The Evolution of Lateral Lumbar Interbody Fusion: A Journey from Past to Present
Abstract
:1. Evolution of Lumbar Interbody Fusion
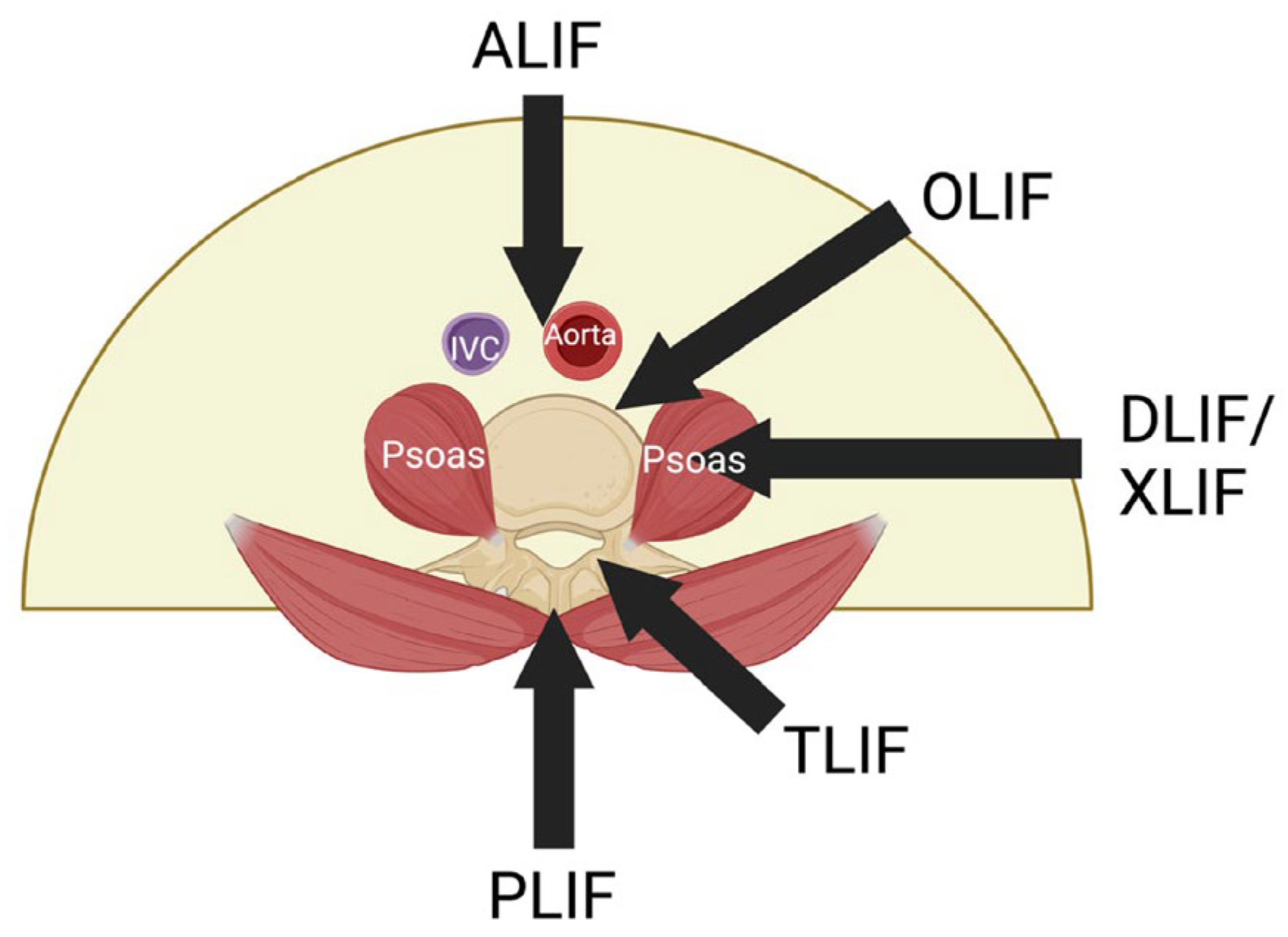
2. A Safer Approach
2.1. The Extreme Lateral Interbody Fusion (XLIF) or Direct Lateral Interbody Fusion (DLIF)
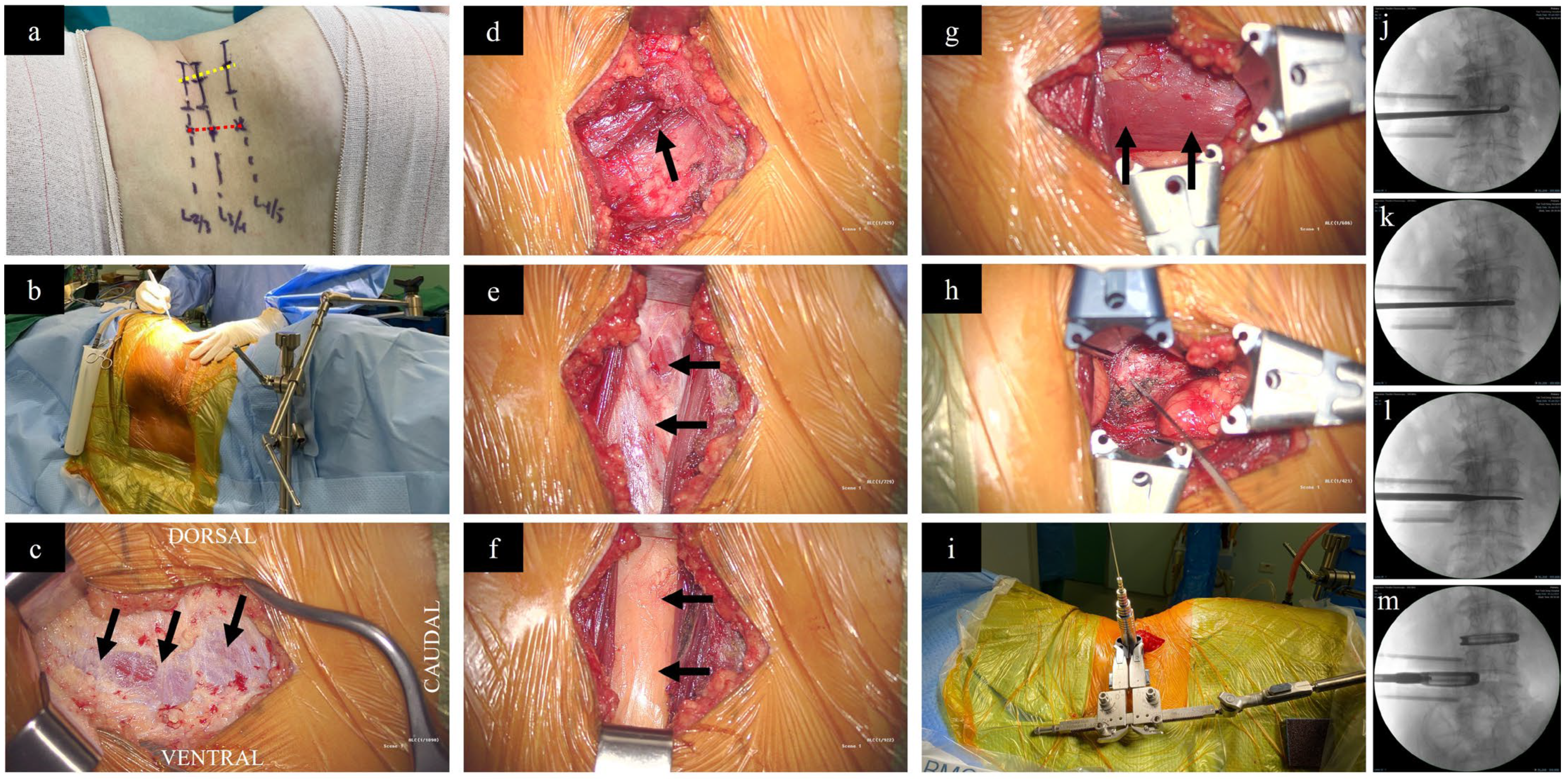
2.2. The Procedure of XLIF/DLIF
2.3. Benefits of XLIF over Other LIF Procedures
2.4. Limitations of Trans-Psoas Approach
3. The Oblique Lumbar Interbody Fusion (OLIF)
3.1. The Procedure of OLIF L2-L5 [82]
3.2. The Procedure of OLIF L5-S1 [82]
3.3. Advantages of OLIF over XLIF
3.4. Surgical Outcomes following OLIF
3.5. Limitations of OLIF and Strategies to Overcome
4. Recent Advances
4.1. Single-Position Surgery (SPS)—LLIF with Posterior Stabilization (PS)
4.1.1. Lateral Single-Position Surgery (L-SPS)
4.1.2. Prone Single-Position Surgery (P-SPS)
4.2. Robot-Assisted L-SPS and P-SPS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howorth, M.B. Evolution of Spinal Fusion. Ann. Surg. 1943, 117, 278–289. [Google Scholar] [CrossRef]
- Albee, F. The fundamental principles involved in the use of the bone graft in surgery. Am. J. Med. Sci. 1915, 149, 313–325. [Google Scholar] [CrossRef]
- Polikeit, A.; Ferguson, S.J.; Nolte, L.P.; Orr, T.E. The importance of the endplate for interbody cages in the lumbar spine. Eur. Spine J. 2003, 12, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.V.; Yoo, J.S.; Karmarkar, S.S.; Lamoutte, E.H.; Singh, K. Interbody options in lumbar fusion. J. Spine Surg. 2019, 5 (Suppl. S1), S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Cloward, R.B. Posterior lumbar interbody fusion updated. Clin. Orthop. Relat. Res. 1985, 193, 16–19. [Google Scholar] [CrossRef]
- Lane, J.D., Jr.; Moore, E.S., Jr. Transperitoneal Approach to the Intervertebral Disc in the Lumbar Area. Ann. Surg. 1948, 127, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Harms, J.; Rolinger, H. A one-stager procedure in operative treatment of spondylolistheses: Dorsal traction-reposition and anterior fusion (author’s transl). Z. Orthop. Ihre Grenzgeb. 1982, 120, 343–347. [Google Scholar] [CrossRef]
- Cole, C.D.; McCall, T.D.; Schmidt, M.H.; Dailey, A.T. Comparison of low back fusion techniques: Transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr. Rev. Musculoskelet. Med. 2009, 2, 118–126. [Google Scholar] [CrossRef]
- Audat, Z.; Moutasem, O.; Yousef, K.; Mohammad, B. Comparison of clinical and radiological results of posterolateral fusion, posterior lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singap. Med. J. 2012, 53, 183–187. [Google Scholar]
- De Kunder, S.L.; van Kuijk, S.M.J.; Rijkers, K.; Caelers, I.; van Hemert, W.L.W.; de Bie, R.A.; van Santbrink, H. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: A systematic review and meta-analysis. Spine J. 2017, 17, 1712–1721. [Google Scholar] [CrossRef]
- Allain, J.; Dufour, T. Anterior lumbar fusion techniques: ALIF, OLIF, DLIF, LLIF, IXLIF. Orthop. Traumatol. Surg. Res. 2020, 106, S149–S157. [Google Scholar] [CrossRef] [PubMed]
- Malham, G.M.; Parker, R.M.; Ellis, N.J.; Blecher, C.M.; Chow, F.Y.; Claydon, M.H. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: A prospective study of complications. J. Neurosurg. Spine 2014, 21, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Lindley, E.M.; McBeth, Z.L.; Henry, S.E.; Cooley, R.; Burger, E.L.; Cain, C.M.; Patel, V.V. Retrograde ejaculation after anterior lumbar spine surgery. Spine (Phila Pa 1976) 2012, 37, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Sasso, R.C.; Kenneth Burkus, J.; LeHuec, J.C. Retrograde ejaculation after anterior lumbar interbody fusion: Transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976) 2003, 28, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Inamasu, J.; Guiot, B.H. Vascular injury and complication in neurosurgical spine surgery. Acta Neurochir. 2006, 148, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Phan, K.; Malham, G.; Seex, K.; Rao, P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015, 1, 2–18. [Google Scholar]
- Okuda, S.; Miyauchi, A.; Oda, T.; Haku, T.; Yamamoto, T.; Iwasaki, M. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J. Neurosurg. Spine 2006, 4, 304–309. [Google Scholar] [CrossRef]
- Hosono, N.; Namekata, M.; Makino, T.; Miwa, T.; Kaito, T.; Kaneko, N.; Fuji, T. Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: Analysis of risk factors. J. Neurosurg. Spine 2008, 9, 403–407. [Google Scholar] [CrossRef]
- Wangaryattawanich, P.; Kale, H.A.; Kanter, A.S.; Agarwal, V. Lateral Lumbar Interbody Fusion: Review of Surgical Technique and Postoperative Multimodality Imaging Findings. AJR Am. J. Roentgenol. 2021, 217, 480–494. [Google Scholar] [CrossRef]
- Abdoli, S.; Sui, J.; Ziegler, K.; Katz, S.; Burnham, W.; Ochoa, C. The periumbilical incision for anterior lumbar interbody fusions. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 384–387. [Google Scholar] [CrossRef]
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion-a literature review. J. Spine Surg. 2020, 6, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L. Less-invasive lateral lumbar interbody fusion (XLIF) surgical technique: Video lecture. Eur. Spine J. 2015, 24 (Suppl. S3), 441–442. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L. Lateral endoscopic transpsoas retroperitoneal approach for lumbar spine surgery. In Proceedings of the VIII Brazilian Spine Society Meeting, Belo Horizonte, Brazil, 4 May 2001. [Google Scholar]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, S.N.; Shue, J.; Hughes, A.P. Lateral Lumbar Interbody Fusion-Outcomes and Complications. Curr. Rev. Musculoskelet. Med. 2017, 10, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, E.; Cardona, R.F.; Smith, D.A.; Uribe, J.S. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg. Focus. 2010, 28, E8. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Hughes, A.; Girardi, F.; Sama, A.; Lebl, D.; Cammisa, F. Lateral Lumbar Interbody Fusion. Asian Spine J. 2015, 9, 978–983. [Google Scholar] [CrossRef]
- Hu, W.K.; He, S.S.; Zhang, S.C.; Liu, Y.B.; Li, M.; Hou, T.S.; Ma, X.L.; Wang, J. An MRI study of psoas major and abdominal large vessels with respect to the X/DLIF approach. Eur. Spine J. 2011, 20, 557–562. [Google Scholar] [CrossRef]
- Patel, V.C.; Park, D.K.; Herkowitz, H.N. Lateral transpsoas fusion: Indications and outcomes. Sci. World J. 2012, 2012, 893608. [Google Scholar] [CrossRef]
- Berjano, P.; Gautschi, O.P.; Schils, F.; Tessitore, E. Extreme lateral interbody fusion (XLIF(R)): How I do it. Acta Neurochir. 2015, 157, 547–551. [Google Scholar] [CrossRef]
