Effect of Drugs Used in Pharmacotherapy of Type 2 Diabetes on Bone Density and Risk of Bone Fractures
Abstract
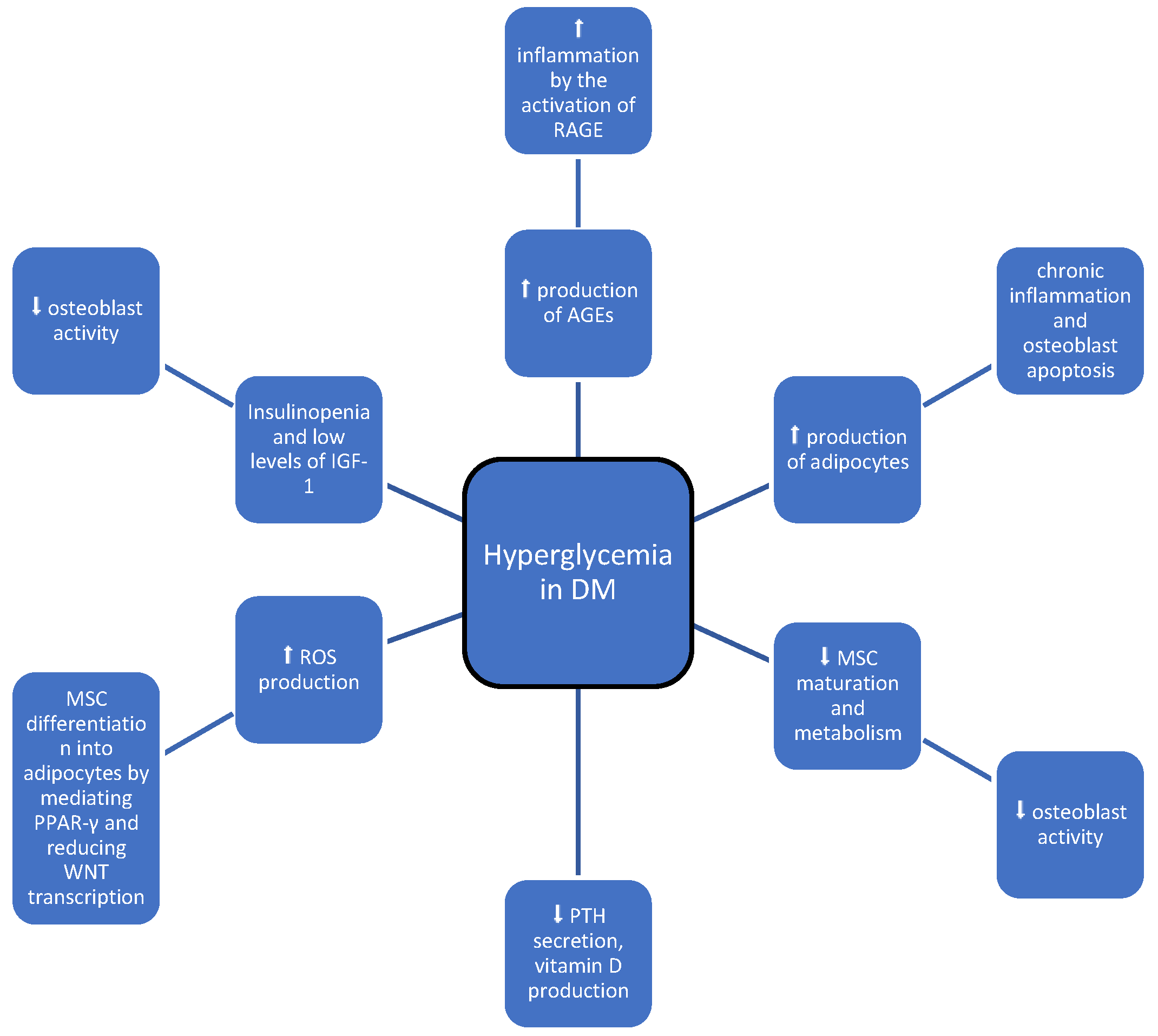
:1. Introduction
2. Methodology
3. Discussion
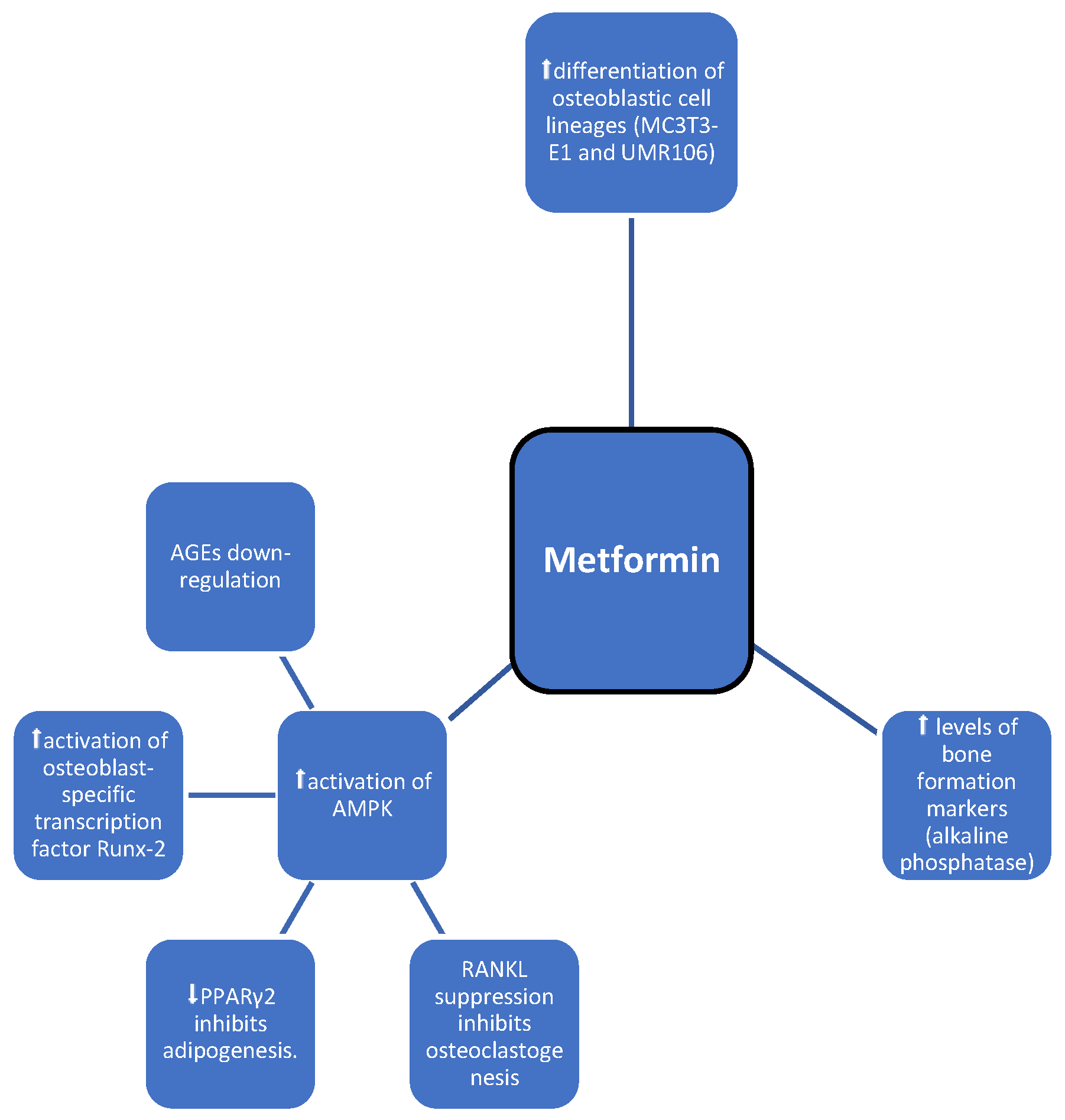
3.1. Metformin
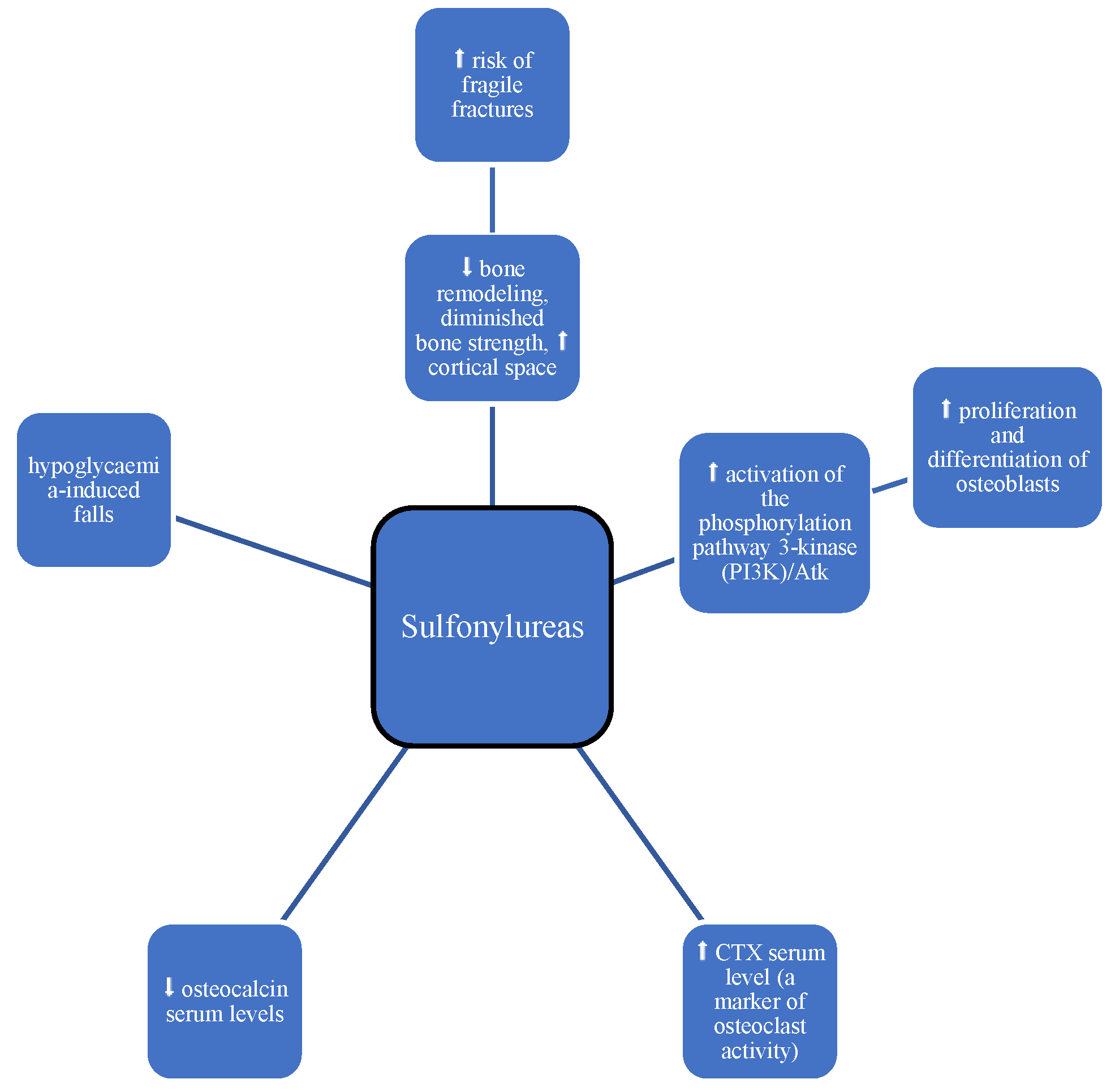
3.2. Sulphonyloureas
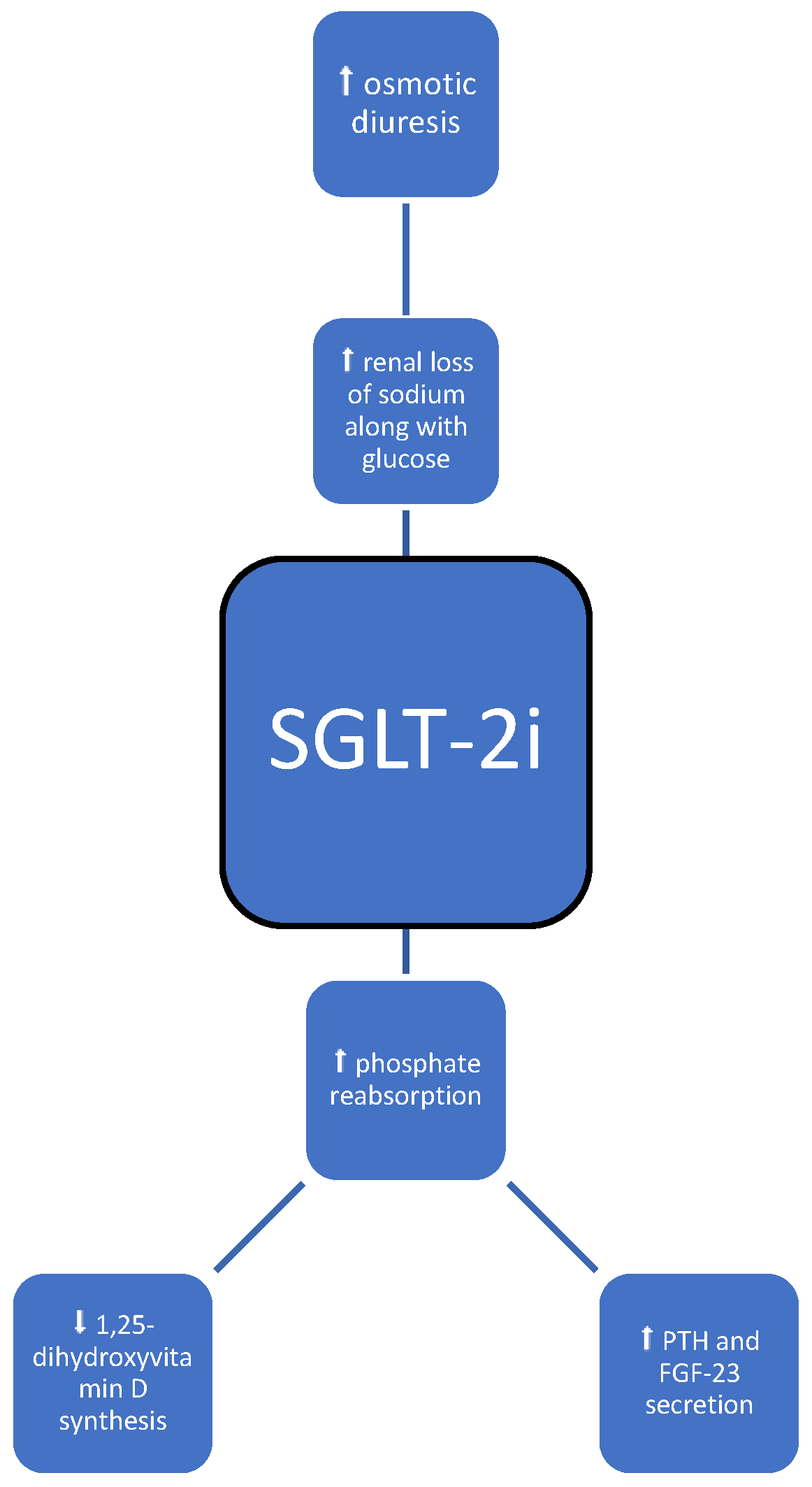
3.3. SGLT-2 Inhibitors
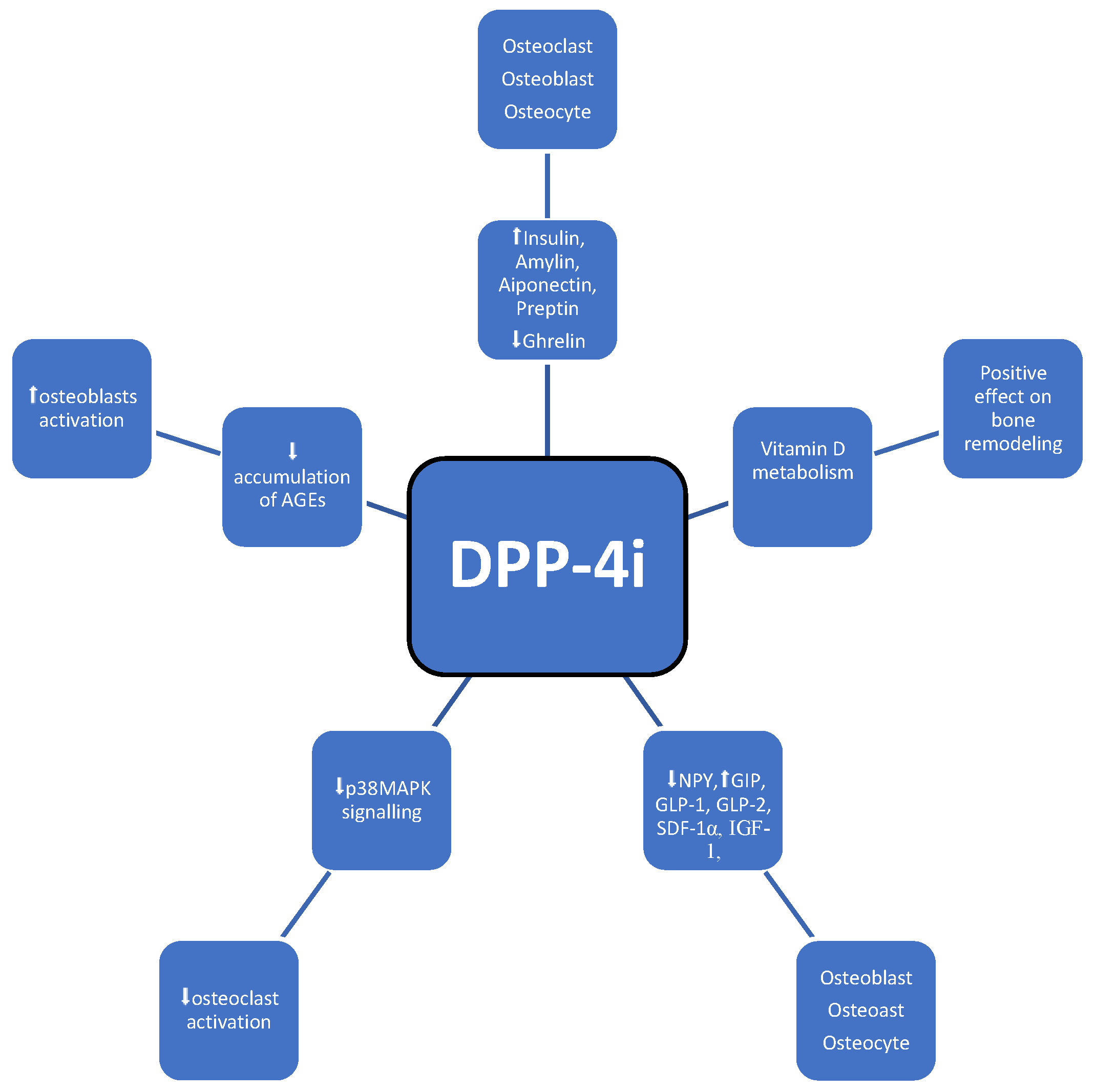
3.4. DPP-4 Inhibitors
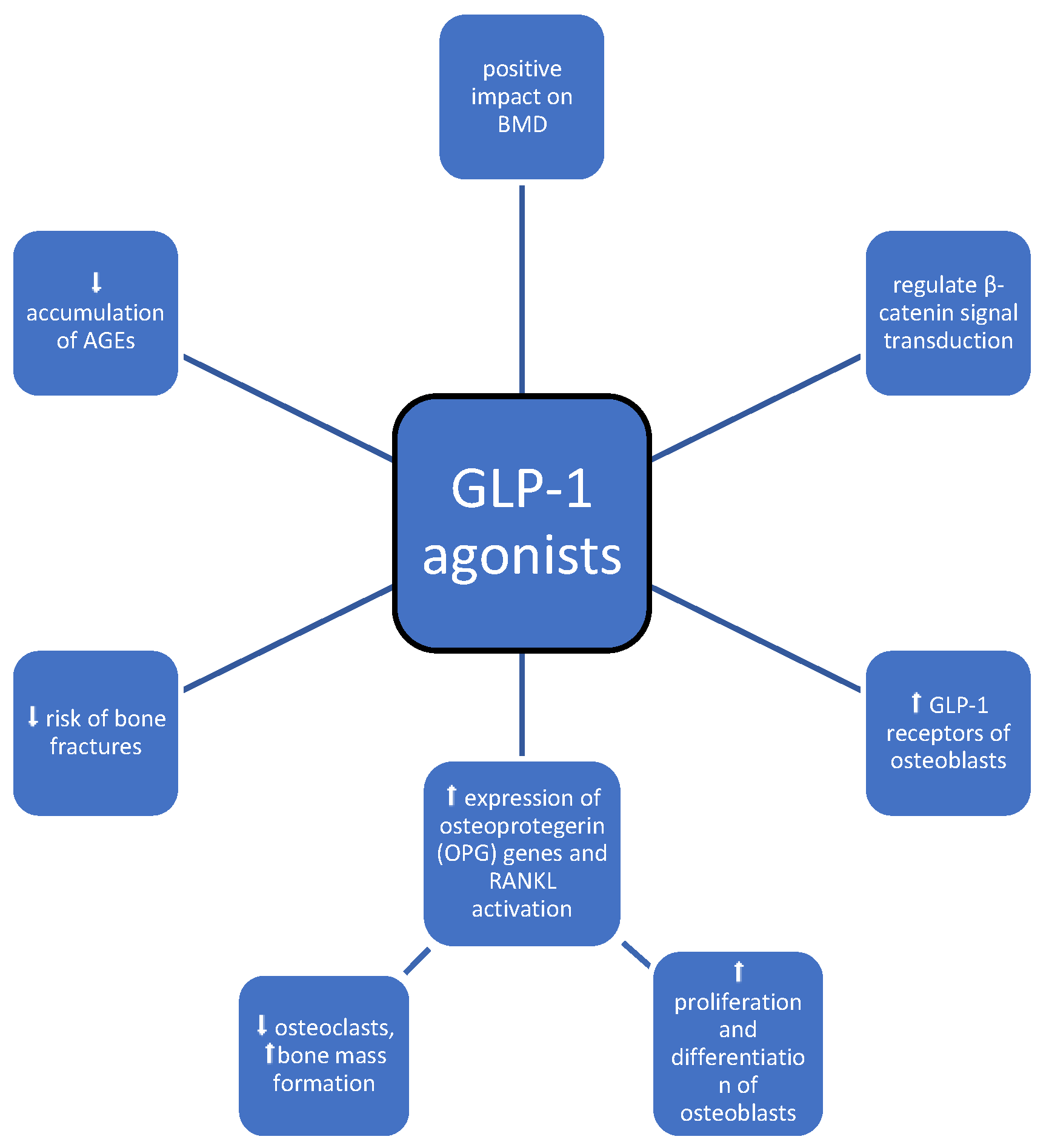
3.5. GLP-1 Agonists
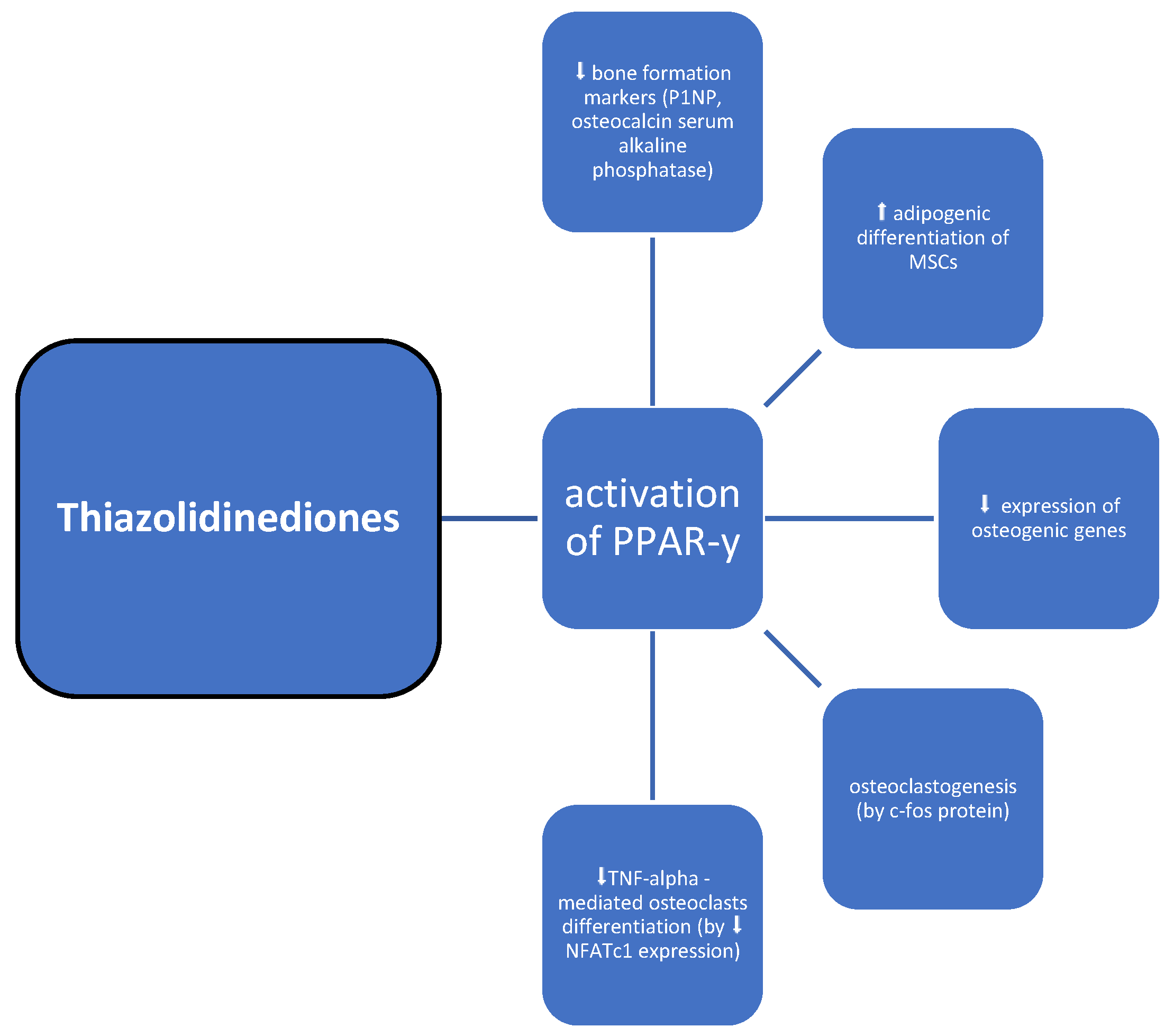
3.6. Thiazolidinediones
3.7. Insulin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Dede, A.D.; Tournis, S.; Dontas, I.; Trovas, G. Type 2 diabetes mellitus and fracture risk. Metabolism 2014, 63, 1480–1490. [Google Scholar] [CrossRef]
- Poiana, C.; Capatina, C. Fracture Risk Assessment in Patients with Diabetes Mellitus. J. Clin. Densitom. 2017, 20, 432–443. [Google Scholar] [CrossRef]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L. IOF Bone and Diabetes Working Group.Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Sheu, A.; Greenfield, J.R.; White, C.P.; Center, J.R. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 2022, 33, 333–344. [Google Scholar] [CrossRef]
- Eller-Vainicher, C.; Cairoli, E.; Grassi, G.; Grassi, F.; Catalano, A.; Merlotti, D.; Falchetti, A.; Gaudio, A.; Chiodini, I.; Gennari, L. Pathophysiology and Management of Type 2 Diabetes Mellitus Bone Fragility. J. Diabetes Res. 2020, 2020, 7608964. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. National Osteoporosis Foundatio. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381, Correction in Osteoporos. Int. 2015, 26, 2045–2047. [Google Scholar] [CrossRef]
- Johnson, K.C.; Bray, G.A.; Cheskin, L.J.; Clark, J.M.; Egan, C.M.; Foreyt, J.P.; Garcia, K.R.; Glasser, S.; Greenway, F.L.; Gregg, E.W.; et al. The Effect of Intentional Weight Loss on Fracture Risk in Persons with Diabetes: Results from the Look AHEAD Randomized Clinical Trial. J. Bone Miner. Res. 2017, 32, 2278–2287. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Z.; Ma, C.; Jiang, Y.; Zhang, N.; Hu, K.; Li, L.; Wang, Z. Metformin promotes the proliferation and differentiation of murine preosteoblast by regulating the expression of sirt6 and oct4. Pharmacol. Res. 2018, 129, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Gu, Y.; Yang, H.; Shi, Q. Metformin Enhances Osteogenesis and Suppresses Adipogenesis of Human Chorionic Villous Mesenchymal Stem Cells. Tohoku J. Exp. Med. 2017, 241, 13–19. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. The impact of diabetes and diabetes medications on bone health. Endocr. Rev. 2015, 36, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Yano, S.; Yamauchi, M.; Sugimoto, T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem. Biophys. Res. Commun. 2008, 375, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, A.M.; Sedlinsky, C.; McCarthy, A.D.; Blanco, A.; Schurman, L. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur. J. Pharmacol. 