive tract cancer (5 cases, 0.6%) Other specified cancer (15 cases, 1.8%) Unspecified neoplasia (419 cases, 51.2%) | ||
| Most common ICI-induced type of psoriasis | Plaque psoriasis | ||
| Type of ICI inducing psoriasis/psoriatic arthritis | Psoriasis | Psoriatic arthritis | |
| Nivolumab | 116 | 7 | |
| Pembrolizumab | 59 | 3 | |
| Cemiplimab | 1 | 0 | |
| Unspecified anti-PD-1 | 151 | 1 | |
| Atezolizumab | 18 | 0 | |
| Avelumab | 1 | 0 | |
| Durvalumab | 12 | 0 | |
| Unspecified anti-PD-L1 | 20 | 0 | |
| Ipilimumab | 1 | 0 | |
| Ipilimumab + Nivolumab | 8 | 3 | |
| Unspecified ICI | 402 | 18 | |
| Treatment needed for psoriatic lesions | Systemic treatment (37.5%), of which:
| Topical treatment (62.5%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, L.G.; Giurcaneanu, C.; Portelli, M.G.; Mihai, M.M.; Beiu, C.; Orzan, O.A.; Ion, A.; Anghel, T.H. Perspectives on Psoriasiform Adverse Events from Immune Checkpoint Inhibitors: Lessons Learned from Our Practice. Medicina 2024, 60, 373. https://doi.org/10.3390/medicina60030373
Popa LG, Giurcaneanu C, Portelli MG, Mihai MM, Beiu C, Orzan OA, Ion A, Anghel TH. Perspectives on Psoriasiform Adverse Events from Immune Checkpoint Inhibitors: Lessons Learned from Our Practice. Medicina. 2024; 60(3):373. https://doi.org/10.3390/medicina60030373
Chicago/Turabian StylePopa, Liliana Gabriela, Calin Giurcaneanu, Mariana Georgiana Portelli, Mara Mădălina Mihai, Cristina Beiu, Olguța Anca Orzan, Ana Ion, and Teodora Hrista Anghel. 2024. "Perspectives on Psoriasiform Adverse Events from Immune Checkpoint Inhibitors: Lessons Learned from Our Practice" Medicina 60, no. 3: 373. https://doi.org/10.3390/medicina60030373
APA StylePopa, L. G., Giurcaneanu, C., Portelli, M. G., Mihai, M. M., Beiu, C., Orzan, O. A., Ion, A., & Anghel, T. H. (2024). Perspectives on Psoriasiform Adverse Events from Immune Checkpoint Inhibitors: Lessons Learned from Our Practice. Medicina, 60(3), 373. https://doi.org/10.3390/medicina60030373