- Armocida, D.; Perna, A.; Cofano, F.; Cimatti, M.; Arcidiacono, U.A.; Marengo, N.; Ajello, M.; Garbossa, D.; Proietti, L.; Tamburrelli, F.C.; et al. Extreme Lateral Interbody Fusion (XLIF) with Lateral Modular Plate Fixation: Preliminary Report on Clinical and Radiological Outcomes. Acta Neurochir. Suppl. 2023, 135, 431–437. [Google Scholar]
- Blizzard, D.J.; Hills, C.P.; Isaacs, R.E.; Brown, C.R. Extreme lateral interbody fusion with posterior instrumentation for spondylodiscitis. J. Clin. Neurosci. 2015, 22, 1758–1761. [Google Scholar] [CrossRef] [PubMed]
- McAfee, P.C.; Shucosky, E.; Chotikul, L.; Salari, B.; Chen, L.; Jerrems, D. Multilevel extreme lateral interbody fusion (XLIF) and osteotomies for 3-dimensional severe deformity: 25 consecutive cases. Int. J. Spine Surg. 2013, 7, e8–e19. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.R.; Smith, B.W.; Patel, R.D.; Park, P. Use of 3D CT-based navigation in minimally invasive lateral lumbar interbody fusion. J. Neurosurg. Spine 2016, 25, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, W.B.; Gerber, E.J.; Patterson, J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: An analysis of 600 cases. Spine (Phila Pa 1976) 2011, 36, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, B.M.; Agarwal, V.; Nail, E.; Pimenta, L. Two-year clinical and radiographic success of minimally invasive lateral transpsoas approach for the treatment of degenerative lumbar conditions. SAS J. 2010, 4, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.M.; Michael, K.W.; Chapman, T.M., Jr.; Massey, G.M.; Howes, C.R.; Isaacs, R.E.; Brown, C.R. Clinical outcomes of extreme lateral interbody fusion in the treatment of adult degenerative scoliosis. Sci. World J. 2012, 2012, 680643. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.A.; McAfee, P.C.; Patty, C.A.; Raley, E.; DeBauche, S.; Shucosky, E.; Chotikul, L. Minimally invasive surgery: Lateral approach interbody fusion: Results and review. Spine (Phila Pa 1976) 2010, 35 (Suppl. S26), S302–S311. [Google Scholar] [CrossRef]
- Formica, M.; Berjano, P.; Cavagnaro, L.; Zanirato, A.; Piazzolla, A.; Formica, C. Extreme lateral approach to the spine in degenerative and post traumatic lumbar diseases: Selection process, results and complications. Eur. Spine J. 2014, 23 (Suppl. S6), 684–692. [Google Scholar] [CrossRef]
- Saadeh, Y.S.; Joseph, J.R.; Smith, B.W.; Kirsch, M.J.; Sabbagh, A.M.; Park, P. Comparison of Segmental Lordosis and Global Spinopelvic Alignment After Single-Level Lateral Lumbar Interbody Fusion or Transforaminal Lumbar Interbody Fusion. World Neurosurg. 2019, 126, e1374–e1378. [Google Scholar] [CrossRef]
- Jain, D.; Verma, K.; Mulvihill, J.; Mizutani, J.; Tay, B.; Burch, S.; Deviren, V. Comparison of Stand-Alone, Transpsoas Lateral Interbody Fusion at L3-4 and Cranial vs Transforaminal Interbody Fusion at L3-4 and L4-5 for the Treatment of Lumbar Adjacent Segment Disease. Int. J. Spine Surg. 2018, 12, 469–474. [Google Scholar] [CrossRef]
- Xu, D.S.; Bach, K.; Uribe, J.S. Minimally invasive anterior and lateral transpsoas approaches for closed reduction of grade II spondylolisthesis: Initial clinical and radiographic experience. Neurosurg. Focus. 2018, 44, E4. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.P.; Hu, J.W.; Zhang, Y.G.; Xu, H. Impact of lumbar interbody fusion surgery on postoperative outcomes in patients with recurrent lumbar disc herniation: Analysis of the US national inpatient sample. J. Clin. Neurosci. 2019, 70, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hrabalek, L.; Sternbersky, J.; Adamus, M. Risk of sympathectomy after anterior and lateral lumbar interbody fusion procedures. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2015, 159, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Sembrano, J.N.; Tohmeh, A.; Isaacs, R.; Group, S.D.S. Two-year Comparative Outcomes of MIS Lateral and MIS Transforaminal Interbody Fusion in the Treatment of Degenerative Spondylolisthesis: Part I: Clinical Findings. Spine (Phila Pa 1976) 2016, 41 (Suppl. S8), S123–S132. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, R.E.; Sembrano, J.N.; Tohmeh, A.G.; Group, S.D.S. Two-Year Comparative Outcomes of MIS Lateral and MIS Transforaminal Interbody Fusion in the Treatment of Degenerative Spondylolisthesis: Part II: Radiographic Findings. Spine (Phila Pa 1976) 2016, 41 (Suppl. S8), S133–S144. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Lu, Y. Comparison of Biomechanical Performance Among Posterolateral Fusion and Transforaminal, Extreme, and Oblique Lumbar Interbody Fusion: A Finite Element Analysis. World Neurosurg. 2019, 129, e890–e899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bai, S.; Dokos, S.; Cheung, J.P.; Diwan, A.D. XLIF interbody cage reduces stress and strain of fixation in spinal reconstructive surgery in comparison with TLIF cage with bilateral or unilateral fixation: A computational analysis. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 1887–1890. [Google Scholar] [PubMed]
- Ohba, T.; Ebata, S.; Haro, H. Comparison of serum markers for muscle damage, surgical blood loss, postoperative recovery, and surgical site pain after extreme lateral interbody fusion with percutaneous pedicle screws or traditional open posterior lumbar interbody fusion. BMC Musculoskelet. Disord. 2017, 18, 415. [Google Scholar] [CrossRef]
- Goodnough, L.H.; Koltsov, J.; Wang, T.; Xiong, G.; Nathan, K.; Cheng, I. Decreased estimated blood loss in lateral trans-psoas versus anterior approach to lumbar interbody fusion for degenerative spondylolisthesis. J. Spine Surg. 2019, 5, 185–193. [Google Scholar] [CrossRef]
- Sembrano, J.N.; Yson, S.C.; Horazdovsky, R.D.; Santos, E.R.; Polly, D.W., Jr. Radiographic Comparison of Lateral Lumbar Interbody Fusion Versus Traditional Fusion Approaches: Analysis of Sagittal Contour Change. Int. J. Spine Surg. 2015, 9, 16. [Google Scholar] [CrossRef]
- Yingsakmongkol, W.; Jitpakdee, K.; Varakornpipat, P.; Choentrakool, C.; Tanasansomboon, T.; Limthongkul, W.; Singhatanadgige, W.; Kotheeranurak, V. Clinical and Radiographic Comparisons among Minimally Invasive Lumbar Interbody Fusion: A Comparison with Three-Way Matching. Asian Spine J. 2022, 16, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Gen, H.; Sakuma, Y.; Koshika, Y. Comparison of Clinical and Radiologic Results of Mini-Open Transforaminal Lumbar Interbody Fusion and Extreme Lateral Interbody Fusion Indirect Decompression for Degenerative Lumbar Spondylolisthesis. Asian Spine J. 2018, 12, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kotheeranurak, V.; Jitpakdee, K.; Lin, G.X.; Mahatthanatrakul, A.; Singhatanadgige, W.; Limthongkul, W.; Yingsakmongkol, W.; Kim, J.S. Subsidence of Interbody Cage Following Oblique Lateral Interbody Fusion: An Analysis and Potential Risk Factors. Glob. Spine J. 2021, 13, 1981–1991. [Google Scholar] [CrossRef]