2006, 536, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Schurman, L.; McCarthy, A.D.; Sedlinsky, C.; Gangoiti, M.V.; Arnol, V.; Bruzzone, L.; Cortizo, A.M. Metformin reverts deleterious effects of advanced glycation end-products (AGEs) on osteoblastic cells. Exp. Clin. Endocrinol. Diabetes 2008, 116, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, M.S.; Schurman, L.; McCarthy, A.D.; Cortizo, A.M.; Tolosa, M.J.; Gangoiti, M.V.; Arnol, V.; Sedlinsky, C. Effect of metformin on bone marrow progenitor cell differentiation: In vivo and in vitro studies. J. Bone Miner. Res. 2010, 25, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A. Metformin therapy and hip fracture risk among patients with type II diabetes mellitus: A population-based cohort study. Bone 2020, 135, 115325. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 2005, 48, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Ye, Z.; Lin, R.; Sun, A.R.; Liu, L.; Wei, J.; Deng, F.; Zhong, X.; Cui, L.; et al. Association of metformin use with fracture risk in type 2 diabetes: A systematic review and meta-analysis of observational studies. Front. Endocrinol. 2023, 13, 1038603. [Google Scholar] [CrossRef]
- Palermo, A.; D’Onofrio, L.; Eastell, R.; Schwartz, A.V.; Pozzilli, P.; Napoli, N. Oral anti-diabetic drugs and fracture risk, cut to the bone: Safe or dangerous? A narrative review. Osteoporos. Int. 2015, 26, 2073–2089. [Google Scholar] [CrossRef]
- Zinman, B.; Haffner, S.M.; Herman, W.H.; Holman, R.R.; Lachin, J.M.; Kravitz, B.G.; Paul, G.; Jones, N.P.; Aftring, R.P.; Viberti, G.; et al. Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 134–142. [Google Scholar] [CrossRef]
- Vianna, A.G.D.; de Lacerda, C.S.; Pechmann, L.M.; Polesel, M.G.; Marino, E.C.; Borba, V.Z.C.; Barreto, F.C. Vildagliptin has the same safety profile as a sulfonylurea on bone metabolism and bone mineral density in post-menopausal women with type 2 diabetes: A randomized controlled trial. Diabetol. Metab. Syndr. 2017, 9, 35. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Wu, M.J.; Shi, B.M. The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: Systematic review and meta-analysis of observational studies. Obes. Rev. 2019, 20, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Lapane, K.L.; Yang, S.; Brown, M.J.; Jawahar, R.; Pagliasotti, C.; Rajpathak, S. Sulfonylureas and risk of falls and fractures: A systematic review. Drugs Aging 2013, 30, 527–547. [Google Scholar] [CrossRef] [PubMed]
- Lecka-Czernik, B. Diabetes, bone and glucose-lowering agents: Basic biology. Diabetologia 2017, 60, 1163–1169. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zheng, Y.D.; Yuan, Y.; Chen, S.C.; Xie, B.C. Effects of Anti-Diabetic Drugs on Fracture Risk: A Systematic Review and Network Meta-Analysis. Front. Endocrinol. 2021, 12, 735824. [Google Scholar] [CrossRef] [PubMed]
- Zinma Rico, H.; Hernandez, E.R.; Cabranes, J.A.; Gomez-Castresana, F. Suggestion of a deficient osteoblastic function in diabetes mellitus: The possible cause of osteopenia in diabetics. Calcif. Tissue Int. 1989, 45, 71–73. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, Y.; Tao, Y.; E, M.; Tang, J.; Liu, Y.; Li, F. Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2020, 159, 107990. [Google Scholar] [CrossRef]