- Tempel, Z.J.; McDowell, M.M.; Panczykowski, D.M.; Gandhoke, G.S.; Hamilton, D.K.; Okonkwo, D.O.; Kanter, A.S. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J. Neurosurg. Spine 2018, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xie, T.; Wang, X.; Yang, Z.; Pu, X.; Lu, Y.; Zeng, J. Clinical and radiological evaluation of cage subsidence following oblique lumbar interbody fusion combined with anterolateral fixation. BMC Musculoskelet. Disord. 2022, 23, 214. [Google Scholar] [CrossRef] [PubMed]
- Amorim-Barbosa, T.; Pereira, C.; Catelas, D.; Rodrigues, C.; Costa, P.; Rodrigues-Pinto, R.; Neves, P. Risk factors for cage subsidence and clinical outcomes after transforaminal and posterior lumbar interbody fusion. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Marchi, L.; Abdala, N.; Oliveira, L.; Amaral, R.; Coutinho, E.; Pimenta, L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J. Neurosurg. Spine 2013, 19, 110–118. [Google Scholar] [CrossRef]
- Lang, G.; Navarro-Ramirez, R.; Gandevia, L.; Hussain, I.; Nakhla, J.; Zubkov, M.; Hartl, R. Elimination of Subsidence with 26-mm-Wide Cages in Extreme Lateral Interbody Fusion. World Neurosurg. 2017, 104, 644–652. [Google Scholar] [CrossRef]
- Alimi, M.; Lang, G.; Navarro-Ramirez, R.; Perrech, M.; Berlin, C.; Hofstetter, C.P.; Moriguchi, Y.; Elowitz, E.; Hartl, R. The Impact of Cage Dimensions, Positioning, and Side of Approach in Extreme Lateral Interbody Fusion. Clin. Spine Surg. 2018, 31, E42–E49. [Google Scholar] [CrossRef]
- Fernandes, R.J.R.; Gee, A.; Kanawati, A.J.; Siddiqi, F.; Rasoulinejad, P.; Zdero, R.; Bailey, C.S. Evaluation of the contact surface between vertebral endplate and 3D printed patient-specific cage vs commercial cage. Sci. Rep. 2022, 12, 12505. [Google Scholar] [CrossRef]
- Le, T.V.; Baaj, A.A.; Dakwar, E.; Burkett, C.J.; Murray, G.; Smith, D.A.; Uribe, J.S. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012, 37, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kepler, C.K.; Girardi, F.P.; Cammisa, F.P.; Huang, R.C.; Sama, A.A. Lateral lumbar interbody fusion: Clinical and radiographic outcomes at 1 year: A preliminary report. J. Spinal Disord. Tech. 2011, 24, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L.; Turner, A.W.; Dooley, Z.A.; Parikh, R.D.; Peterson, M.D. Biomechanics of lateral interbody spacers: Going wider for going stiffer. Sci. World J. 2012, 2012, 381814. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Kaliya-Perumal, A.K.; Chou, S.M.; Oh, J.Y. Does Lumbar Interbody Cage Size Influence Subsidence? A Biomechanical Study. Spine (Phila Pa 1976) 2020, 45, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hijji, F.Y.; Narain, A.S.; Bohl, D.D.; Ahn, J.; Long, W.W.; DiBattista, J.V.; Kudaravalli, K.T.; Singh, K. Lateral lumbar interbody fusion: A systematic review of complication rates. Spine J. 2017, 17, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Review of Risks and Complications of Extreme Lateral Interbody Fusion (XLIF). Surg. Neurol. Int. 2019, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, K.; Shen, A.; Lagina, M.; Hutchison, A. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur. Spine J. 2015, 24 (Suppl. S3), 322–330. [Google Scholar] [CrossRef] [PubMed]
- Uribe, J.S.; Isaacs, R.E.; Youssef, J.A.; Khajavi, K.; Balzer, J.R.; Kanter, A.S.; Kuelling, F.A.; Peterson, M.D.; Group, S.D.S. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur. Spine J. 2015, 24 (Suppl. S3), 378–385. [Google Scholar] [CrossRef]
- O’Brien, J.R. Nerve Injury in Lateral Lumbar Interbody Fusion. Spine (Phila Pa 1976) 2017, 42 (Suppl. S7), S24. [Google Scholar] [CrossRef]
- Abel, N.A.; Januszewski, J.; Vivas, A.C.; Uribe, J.S. Femoral nerve and lumbar plexus injury after minimally invasive lateral retroperitoneal transpsoas approach: Electrodiagnostic prognostic indicators and a roadmap to recovery. Neurosurg. Rev. 2018, 41, 457–464. [Google Scholar] [CrossRef]
- Cummock, M.D.; Vanni, S.; Levi, A.D.; Yu, Y.; Wang, M.Y. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J. Neurosurg. Spine 2011, 15, 11–18. [Google Scholar] [CrossRef]
- Tohmeh, A.G.; Rodgers, W.B.; Peterson, M.D. Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J. Neurosurg. Spine 2011, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Regev, G.J.; Chan, J.; Zhang, B.; Taylor, W.; Kim, C.W.; Garfin, S.R. Evaluation of hip flexion strength following lateral lumbar interbody fusion. Spine J. 2013, 13, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. High neurological complication rates for extreme lateral lumbar interbody fusion and related techniques: A review of safety concerns. Surg. Neurol. Int. 2016, 7 (Suppl. S25), S652–S655. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, A.; Deukmedjian, A.R.; Abel, N.; Dakwar, E.; Uribe, J.S. Analysis of lumbar plexopathies and nerve injury after lateral retroperitoneal transpsoas approach: Diagnostic standardization. J. Neurosurg. Spine 2013, 18, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, H.; Okuda, T.; Takano, H.; Gomi, M.; Takahashi, R.; Shimura, A.; Tamagawa, S.; Hara, T.; Ohara, Y.; Ishijima, M. Elimination of Lumbar Plexus Injury by Changing the Entry Point and Traction Direction of the Psoas Major Muscle in Transpsoas Lateral Lumbar Spine Surgery. Medicina 2023, 59, 730. [Google Scholar] [CrossRef] [PubMed]
- Buric, J.; Bombardieri, D. Direct lesion and repair of a common iliac vein during XLIF approach. Eur. Spine J. 2016, 25 (Suppl. S1), 89–93. [Google Scholar] [CrossRef]
- Assina, R.; Majmundar, N.J.; Herschman, Y.; Heary, R.F. First report of major vascular injury due to lateral transpsoas approach leading to fatality. J. Neurosurg. Spine 2014, 21, 794–798. [Google Scholar] [CrossRef]
- Epstein, N.E. Incidence of Major Vascular Injuries with Extreme Lateral Interbody Fusion (XLIF). Surg. Neurol. Int. 2020, 11, 70. [Google Scholar] [CrossRef]
- Xu, J.; Chen, E.; Wang, L.; Zou, X.; Deng, C.; Chen, J.; Ma, R.; Ma, X.; Wu, Z. Extreme lateral interbody fusion (XLIF) approach for L5-S1: Preliminary experience. Front. Surg. 2022, 9, 995662. [Google Scholar] [CrossRef]
- Woods, K.R.; Billys, J.B.; Hynes, R.A. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J. 2017, 17, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Zhou, H.; Jiang, W. Development and Application of Oblique Lumbar Interbody Fusion. Orthop. Surg. 2020, 12, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.R.; Wong, R.A.; Kaliya-Perumal, A.K.; Oh, J.Y.L. Complications Associated with Oblique Lumbar Interbody Fusion: A Systematic Review. Surg. Tech. Dev. 2023, 12, 211–223. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Wu, H.; Chen, Z.; Chou, D.; Jian, F. The Anatomic Characteristics of the Retroperitoneal Oblique Corridor to the L1-S1 Intervertebral Disc Spaces. Spine (Phila Pa 1976) 2019, 44, E697–E706. [Google Scholar] [CrossRef] [PubMed]
- Razzouk, J.; Ramos, O.; Mehta, S.; Harianja, G.; Wycliffe, N.; Danisa, O.; Cheng, W. Anterior-To-Psoas Approach Measurements, Feasibility, Non-Neurological Structures at Risk and Influencing Factors: A Bilateral Analysis From L1-L5 Using Computed Tomography Imaging. Oper. Neurosurg. 2023, 25, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Zhong, L.; Min, J.K.; Fang, X.Q.; Jiang, L.S. Oblique lateral interbody fusion combined with lateral plate fixation for the treatment of degenerative diseases of the lumbar spine: A retrospective study. Medicine 2022, 101, e28784. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, B.; Wang, H.; Qi, J.; Gu, X.; Yu, J.; Ye, X.; Xu, G.; Xi, Y. Oblique lateral interbody fusion stand-alone vs. combined with percutaneous pedicle screw fixation in the treatment of discogenic low back pain. Front. Surg. 2022, 9, 1013431. [Google Scholar] [CrossRef]
- Phan, K.; Mobbs, R.J. Oblique Lumbar Interbody Fusion for Revision of Non-union Following Prior Posterior Surgery: A Case Report. Orthop. Surg. 2015, 7, 364–367. [Google Scholar] [CrossRef]
- Chung, H.W.; Lee, H.D.; Jeon, C.H.; Chung, N.S. Comparison of surgical outcomes between oblique lateral interbody fusion (OLIF) and anterior lumbar interbody fusion (ALIF). Clin. Neurol. Neurosurg. 2021, 209, 106901. [Google Scholar] [CrossRef]
- Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Ishikawa, T.; et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Lumbar Spinal Degeneration Disease. Yonsei Med. J. 2015, 56, 1051–1059. [Google Scholar] [CrossRef]
- Sato, J.; Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Miyagi, M.; et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: Oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur. Spine J. 2017, 26, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, C.; Mac-Thiong, J.M.; Hilmi, R.; Roussouly, P. Complications and Morbidities of Mini-open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lumbar Interbody Fusion in 179 Patients. Asian Spine J. 2012, 6, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gragnaniello, C.; Seex, K. Anterior to psoas (ATP) fusion of the lumbar spine: Evolution of a technique facilitated by changes in equipment. J. Spine Surg. 2016, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Orita, S.; Mannoji, C.; Motegi, H.; Aramomi, M.; Ishikawa, T.; Kotani, T.; Akazawa, T.; Morinaga, T.; Fujiyoshi, T.; et al. Perioperative Complications in 155 Patients Who Underwent Oblique Lateral Interbody Fusion Surgery: Perspectives and Indications From a Retrospective, Multicenter Survey. Spine (Phila Pa 1976) 2017, 42, 55–62. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Xu, Z.W.; He, D.W.; Zhao, X.; Ma, W.H.; Ni, W.F.; Song, Y.X.; Zhang, J.Q.; Yu, W.; Fang, X.Q.; et al. Complications and Prevention Strategies of Oblique Lateral Interbody Fusion Technique. Orthop. Surg. 2018, 10, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mahan, M.A.; Sanders, L.E.; Guan, J.; Dailey, A.T.; Taylor, W.; Morton, D.A. Anatomy of psoas muscle innervation: Cadaveric study. Clin. Anat. 2017, 30, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Kawakami, N.; Asazuma, T.; Ito, M.; Mizutani, J.; Nagashima, H.; Nakamura, M.; Sairyo, K.; Takemasa, R.; Iwasaki, M. Complications Associated With Lateral Interbody Fusion: Nationwide Survey of 2998 Cases During the First 2 Years of Its Use in Japan. Spine (Phila Pa 1976) 2017, 42, 1478–1484. [Google Scholar] [CrossRef]
- Lee, S.; Kim, A.R.; Bang, W.S.; Park, J.H.; Lee, S.W.; Kim, K.T.; Cho, D.C. Psoas weakness following oblique lateral interbody fusion surgery: A prospective observational study with an isokinetic dynamometer. Spine J. 2022, 22, 1990–1999. [Google Scholar] [CrossRef]
- Sadrameli, S.S.; Davidov, V.; Huang, M.; Lee, J.J.; Ramesh, S.; Guerrero, J.R.; Wong, M.S.; Boghani, Z.; Ordonez, A.; Barber, S.M.; et al. Complications associated with L4-5 anterior retroperitoneal trans-psoas interbody fusion: A single institution series. J. Spine Surg. 2020, 6, 562–571. [Google Scholar] [CrossRef]
- Li, J.X.; Phan, K.; Mobbs, R. Oblique Lumbar Interbody Fusion: Technical Aspects, Operative Outcomes, and Complications. World Neurosurg. 2017, 98, 113–123. [Google Scholar] [CrossRef]
- Li, H.M.; Zhang, R.J.; Shen, C.L. Differences in radiographic and clinical outcomes of oblique lateral interbody fusion and lateral lumbar interbody fusion for degenerative lumbar disease: A meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 582. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.T.; Farber, S.H.; Cole, T.S.; Xu, D.S.; Godzik, J.; Whiting, A.C.; Hartman, C.; Porter, R.W.; Turner, J.D.; Uribe, J. Complications for minimally invasive lateral interbody arthrodesis: A systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J. Neurosurg. Spine 2019, 30, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Aleinik, A.Y.; Mlyavykh, S.G.; Qureshi, S. Lumbar Spinal Fusion Using Lateral Oblique (Pre-psoas) Approach (Review). Sovrem. Tekhnologii Med. 2021, 13, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Rabau, O.; Navarro-Ramirez, R.; Aziz, M.; Teles, A.; Mengxiao Ge, S.; Quillo-Olvera, J.; Ouellet, J. Lateral Lumbar Interbody Fusion (LLIF): An Update. Glob. Spine J. 2020, 10 (Suppl. S2), 17S–21S. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Park, C.W.; Sharma, S.; Kotheeranurak, V.; Kim, J.S. Endoscopic anterior to psoas lumbar interbody fusion: Indications, techniques, and clinical outcomes. Eur. Spine J. 2023, 32, 2776–2795. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.S.; Walker, C.T.; Godzik, J.; Turner, J.D.; Smith, W.; Uribe, J.S. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: A literature review. Ann. Transl. Med. 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- He, W.; He, D.; Tian, W. Evaluation of lumbar fusion using the anterior to psoas approach for the treatment of L5/S1 spondylolisthesis. Medicine 2020, 99, e20014. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Virk, S.; Qureshi, S. Interbody Fusions in the Lumbar Spine: A Review. HSS J. 2020, 16, 162–167. [Google Scholar] [CrossRef]
- Jin, C.; Jaiswal, M.S.; Jeun, S.S.; Ryu, K.S.; Hur, J.W.; Kim, J.S. Outcomes of oblique lateral interbody fusion for degenerative lumbar disease in patients under or over 65 years of age. J. Orthop. Surg. Res. 2018, 13, 38. [Google Scholar] [CrossRef]