- Tao, Y.; E, M.; Shi, J.; Zhang, Z. Sulfonylureas use and fractures risk in elderly patients with type 2 diabetes mellitus: A meta-analysis study. Aging Clin. Exp. Res. 2021, 33, 2133–2139. [Google Scholar] [CrossRef]
- Fonseca-Correa, J.I.; Correa-Rotter, R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front. Med. 2021, 8, 777861. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Rau, M.; Thiele, K.; Hartmann, N.K.; Möllmann, J.; Wied, S.; Hohl, M.; Marx, N.; Lehrke, M. Effects of empagliflozin on markers of calcium and phosphate homeostasis in patients with type 2 diabetes—Data from a randomized, placebo-controlled study. Bone Rep. 2022, 16, 101175. [Google Scholar] [CrossRef]
- de Jong, M.A.; Petrykiv, S.I.; Laverman, G.D.; van Herwaarden, A.E.; de Zeeuw, D.; Bakker, S.J.L.; Heerspink, H.J.L.; de Borst, M.H. Effects of Dapagliflozin on Circulating Markers of Phosphate Homeostasis. Clin. J. Am. Soc. Nephrol. 2019, 14, 66–73. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef]
- Goldman, A.; Fishman, B.; Twig, G.; Raschi, E.; Cukierman-Yaffe, T.; Moshkovits, Y.; Pomerantz, A.; Ben-Zvi, I.; Dankner, R.; Maor, E. The real-world safety profile of sodium-glucose co-transporter-2 inhibitors among older adults (≥75 years): A retrospective, pharmacovigilance study. Cardiovasc. Diabetol. 2023, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Nashar, K.; Khalil, P. Clinical Evaluation of Dapagliflozin in the Management of CKD: Focus on Patient Selection and Clinical Perspectives. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Zhang, Y.; Zhang, J.; Sheng, Y.; Wang, W.; Li, Y. Effect of SGLT2 inhibitors on fractures, BMD, and bone metabolism markers in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Osteoporos. Int. 2023, 34, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- List, J.F.; Woo, V.; Morales, E.; Tang, W.; Fiedorek, F.T. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009, 32, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, Ö.; Bolinder, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sjöström, C.D.; Sugg, J.; Parikh, S. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes. Metab. 2012, 14, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Yu, Y.; Duan, J.; Bi, S.; Swe, K.N.C.; Xi, Z.; Gao, Y.; Zhou, Y.; Nie, X.; Liu, W. Sodium-glucose cotransporter 2 inhibitors and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Ther. Adv. Chronic Dis. 2020, 11, 2040622320961599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jardine, M.; Perkovic, V.; Matthews, D.R.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Desai, M.; Oh, R.; Simpson, R.; et al. Canagliflozin and fracture risk in individuals with type 2 diabetes: Results from the CANVAS Program. Diabetologia 2019, 62, 1854–1867. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Lupsa, B.C.; Inzucchi, S.E. Use of SGLT2 inhibitors in type 2 diabetes: Weighing the risks and benefits. Diabetologia 2018, 61, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, C.; Liang, J.; Yu, M.; Qu, X. Effect of Dipeptidyl Peptidase-4 Inhibitors on Bone Metabolism and the Possible Underlying Mechanisms. Front. Pharmacol. 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; et al. Effects of Incretin-Related Diabetes Drugs on Bone Formation and Bone Resorption. Int. J. Mol. Sci. 2021, 22, 6578. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhong, W.; Liang, X.; Wang, H.; Fu, S.; Luo, Z. Meta-Analysis on the Association Between DPP-4 Inhibitors and Bone Mineral Density and Osteoporosis. J. Clin. Densitom. 2024, 27, 101455. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Dicembrini, I.; Antenore, A.; Mannucci, E. Dipeptidyl peptidase-4 inhibitors and bone fractures: A meta-analysis of randomized clinical trials. Diabetes Care 2011, 34, 2474–2476, Correction in Diabetes Care 2014, 37, 312. [Google Scholar] [CrossRef]
- Dombrowski, S.; Kostev, K.; Jacob, L. Use of dipeptidyl peptidase-4 inhibitors and risk of bone fracture in patients with type 2 diabetes in Germany-A retrospective analysis of real-world data. Osteoporos. Int. 2017, 28, 2421–2428. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, C.; Lee, Y.K.; Ha, Y.C.; Jang, S.; Shin, C.S. Risk of fractures and diabetes medications: A nationwide cohort study. Osteoporos. Int. 2016, 27, 2709–2715. [Google Scholar] [CrossRef]
- Han, S.J.; Ha, K.H.; Lee, N.; Kim, D.J. Effectiveness and safety of sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in older adults with type 2 diabetes: A nationwide population-based study. Diabetes Obes. Metab. 2021, 23, 682–691. [Google Scholar] [CrossRef]
- Cai, T.T.; Li, H.Q.; Jiang, L.L.; Wang, H.Y.; Luo, M.H.; Su, X.F.; Ma, J.H. Effects of GLP-1 Receptor Agonists on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus: A 52-Week Clinical Study. BioMed Res. Int. 2021, 2021, 3361309. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, Y.; Li, Y.; Cao, X.; Bai, N.; Lu, T.; Li, G.; Li, N.; Wang, A.; Mao, X. Glucagon-like peptide-1 receptor agonists and risk of bone fracture in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab. Res. Rev. 2019, 35, e3168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, P.; Tang, Z.; Dou, Q.; Feng, B. Effects of GLP-1 receptor analogue liraglutide and DPP-4 inhibitor vildagliptin on the bone metabolism in ApoE−/− mice. Ann. Transl. Med. 2019, 7, 369. [Google Scholar] [CrossRef]
- Nuche-Berenguer, B.; Portal-Núñez, S.; Moreno, P.; González, N.; Acitores, A.; López-Herradón, A.; Esbrit, P.; Valverde, I.; Villanueva-Peñacarrillo, M.L. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J. Cell. Physiol. 2010, 225, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, E.; Guarino, E.G.; Merlotti, D.; Patti, A.; Gennari, L.; Nuti, R.; Dotta, F. Beyond glycemic control in diabetes mellitus: Effects of incretin-based therapies on bone metabolism. Front. Endocrinol. 2013, 4, 73. [Google Scholar] [CrossRef]
- Meng, J.; Ma, X.; Wang, N.; Jia, M.; Bi, L.; Wang, Y.; Li, M.; Zhang, H.; Xue, X.; Hou, Z.; et al. Activation of GLP-1 receptor promotes bone marrow stromal cell osteogenic differentiation through β-catenin. Stem Cell Rep. 2016, 6, 579–591. [Google Scholar] [CrossRef]
- Luo, G.; Liu, H.; Lu, H. Glucagon-like peptide-1(GLP-1) receptor agonists: Potential to reduce fracture risk in diabetic patients? Br. J. Clin. Pharmacol. 2016, 81, 78–88. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Plutzky, J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007, 115, 518–533. [Google Scholar] [CrossRef]