- Schwab, F.; Ungar, B.; Blondel, B.; Buchowski, J.; Coe, J.; Deinlein, D.; DeWald, C.; Mehdian, H.; Shaffrey, C.; Tribus, C.; et al. Scoliosis Research Society-Schwab adult spinal deformity classification: A validation study. Spine (Phila Pa 1976) 2012, 37, 1077–1082. [Google Scholar] [CrossRef]
- Tung, K.K.; Tseng, W.C.; Wu, Y.C.; Chen, K.H.; Pan, C.C.; Lu, W.X.; Shih, C.M.; Lee, C.H. Comparison of radiographic and clinical outcomes between ALIF, OLIF, and TLIF over 2-year follow-up: A comparative study. J. Orthop. Surg. Res. 2023, 18, 158. [Google Scholar] [CrossRef]
- Ohtori, S.; Mannoji, C.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Degenerated Lumbar Spinal Kyphoscoliosis. Asian Spine J. 2015, 9, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kwon, S.W.; Moon, J.H.; Kim, C.H.; Chung, C.K.; Park, S.B.; Heo, W. Lateral Lumbar Interbody Fusion and in Situ Screw Fixation for Rostral Adjacent Segment Stenosis of the Lumbar Spine. J. Korean Neurosurg. Soc. 2017, 60, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Patel, A.; Ungar, B.; Farcy, J.P.; Lafage, V. Adult spinal deformity-postoperative standing imbalance: How much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010, 35, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Harroud, A.; Labelle, H.; Joncas, J.; Mac-Thiong, J.M. Global sagittal alignment and health-related quality of life in lumbosacral spondylolisthesis. Eur. Spine J. 2013, 22, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Seo, E.M. Efficacy and radiographic analysis of oblique lumbar interbody fusion in treating lumbar degenerative spondylolisthesis with sagittal imbalance. Neurosurg. Rev. 2021, 44, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.J.; Park, S.W.; Kim, Y.B. Effect of Cage in Radiological Differences between Direct and Oblique Lateral Interbody Fusion Techniques. J. Korean Neurosurg. Soc. 2019, 62, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Emami, A.; Patel, N.; Coban, D.; Saela, S.; Sinha, K.; Faloon, M.; Hwang, K.S. Comparing clinical and radiological outcomes between single-level OLIF and XLIF: A systematic review and meta-analysis. N. Am. Spine Soc. J. 2023, 14, 100216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, B.S.; Chang, S.Y. Pearls and Pitfalls of Oblique Lateral Interbody Fusion: A Comprehensive Narrative Review. Neurospine 2022, 19, 163–176. [Google Scholar] [CrossRef]
- Jin, J.; Ryu, K.S.; Hur, J.W.; Seong, J.H.; Kim, J.S.; Cho, H.J. Comparative Study of the Difference of Perioperative Complication and Radiologic Results: MIS-DLIF (Minimally Invasive Direct Lateral Lumbar Interbody Fusion) Versus MIS-OLIF (Minimally Invasive Oblique Lateral Lumbar Interbody Fusion). Clin. Spine Surg. 2018, 31, 31–36. [Google Scholar] [CrossRef]
- Miscusi, M.; Ramieri, A.; Forcato, S.; Giuffre, M.; Trungu, S.; Cimatti, M.; Pesce, A.; Familiari, P.; Piazza, A.; Carnevali, C.; et al. Comparison of pure lateral and oblique lateral inter-body fusion for treatment of lumbar degenerative disk disease: A multicentric cohort study. Eur. Spine J. 2018, 27 (Suppl. S2), 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, L.; Piazza, A.; Capobianco, M.; Della Pepa, G.M.; Miscusi, M.; Raco, A.; Scerrati, A.; Somma, T.; Lofrese, G.; Sturiale, C.L. Lumbar interbody fusion using oblique (OLIF) and lateral (LLIF) approaches for degenerative spine disorders: A meta-analysis of the comparative studies. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.U.R.; Cesare, J.; Wahood, W.; Alvi, M.A.; Onyedimma, C.E.; Ghaith, A.K.; Akinnusotu, O.; El Sammak, S.; Freedman, B.A.; Sebastian, A.S.; et al. Assessing the differences in operative and patient-reported outcomes between lateral approaches for lumbar fusion: A systematic review and indirect meta-analysis. J. Neurosurg. Spine 2022, 37, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Uribe, J.S. Neural anatomy, neuromonitoring and related complications in extreme lateral interbody fusion: Video lecture. Eur. Spine J. 2015, 24 (Suppl. S3), 445–446. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Hassan, O.; Diaz-Aguilar, L.D.; Lehman, R.A. Complications Associated With Oblique Lumbar Interbody Fusion at L5-S1: A Systematic Review of the Literature. Neurosurg. Pract. 2021, 2, okab018. [Google Scholar] [CrossRef]
- Chang, S.Y.; Lee, W.S.; Mok, S.; Park, S.C.; Kim, H.; Chang, B.S. Anterior Thigh Pain Following Minimally Invasive Oblique Lateral Interbody Fusion: Multivariate Analysis from a Prospective Case Series. Clin. Orthop. Surg. 2022, 14, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.; Phan, K.; Smith, A.; Stewart, F.; Seex, K.; Gragnaniello, C. Morphometric anatomy of the lumbar sympathetic trunk with respect to the anterolateral approach to lumbar interbody fusion: A cadaver study. J. Spine Surg. 2017, 3, 419–425. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Zhao, Z.; Chou, D.; Jian, F.; Wu, H. A modified oblique lumbar interbody fusion: A better way to establish an exposure under direct microscopic vision. Front. Surg. 2023, 10, 1130489. [Google Scholar] [CrossRef]
- Quillo-Olvera, J.; Lin, G.X.; Jo, H.J.; Kim, J.S. Complications on minimally invasive oblique lumbar interbody fusion at L2-L5 levels: A review of the literature and surgical strategies. Ann. Transl. Med. 2018, 6, 101. [Google Scholar] [CrossRef]
- Liu, L.; Liang, Y.; Zhang, H.; Wang, H.; Guo, C.; Pu, X.; Zhang, C.; Wang, L.; Wang, J.; Lv, Y.; et al. Imaging Anatomical Research on the Operative Windows of Oblique Lumbar Interbody Fusion. PLoS ONE 2016, 11, e0163452. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, G.; Liang, Z.; Zhang, P.; Ge, Z.; Lin, S.; Wang, X.; Yu, X.; Tang, J.; Ren, H.; et al. Application of offset Dingo instruments in Anterior to Psoas (ATP)/Oblique Lumbar Interbody Fusion (OLIF) procedure: A retrospective study of 80 patients. Neurochirurgie 2022, 68, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Otsuki, B.; Kimura, H.; Tanida, S.; Masamoto, K.; Matsuda, S. Preoperative assessment of the ureter with dual-phase contrast-enhanced computed tomography for lateral lumbar interbody fusion procedures. J. Orthop. Sci. 2017, 22, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Menezes, C.; Buckland, A.J.; Khajavi, K.; Ashayeri, K.; Braly, B.A.; Kwon, B.; Cheng, I.; Berjano, P. Single-position circumferential lumbar spinal fusion: An overview of terminology, concepts, rationale and the current evidence base. Eur. Spine J. 2022, 31, 2167–2174. [Google Scholar] [CrossRef]