- Nuttall, M.E.; Patton, A.J.; Olivera, D.L.; Nadeau, D.P.; Gowen, M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: Implications for osteopenic disorders. J. Bone Miner. Res. 1998, 13, 371–382. [Google Scholar] [CrossRef]
- Ali, A.A.; Weinstein, R.S.; Stewart, S.A.; Parfitt, A.M.; Manolagas, S.C.; Jilka, R.L. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 2005, 146, 1226–1235. [Google Scholar] [CrossRef]
- Wan, Y.; Chong, L.W.; Evans, R.M. PPAR-gamma regulates osteoclastogenesis in mice. Nat. Med. 2007, 13, 1496–1503. [Google Scholar] [CrossRef]
- Yang, C.R.; Lai, C.C. Thiazolidinediones inhibit TNF-alpha-mediated osteoclast differentiation of RAW264.7 macrophages and mouse bone marrow cells through downregulation of NFATc1. Shock 2010, 33, 662–667. [Google Scholar] [CrossRef]
- Zhu, Z.N.; Jiang, Y.F.; Ding, T. Risk of fracture with thiazolidinediones: An updated meta-analysis of randomized clinical trials. Bone 2014, 68, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Bolland, M.J.; Gamble, G.D.; Wattie, D.; Horne, A.M.; Davidson, J.S.; Reid, I.R. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: A randomized, controlled trial. J. Clin. Endocrinol. Metab. 2007, 92, 1305–1310. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Sellmeyer, D.E.; Vittinghoff, E.; Palermo, L.; Lecka-Czernik, B.; Feingold, K.R.; Strotmeyer, E.S.; Resnick, H.E.; Carbone, L.; Beamer, B.A.; et al. Thiazolidinedione use and bone loss in older diabetic adults. J. Clin. Endocrinol. Metab. 2006, 91, 3349–3354. [Google Scholar] [CrossRef] [PubMed]
- Billington, E.O.; Grey, A.; Bolland, M.J. The effect of thiazolidinediones on bone mineral density and bone turnover: Systematic review and meta-analysis. Diabetologia 2015, 58, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Wang, W.; Liu, J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem. Funct. 2010, 28, 334–341. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Reid, I.R. Insulin increases histomorphometric indices of bone formation In vivo. Calcif. Tissue Int. 1996, 59, 492–495. [Google Scholar] [CrossRef]
- Kemink, S.A.; Hermus, A.R.; Swinkels, L.M.; Lutterman, J.A.; Smals, A.G. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J. Endocrinol. Investig. 2000, 23, 295–303. [Google Scholar] [CrossRef]
- Pun, K.K.; Lau, P.; Ho, P.W. The characterization, regulation, and function of insulin receptors on osteoblast-like clonal osteosarcoma cell line. J. Bone Miner. Res. 1989, 4, 853–862. [Google Scholar] [CrossRef]
- Fulzele, K.; Riddle, R.C.; DiGirolamo, D.J.; Cao, X.; Wan, C.; Chen, D.; Faugere, M.C.; Aja, S.; Hussain, M.A.; Brüning, J.C.; et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 2010, 142, 309–319, Correction in Cell 2022, 185, 746. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Karsenty, G. The osteoblast: An insulin target cell controlling glucose homeostasis. J. Bone Miner. Res. 2011, 26, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Thrailkill, K.M.; Lumpkin, C.K., Jr.; Bunn, R.C.; Kemp, S.F.; Fowlkes, J.L. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E735–E745. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wan, Z.H.; Cheng, S.L.; Michaëlsson, K.; Larsson, S.C. Insulin-like Growth Factor-1, Bone Mineral Density, and Fracture: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, e1552–e1558. [Google Scholar] [CrossRef] [PubMed]
- Dennison, E.M.; Syddall, H.E.; Aihie Sayer, A.; Craighead, S.; Phillips, D.I.; Cooper, C. Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: Evidence for an indirect effect of insulin resistance? Diabetologia 2004, 47, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, V.V.; Finkelstein, J.S.; Bouxsein, M.L.; Yu, E.W. Association Between Insulin Resistance and Bone Structure in Nondiabetic Postmenopausal Women. J. Clin. Endocrinol. Metab. 2016, 101, 3114–3122. [Google Scholar] [CrossRef]
- Yang, J.; Hong, N.; Shim, J.S.; Rhee, Y.; Kim, H.C. Association of Insulin Resistance with Lower Bone Volume and Strength Index of the Proximal Femur in Nondiabetic Postmenopausal Women. J. Bone Metab. 2018, 25, 123–132. [Google Scholar] [CrossRef]
- Dutta, M.; Pakhetra, R.; Garg, M. Evaluation of bone mineral density in type 2 diabetes mellitus patients before and after treatment. Med. J. Armed Forces India 2012, 68, 48–52. [Google Scholar] [CrossRef]
- Ruppert, K.; Cauley, J.; Lian, Y.; Zgibor, J.C.; Derby, C.; Solomon, D.H. The effect of insulin on bone mineral density among women with type 2 diabetes: A SWAN Pharmacoepidemiology study. Osteoporos. Int. 2018, 29, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Leibson, C.L.; Achenbach, S.J.; Therneau, T.M.; Khosla, S. Fracture risk in type 2 diabetes: Update of a population-based study. J. Bone Miner. Res. 2008, 23, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Sloane, R.; Pieper, C.; Lyles, K.W.; Adler, R.A.; Van Houtven, C.; LaFleur, J.; Colón-Emeric, C. Glycemic Control and Insulin Treatment Alter Fracture Risk in Older Men with Type 2 Diabetes Mellitus. J. Bone Miner. Res. 2019, 34, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Strotmeyer, E.S.; Ensrud, K.E.; Sellmeyer, D.E.; Bauer, D.C.; Hoffman, A.R.; Dam, T.T.; Barrett-Connor, E.; Palermo, L.; Orwoll, E.S.; et al. Fracture risk in diabetic elderly men: The MrOS study. Diabetologia 2014, 57, 2057–2065. [Google Scholar] [CrossRef]
- Pscherer, S.; Kostev, K.; Dippel, F.W.; Rathmann, W. Fracture risk in patients with type 2 diabetes under different antidiabetic treatment regimens: A retrospective database analysis in primary care. Diabetes Metab. Syndr. Obes. 2016, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Fonseca, V.; Schinzel, S.; Dain, M.P.; Mullins, P.; Riddle, M. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: Evidence from a long-term controlled trial. J. Diabetes Complicat. 2014, 28, 742–749. [Google Scholar] [CrossRef]
- Ivers, R.Q.; Cumming, R.G.; Mitchell, P.; Peduto, A.J.; Blue Mountains Eye Study. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care 2001, 24, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Prior, J.C.; Leslie, W.D.; Thabane, L.; Papaioannou, A.; Josse, R.G.; Kaiser, S.M.; Kovacs, C.S.; Anastassiades, T.; Towheed, T.; et al. Frailty and Risk of Fractures in Patients with Type 2 Diabetes. Diabetes Care 2019, 42, 507–513. [Google Scholar] [CrossRef]