- Ziino, C.; Konopka, J.A.; Ajiboye, R.M.; Ledesma, J.B.; Koltsov, J.C.B.; Cheng, I. Single position versus lateral-then-prone positioning for lateral interbody fusion and pedicle screw fixation. J. Spine Surg. 2018, 4, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ziino, C.; Arzeno, A.; Cheng, I. Analysis of single-position for revision surgery using lateral interbody fusion and pedicle screw fixation: Feasibility and perioperative results. J. Spine Surg. 2019, 5, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Blizzard, D.J.; Thomas, J.A. MIS Single-position Lateral and Oblique Lateral Lumbar Interbody Fusion and Bilateral Pedicle Screw Fixation: Feasibility and Perioperative Results. Spine (Phila Pa 1976) 2018, 43, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, B.P.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef]
- Ogihara, S.; Yamazaki, T.; Maruyama, T.; Oka, H.; Miyoshi, K.; Azuma, S.; Yamada, T.; Murakami, M.; Kawamura, N.; Hara, N.; et al. Prospective multicenter surveillance and risk factor analysis of deep surgical site infection after posterior thoracic and/or lumbar spinal surgery in adults. J. Orthop. Sci. 2015, 20, 71–77. [Google Scholar] [CrossRef]
- Kim, B.D.; Hsu, W.K.; De Oliveira, G.S., Jr.; Saha, S.; Kim, J.Y. Operative duration as an independent risk factor for postoperative complications in single-level lumbar fusion: An analysis of 4588 surgical cases. Spine (Phila Pa 1976) 2014, 39, 510–520. [Google Scholar] [CrossRef]
- Cheng, P.; Zhang, X.B.; Zhao, Q.M.; Zhang, H.H. Efficacy of Single-Position Oblique Lateral Interbody Fusion Combined with Percutaneous Pedicle Screw Fixation in Treating Degenerative Lumbar Spondylolisthesis: A Cohort Study. Front. Neurol. 2022, 13, 856022. [Google Scholar] [CrossRef]
- Guiroy, A.; Carazzo, C.; Camino-Willhuber, G.; Gagliardi, M.; Fernandes-Joaquim, A.; Cabrera, J.P.; Menezes, C.; Asghar, J. Single-Position Surgery versus Lateral-Then-Prone-Position Circumferential Lumbar Interbody Fusion: A Systematic Literature Review. World Neurosurg. 2021, 151, e379–e386. [Google Scholar] [CrossRef] [PubMed]
- Godzik, J.; Ohiorhenuan, I.E.; Xu, D.S.; de Andrada Pereira, B.; Walker, C.T.; Whiting, A.C.; Turner, J.D.; Uribe, J.S. Single-position prone lateral approach: Cadaveric feasibility study and early clinical experience. Neurosurg. Focus. 2020, 49, E15. [Google Scholar] [CrossRef] [PubMed]
- Drazin, D.; Kim, T.T.; Johnson, J.P. Simultaneous Lateral Interbody Fusion and Posterior Percutaneous Instrumentation: Early Experience and Technical Considerations. Biomed. Res. Int. 2015, 2015, 458284. [Google Scholar] [CrossRef]
- Keorochana, G.; Muljadi, J.A.; Kongtharvonskul, J. Perioperative and Radiographic Outcomes Between Single-Position Surgery (Lateral Decubitus) and Dual-Position Surgery for Lateral Lumbar Interbody Fusion and Percutaneous Pedicle Screw Fixation: Meta-Analysis. World Neurosurg. 2022, 165, e282–e291. [Google Scholar] [CrossRef] [PubMed]
- Ouchida, J.; Kanemura, T.; Satake, K.; Nakashima, H.; Ishikawa, Y.; Imagama, S. Simultaneous single-position lateral interbody fusion and percutaneous pedicle screw fixation using O-arm-based navigation reduces the occupancy time of the operating room. Eur. Spine J. 2020, 29, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Buckland, A.J.; Proctor, D.; Thomas, J.A.; Protopsaltis, T.S.; Ashayeri, K.; Braly, B.A. Single-Position Prone Lateral Lumbar Interbody Fusion Increases Operative Efficiency and Maintains Safety in Revision Lumbar Spinal Fusion. Spine (Phila Pa 1976) 2023, 49, E19–E24. [Google Scholar] [CrossRef] [PubMed]
- Barkay, G.; Wellington, I.; Mallozzi, S.; Singh, H.; Moss, I.L. The Prone Lateral Approach for Lumbar Fusion-A Review of the Literature and Case Series. Medicina 2023, 59, 251. [Google Scholar] [CrossRef]
- Wellington, I.J.; Antonacci, C.L.; Chaudhary, C.; Coskun, E.; Cote, M.P.; Singh, H.; Mallozzi, S.S.; Moss, I.L. Early Clinical Outcomes of the Prone Transpsoas Lumbar Interbody Fusion Technique. Int. J. Spine Surg. 2023, 17, 112–121. [Google Scholar] [CrossRef]
- Hiyama, A.; Katoh, H.; Sakai, D.; Tanaka, M.; Sato, M.; Watanabe, M. Facet joint violation after single-position versus dual-position lateral interbody fusion and percutaneous pedicle screw fixation: A comparison of two techniques. J. Clin. Neurosci. 2020, 78, 47–52. [Google Scholar] [CrossRef]
- Lopez, G.; Sayari, A.J.; Phillips, F. Single-Position Anterior Column Lateral Lumbar Interbody Fusion. Int. J. Spine Surg. 2022, 16, S17–S25. [Google Scholar] [CrossRef]
- Pimenta, L.; Pokorny, G.; Amaral, R.; Ditty, B.; Batista, M.; Moriguchi, R.; Filho, F.M.; Taylor, W.R. Single-Position Prone Transpsoas Lateral Interbody Fusion Including L4L5: Early Postoperative Outcomes. World Neurosurg. 2021, 149, e664–e668. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Joseph, S.A., Jr.; Ditty, B.; Amaral, R.; Tohmeh, A.; Taylor, W.R.; Pimenta, L. Initial multi-centre clinical experience with prone transpsoas lateral interbody fusion: Feasibility, perioperative outcomes, and lessons learned. N. Am. Spine Soc. J. 2021, 6, 100056. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, N.L.; Uribe, J.S.; Randolph, B.M.; Buchanan, R.I. Prone Lateral Lumbar Interbody Fusion: Case Report and Technical Note. World Neurosurg. 2020, 144, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.; Moriguchi, R.; Pokorny, G.; Arnoni, D.; Barreira, I.; Marcelino, F.; Pokorny, J.; Pimenta, L. Comparison of segmental lordosis gain of prone transpsoas (PTP) vs. lateral lumbar interbody fusion. Arch. Orthop. Trauma. Surg. 2023, 143, 5485–5490. [Google Scholar] [CrossRef] [PubMed]
- Alan, N.; Kanter, J.J.; Puccio, L.; Anand, S.K.; Kanter, A.S. Transitioning from lateral to the prone transpsoas approach: Flatten the learning curve by knowing the nuances. Neurosurg. Focus. Video 2022, 7, V8. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, C.; Berjano, P. Prone single-position extreme lateral interbody fusion (Pro-XLIF): Preliminary results. Eur. Spine J. 2020, 29 (Suppl. S1), 6–13. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, L.; Amaral, R.; Taylor, W.; Tohmeh, A.; Pokorny, G.; Rodrigues, R.; Arnoni, D.; Guirelli, T.; Batista, M. The prone transpsoas technique: Preliminary radiographic results of a multicenter experience. Eur. Spine J. 2021, 30, 108–113. [Google Scholar] [CrossRef]
- Soliman, M.A.R.; Aguirre, A.O.; Ruggiero, N.; Kuo, C.C.; Mariotti, B.L.; Khan, A.; Mullin, J.P.; Pollina, J. Comparison of prone transpsoas lateral lumbar interbody fusion and transforaminal lumbar interbody fusion for degenerative lumbar spine disease: A retrospective radiographic propensity score-matched analysis. Clin. Neurol. Neurosurg. 2022, 213, 107105. [Google Scholar] [CrossRef]