- Ohira, M.; Suzuki, S.; Yoshida, T.; Koide, H.; Tanaka, T.; Tatsuno, I. Fracture Risk Assessment Tool May Not Indicate Bone Fragility in Women with Type 2 Diabetes. Am. J. Med. Sci. 2020, 360, 552–559. [Google Scholar] [CrossRef]
- Hidayat, K.; Fang, Q.L.; Shi, B.M.; Qin, L.Q. Influence of glycemic control and hypoglycemia on the risk of fracture in patients with diabetes mellitus: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2021, 32, 1693–1704. [Google Scholar] [CrossRef]
- Rasmussen, N.H.; Vestergaard, P. Diabetes and osteoporosis—Treating two entities: A challenge or cause for concern? Best Pract. Res. Clin. Rheumatol. 2022, 36, 101779. [Google Scholar] [CrossRef]
- Schacter, G.I.; Leslie, W.D. Diabetes and Osteoporosis: Part I, Epidemiology and Pathophysiology. Endocrinol. Metab. Clin. N. Am. 2021, 50, 275–285. [Google Scholar] [CrossRef]
- Schacter, G.I.; Leslie, W.D. Diabetes and Osteoporosis: Part II, Clinical Management. Endocrinol. Metab. Clin. N. Am. 2021, 50, 287–297. [Google Scholar] [CrossRef] [PubMed]

| Metformin | Sulphonylureas | SGLT-2 Inhibitors | DPP-4 Inhibitors | GLP-1 Agonists | Thiazolidinediones | Insulin |
|---|---|---|---|---|---|---|
| ↓ intestinal glucose absorption | ↑ insulin release by pancreatic B-cells | ↑ urinary glucose excretion | ↑ endogenous incretin concentration of GLP-1 and GIP | ↑ GLP-1 receptor activation | ↑ uptake of free fatty acids by adipocytes | ↑ glucose utilization and storage by increasing glucose transport and net glycogen synthesis |
| ↑ glucose utilization by intestinal cells | ↑ tissue sensitivity to insulin | ↑ glucose utilization | ↑ sensitivity of pancreatic B-cells to glucose and ↑ glucose dependent insulin secretion | ↓ glucagon secretion in a-cells | ↑ secretion of adiponectin | ↑ glucose transport into cells and net glycogen synthesis |
| ↓ hepatic gluconeogenesis and glycogenolysis | ↑ glucose transport into adipose tissue and muscles | ↓ insulin resistance | ↑ sensitivity of a-cells to glucose, glucagon secretion | ↑ glucose-dependent insulin secretion | ↓ production of TNF-a | white adipose tissue (WAT): ↓ lipolysis, ↑ glucose transport, ↑ lipogenesis |
| ↑ glucose uptake and utilization by peripheral tissues | ↑ glycogenesis in liver and muscles | ↓ glucotoxicity | ↓ hepatic glucose secretion both in fasting and postprandial states | ↓ B-cell death, ↑B-cell proliferation, ↑ expansion of B-cell mass | ↓ production of resistin | Liver: ↑ activation of glycogen synthesis, ↑ lipogenic gene expression, ↓ gluconeogenic gene expression |
| ↑ peripheral insulin sensitivity | ↓ synthesis of glucose and oxidation of fatty acids in the liver | adipose tissue: ↑ lipolysis, fatty acid oxidation and ketone body formation, ↓ visceral and epicardial fat mass | delay in gastric emptying, ↓ caloric intake and weight loss | ↓ islet inflammation | ↑ HDL-cholesterol concentration | Muscle cells: ↑ glycogenesis and ↓ protein synthesis, protein catabolism |