- Soliman, M.A.R.; Khan, A.; Pollina, J. Comparison of Prone Transpsoas and Standard Lateral Lumbar Interbody Fusion Surgery for Degenerative Lumbar Spine Disease: A Retrospective Radiographic Propensity Score-Matched Analysis. World Neurosurg. 2022, 157, e11–e21. [Google Scholar] [CrossRef]
- Lieberman, I.H.; Kisinde, S.; Hesselbacher, S. Robotic-Assisted Pedicle Screw Placement During Spine Surgery. JBJS Essent. Surg. Tech. 2020, 10, e0020. [Google Scholar] [CrossRef]
- Asada, T.; Simon, C.Z.; Lu, A.Z.; Adida, S.; Dupont, M.; Parel, P.M.; Zhang, J.; Bhargava, S.; Morse, K.W.; Dowdell, J.E.; et al. Robot-Navigated Pedicle Screw Insertion Can Reduce Intraoperative Blood Loss and Length of Hospital Stay: Analysis of 1633 Patients Utilizing Propensity Score Matching. Spine J. 2023, 24, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Sinkov, V.; Lockey, S.D.; Cunningham, B.W. Single Position Lateral Lumbar Interbody Fusion With Posterior Instrumentation Utilizing Computer Navigation and Robotic Assistance: Retrospective case review and surgical technique considerations. Glob. Spine J. 2022, 12, 75S–81S. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Aguilar, L.D.; Shah, V.; Himstead, A.; Brown, N.J.; Abraham, M.E.; Pham, M.H. Simultaneous Robotic Single-Position Surgery (SR-SPS) with Oblique Lumbar Interbody Fusion: A Case Series. World Neurosurg. 2021, 151, e1036–e1043. [Google Scholar] [CrossRef] [PubMed]
- Huntsman, K.T.; Riggleman, J.R.; Ahrendtsen, L.A.; Ledonio, C.G. Navigated robot-guided pedicle screws placed successfully in single-position lateral lumbar interbody fusion. J. Robot. Surg. 2020, 14, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.L.; Yu, H.M.; Zhang, R.M. Single oblique lumbar interbody fusion with robot-assisted posterior internal fixation for lumbar degenerative diseases. China J. Orthop. Traumatol. 2022, 35, 128–131. [Google Scholar]
- Pham, M.H.; Diaz-Aguilar, L.D.; Shah, V.; Brandel, M.; Loya, J.; Lehman, R.A. Simultaneous Robotic Single Position Oblique Lumbar Interbody Fusion with Bilateral Sacropelvic Fixation in Lateral Decubitus. Neurospine 2021, 18, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Menger, R.P.; Savardekar, A.R.; Farokhi, F.; Sin, A. A Cost-Effectiveness Analysis of the Integration of Robotic Spine Technology in Spine Surgery. Neurospine 2018, 15, 216–224. [Google Scholar] [CrossRef]



| Procedure Description | Advantages | Disadvantages | |
|---|---|---|---|
| PLIF | Posterior midline incision in prone position; requires laminectomy and retraction of thecal sac and nerve roots to reach the intervertebral disc space |
|
|
| TLIF | Posterior incision with a more lateral trajectory; requires facetectomy to allow visualization of nerve roots and perform discectomy |
| |
| ALIF | Longitudinal midline or paramedian incision to access retroperitoneal space in supine position |
|
|
| Study | Patients | Results | Conclusions |
|---|---|---|---|
| Saadeh et al. (2019) [40] | 40 (20 XLIF, 20 TLIF) | Segmental lordosis change: XLIF: 4.9° TLIF: 2.6° | XLIF achieved greater segmental lordosis correction as compared to TLIF |
| Jain et al. (2018) [41] | 33 (17 XLIF, 16 TLIF) | Blood loss: XLIF: 36 ± 16 mL TLIF: 700 ± 767 mL Length of hospital stay: XLIF: 2.6 ± 2.9 days TLIF: 3.3 ± 0.9 days | The XLIF group experienced decreased blood loss and shorter hospital stays compared to the TLIF group |
| Xu, Bach, and Uribe. (2018) [42] | 24 (16 XLIF, 8 ALIF) | Blood loss: XLIF: 60.6 mL ALIF: 106.3 mL | The XLIF group experienced decreased blood loss compared to the ALIF group |
| Ye, Hu, Zhang, and Xu. (2019) [43] | 2625 (1081 XLIF, 241 ALIF, 1303 PLIF/TLIF) | Length of hospital stay: XLIF: 3.77 days PLIF/TLIF: 4.04 days ALIF: 4.31 days | The XLIF group had a shorter hospital stay in comparison to the PLIF/TLIF and ALIF groups |
| Hrabek et al. (2015) [44] | 431 (101 XLIF, 330 ALIF) | Sympathectomy risk percentages: XLIF: 4% ALIF: 15% | XLIF has a lower sympathectomy risk than ALIF |
| Sembrano et al. (2016) [45] | 55 (29 MIS XLIF, 26 MIS TLIF) | Surgical duration: MIS XLIF: 171 min MIS TLIF: 186 min Blood loss < 100 mL: MIS XLIF: 79% MIS TLIF: 27% | The XLIF group had notably less blood loss in comparison to the MIS TLIF group |
| Isaacs, Sembrano, Tohmeh, and Group. (2016) [46] | 55 (29 XLIF, 26 MIS TLIF) | Average disc height change (24-month assessment): XLIF: −0.9 MIS TLIF: −1.7 Graft subsidence percentage (24-month assessment): XLIF 3% MIS TLIF 10% | XLIF demonstrates reduced subsidence and less resultant disc height loss compared to the MIS TLIF group at 24 months post-surgery |
| Lu and Lu. (2019) [47] | Finite element analysis (computerized models) | Stress peaks in endplate and cancellous bone, respectively: TLIF: 24.94–60.03 MPa, 0.72–1.96 MPa; XLIF: 17.01–35.32 MPa, 0.56–1.12 MPa | XLIF exhibits fewer stress peaks in the cortical endplate and cancellous bone in comparison to TLIF, which helps lower the risk of subsidence while preserving disc height and segmental angle |
| Zhang, Bai, Dokos, Cheung, and Diwan. (2019) [48] | Finite element analysis (computerized models) | Maximum stress and strain presented at the rods and facet joint, respectively, while utilizing an XLIF cage was less compared to when utilizing a TLIF cage | XLIF induces lower stress and strain on the fixed segments compared to TLIF and, hence, achieves greater stability |
| Ohba, Ebata, and Haro. (2017) [49] | 102 (46 XLIF, 56 PLIF) | Blood loss: XLIF: 51 ± 41 mL PLIF: 206 ± 191 mL | The XLIF group had notably less intraoperative blood loss in comparison to the PLIF group |
| Goodnough et al. (2019) [50] | 75 (21 XLIF, 54 ALIF) | Blood loss: XLIF: 50–100 mL ALIF: 150–400 mL | The XLIF group had notably less intraoperative blood loss in comparison to the ALIF group |
| Sembrano, Yson, Horazdovsky, Santos, and Polly, Jr. (2015) [51] | 85 (35 XLIF, 50 TLIF) | Mean operative level segmental lordosis: XLIF: 3.2 ± 3.6° TLIF: 1.9 ± 3.9° Overall lumbar lordosis change: XLIF: 2.5 ± 4.1° TLIF: 2.1 ± 6.0° | XLIF provided better segmental lordosis in comparison to TLIF |
| Yingsakmongkol et al. (2022) [52] | 60 (30 XLIF, 30 MIS TLIF) | Estimated blood loss: MIS TLIF: 200.33 ± 59.22 mL XLIF: 49.17 ± 32.91 mL Duration of hospital stay: MIS TLIF: 4.33 ± 0.61 days XLIF: 3.6 ± 0.62 days Operative time: MIS TLIF: 2.82 ± 0.47 h XLIF: 2.4 ± 0.81 h | The XLIF group exhibited decreased blood loss, as well as shorter postoperative hospital stays and operating times, when compared to TLIF |