| ↑ fatty acid oxidation in adipose tissue and skeletal muscles | Hepatic: ↑ gluconeogenesis, ↑ ketogenesis, ↑ hepatic glucose output, ↓ hepatic steatosis | ↑ delayed gastric emptying, ↓ food intake, ↑ weight loss | ↑ LDL-cholesterol concentration and particle size | Pancreatic beta cells: ↓ glucagon release | ||
| ↑ lipolysis and inhibits lipogenesis | Cardiovascular: ↓ intravascular volume, ↓ blood pressure, ↓ cardiac preload and afterload, improves endothelial and ↓ vascular stiffness | ↓ triglyceride concentration | ||||
| ↓ the nuclear factor KB pathway in immune cells | ↓ plasminogen activator inhibitor-1 and fibrinogen | |||||
| ↓ the differentiation of monocytes to macrophages | anti-inflammatory effects | |||||
| ↓ inflammation |
| Antidiabetic Medication | BMD | Fracture Risk | Overall Impact |
|---|---|---|---|
| Metformin | ↑/↔ | ↓/↔ | Most studies have shown beneficial effects on bone metabolism. Clinical data indicate neutral or even positive effects on bone and fracture risk, although metformin is usually used in individuals with a shorter history of diabetes with fewer complications. |
| Sulphonyloureas | limited data | ↑ in at-risk individuals (elderly, frail, and post-menopausal women); results might be confounded by an increased risk of hypoglycemia-induced falls | Data on bone metabolism are very limited. Attention must be paid to the higher risk of hypoglycemia-induced falls. |
| SGLT-2 inhibitors | ↔ | ↔, ↑ with canaglifozin | SGLT2 inhibitors are not significantly linked to an elevated risk of fractures; caution is advised with canagliflozin, which has raised concerns regarding potential detrimental effects on bone health. |
| DPP-4 inhibitors | ↑, ↔ | ↔, ↓ | DPP-4 inhibitors have been reported to have neutral or beneficial effects on bone by the majority of studies and have been associated with a lower incidence of fractures. |
| GLP-1 receptor agonists | ↔ | ↔ | Preclinical models show a beneficial effect on bone. Clinical data show mostly neutral effects, although a few studies have shown harmful or beneficial effects on risk for fracture. |
| Thiazolidinediones | ↓ | ↑ | There are negative effects on bone metabolism and an increase in fracture risk. |
| Insulin | ↑ | ↑ in T2D | Insulin use in T2D is associated with ↑ fracture risk. Maintenance of tight glycemic control should be avoided due to increased episodes of hypoglycemia, falls, and fractures in at-risk populations. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikarek, A.; Grabarczyk, M.; Klimek, K.; Janoska-Gawrońska, A.; Suchodolska, M.; Holecki, M. Effect of Drugs Used in Pharmacotherapy of Type 2 Diabetes on Bone Density and Risk of Bone Fractures. Medicina 2024, 60, 393. https://doi.org/10.3390/medicina60030393
Wikarek A, Grabarczyk M, Klimek K, Janoska-Gawrońska A, Suchodolska M, Holecki M. Effect of Drugs Used in Pharmacotherapy of Type 2 Diabetes on Bone Density and Risk of Bone Fractures. Medicina. 2024; 60(3):393. https://doi.org/10.3390/medicina60030393
Chicago/Turabian StyleWikarek, Agnieszka, Małgorzata Grabarczyk, Katarzyna Klimek, Agata Janoska-Gawrońska, Magdalena Suchodolska, and Michał Holecki. 2024. "Effect of Drugs Used in Pharmacotherapy of Type 2 Diabetes on Bone Density and Risk of Bone Fractures" Medicina 60, no. 3: 393. https://doi.org/10.3390/medicina60030393