| Kono, Gen, Sakuma, and Koshika. (2018) [53] | 40 (20 XLIF, 20 TLIF) | Estimated blood loss: XLIF: 36.1 ± 15.3 mL TLIF: 225.7 ± 215.9 mL Change in disc height (12-month assessment in comparison to pre-op status): XLIF: 1.8 ± 1.9 mm TLIF: 0.7 ± 1.4 mm | The XLIF group had significantly lower blood loss compared to the TLIF group, and the post-procedure disc height remained well-maintained at the 12-month assessment |
| Study | Patients | Results | Conclusions |
|---|---|---|---|
| Li, Zhang, and Shen. (2019) [102] | 2605 (1043 OLIF, 1562 XLIF) | Transient psoas weakness: OLIF: 8.8% XLIF: 21.2% | The OLIF group experienced less transient psoas weakness than the XLIF group |
| Walker et al. (2019) [103] | 6481 (1874 OLIF, 4607 XLIF) | Transient psoas weakness: OLIF: 5.7% XLIF: 19.7% | |
| Emami et al. (2023) [119] | 1010 (408 OLIF, 602 XLIF) | Rate of neuropraxia: OLIF: 10.9% XLIF: 21.2% | OLIF group experienced a lower rate of neurological complications than the XLIF group |
| Kim, Chang, Chang. (2022) [120] | 287 OLIF 584 XLIF | Fusion rates: OLIF: 96.9% XLIF: 91.6% | Similar fusion rates between OLIF and XLIF |
| Ko, Park, Kim. (2019) [118] | 343 (142 OLIF, 201 XLIF) | OLIF group:
| OLIF has similar CDA, MDH, and FH but higher SDA as compared to XLIF |
| Yingsakmongkol et al. (2022) [52] | 60 patients (30 OLIF, 30 XLIF) | Estimated blood loss: OLIF: 48.67 ± 33.4 mL XLIF: 49.17 ± 32.91 mL Difference in clinical pain parameters (1-year assessment) OLIF:
| OLIF showed comparable blood loss to that of XLIF, and there is no significant contrast in postoperative clinical pain parameters between the two procedures at the 1-year assessment |
| Jin et al. (2018) [121] | 43 patients (22 MIS XLIF, 21 MIS OLIF) | Persistent postoperative complications: MIS XLIF: 13.6% MIS OLIF: NIL | MIS OLIF results in fewer long-lasting postoperative complications compared to MIS XLIF |
| Miscusi et al. (2018) [122] | 45 patients (31 XLIF, 14 OLIF) | Post-op ODI scores: OLIF: Better (71.4%); Stable (21.5%) XLIF: Better (22.6%); Stable (61.2%) SF-36 Mental scale (at follow-up): OLIF: 70.00% XLIF: 60.22% SF-36 Physical scale (at follow-up): OLIF: 55.00% XLIF: 51.15% | The OLIF group demonstrates superior ODI and SF-36 scores when compared to the XLIF group |
| Fujibayashi et al. (2017) [98] | 2998 patients (1995 XLIF, 1003 OLIF) | Complication rates: OLIF: 15.3% XLIF: 19.4% Psoas weakness: OLIF: 3.0% XLIF: 4.9% Neuromonitoring usage: OLIF: 18.8% XLIF: 99.3% | The OLIF group exhibits a reduced overall complication rate in comparison to the XLIF group The incidence of psoas weakness is lower in the OLIF group than in the XLIF group OLIF necessitates limited neuromonitoring compared to XLIF |
| Ricciardi et al. (2023) [123] | 318 patients (128 OLIF, 190 XLIF) | Psoas weakness: OLIF: 1.56% XLIF: 7.37% Neurological symptoms: OLIF: 3.9% XLIF: 13.1% | The OLIF group had fewer patients with psoas weakness and neurological symptoms compared to the XLIF group |
| Aleinik et al. (2021) [104] | Review of 2900 patients (17 sources) | Overall complication rate following OLIF is 13.9% Incidence of severe persistent complications following OLIF is less than 1% | The relatively low complication rate makes OLIF superior to other approaches |
| Study | Patients | Results | Conclusions |
|---|---|---|---|
| Lamartina et al. (2020) [157] | 17 patients; 7 P-SPS and 10 conventional XLIF with PS | Oswestry Disability Index (preoperative to postoperative): P-SPS: 48.5 ± 21 to 14.57 ± 18.54 Conventional XLIF: 50.8 ± 11.7 to 22.50 ± 13.9 Back Pain Numeric Rating Scale (preoperative to postoperative): P-SPS: 7.7 ± 1.7 to 1.71 ± 2.91 Conventional XLIF: 5.7 ± 1.2 to 3.7 ± 2.91 Leg Pain Numeric Rating Scale (preoperative to postoperative): P-SPS: 8.5 ± 1.2 to 2.71 ± 3.25 Conventional XLIF: 7.2 ± 1.3 to 2.50 ± 3.03 | P-SPS is feasible and safe; results are comparable to the standard technique |
| Pimenta et al. (2021) [158] | 32 patients; 45 levels | Index level segmental lordosis increased from 8.7° pre-operatively to 14.8° postoperatively; lumbar lordosis (L1-S1) increased from 41.9° pre-operatively to 46.7° postoperatively; preoperatively, 22 patients had a pelvic incidence (PI)–lumber lordosis (LL) mismatch of 10° or more, while postoperatively, only 12 patients had a mismatch beyond 10° | P-SPS is associated with a significant gain of segmental lordosis and correction of spinopelvic alignment parameters |
| Pimenta et al. (2021) [152] | 27 patients (L4-5 only: 18 patients; L3-5: 8 patients and L2-5: 1 patient) | Posterior stabilization: 22 patients (81.5%) Mean surgical duration: 182 ± 72 min Mean trans-psoas time: 29 ± 14 min Estimated blood loss: 200 ± 166 mL Median hospitalization time: 2 days Onset of sensory deficit: 2 patients Onset of a motor and sensory deficit: 1 patient | P-SPS is safe and feasible for approaching the L4-5 disk, presenting with low rate of complications and new-onset neurologic deficits |
| Smith et al. (2021) [153] | 120 patients; 176 levels; 22 surgeons | Lateral exposure: 18 min/level Retraction time: 25 min/level Percutaneous pedicle screws: 65% Open pedicle screws: 24% Direct decompression: 37% Osteotomy or bony releases: 9% | Perioperative outcomes of P-SPS are consistent with lateral decubitus experience |
| Soliman et al. (2022) [159] | 22 patients; 11 P-SPS and 11 TLIF | Improvement in LL: P-SPS: 11.5 ± 9.5 TLIF: 0.1 ± 15.1 Postoperative PI-LL: P-SPS: 3 ± 10.3 TLIF: 14.9 ± 14.1 Change in PI-LL: P-SPS: 15.5 ± 7.7 TLIF: 3.8 ± 15.2 | P-SPS led to superior enhancements in both postoperative radiographic parameters and patient-reported outcomes |
| Soliman et al. (2022) [160] | 20 patients; 10 P-SPS and 10 conventional XLIF with PS | Improvement in LL: P-SPS: 9.9 ± 8.5 Conventional XLIF: 0.5 ± 11 | P-SPS group demonstrated a significantly better improvement in lumbar lordosis |
| Amaral et al. (2023) [155] | 71 patients; 18 P-SPS and 53 conventional XLIF with PS | After propensity score matching: P-SPS (n = 18): 6.6° ± 5.5° Conventional XLIF (n = 18): 1.9° ± 4.7° | P-SPS can significantly enhance segmental lordosis correction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, A.X.J.; Tang, D.H.; Kaliya-Perumal, A.-K.; Oh, J.Y.-L. The Evolution of Lateral Lumbar Interbody Fusion: A Journey from Past to Present. Medicina 2024, 60, 378. https://doi.org/10.3390/medicina60030378
Wong AXJ, Tang DH, Kaliya-Perumal A-K, Oh JY-L. The Evolution of Lateral Lumbar Interbody Fusion: A Journey from Past to Present. Medicina. 2024; 60(3):378. https://doi.org/10.3390/medicina60030378
Chicago/Turabian StyleWong, Anthony Xi Jie, Derek Haowen Tang, Arun-Kumar Kaliya-Perumal, and Jacob Yoong-Leong Oh. 2024. "The Evolution of Lateral Lumbar Interbody Fusion: A Journey from Past to Present" Medicina 60, no. 3: 378. https://doi.org/10.3390/medicina60030378





