Local Oxygen-Based Therapy (blue®m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies
Abstract
1. Introduction
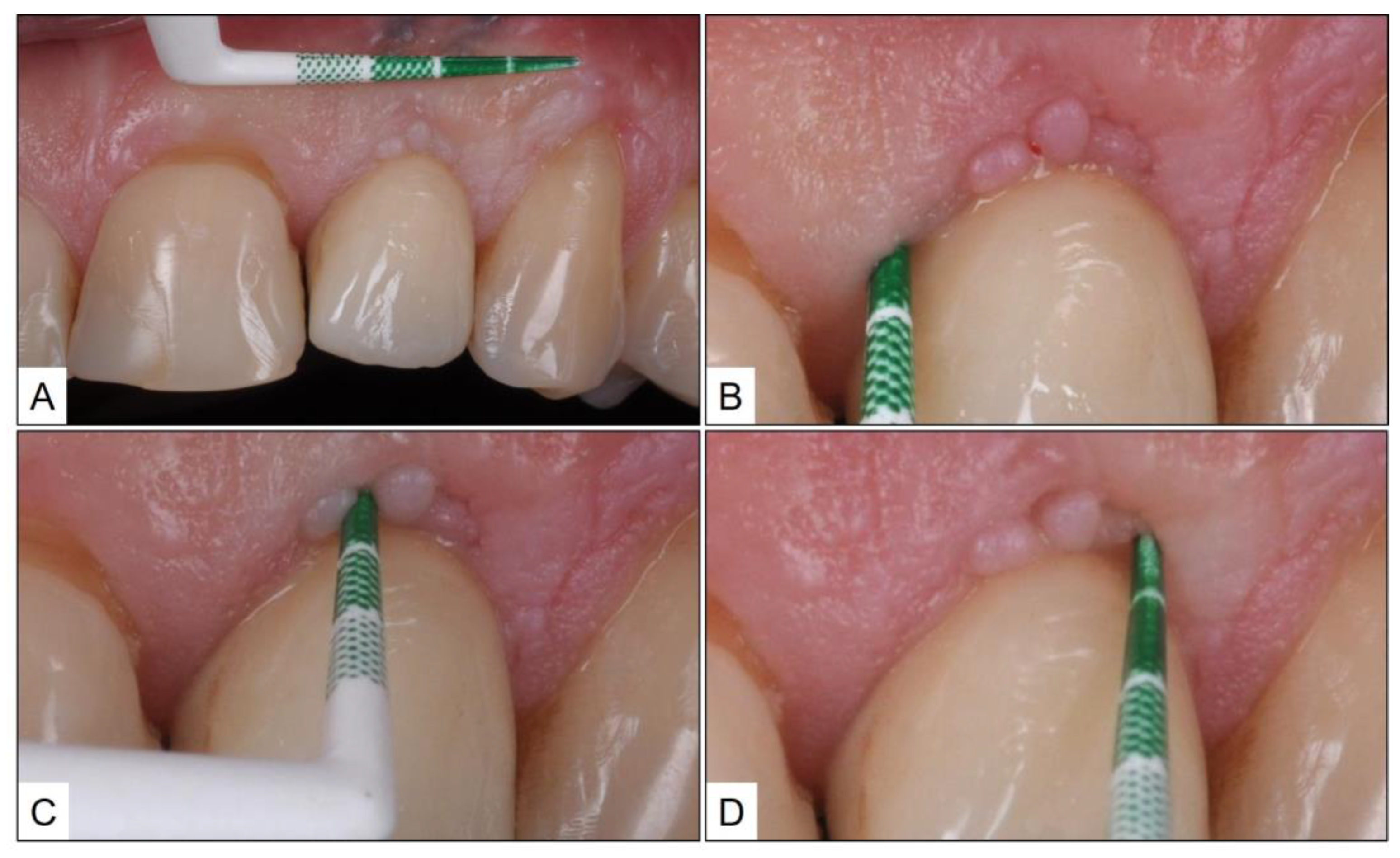
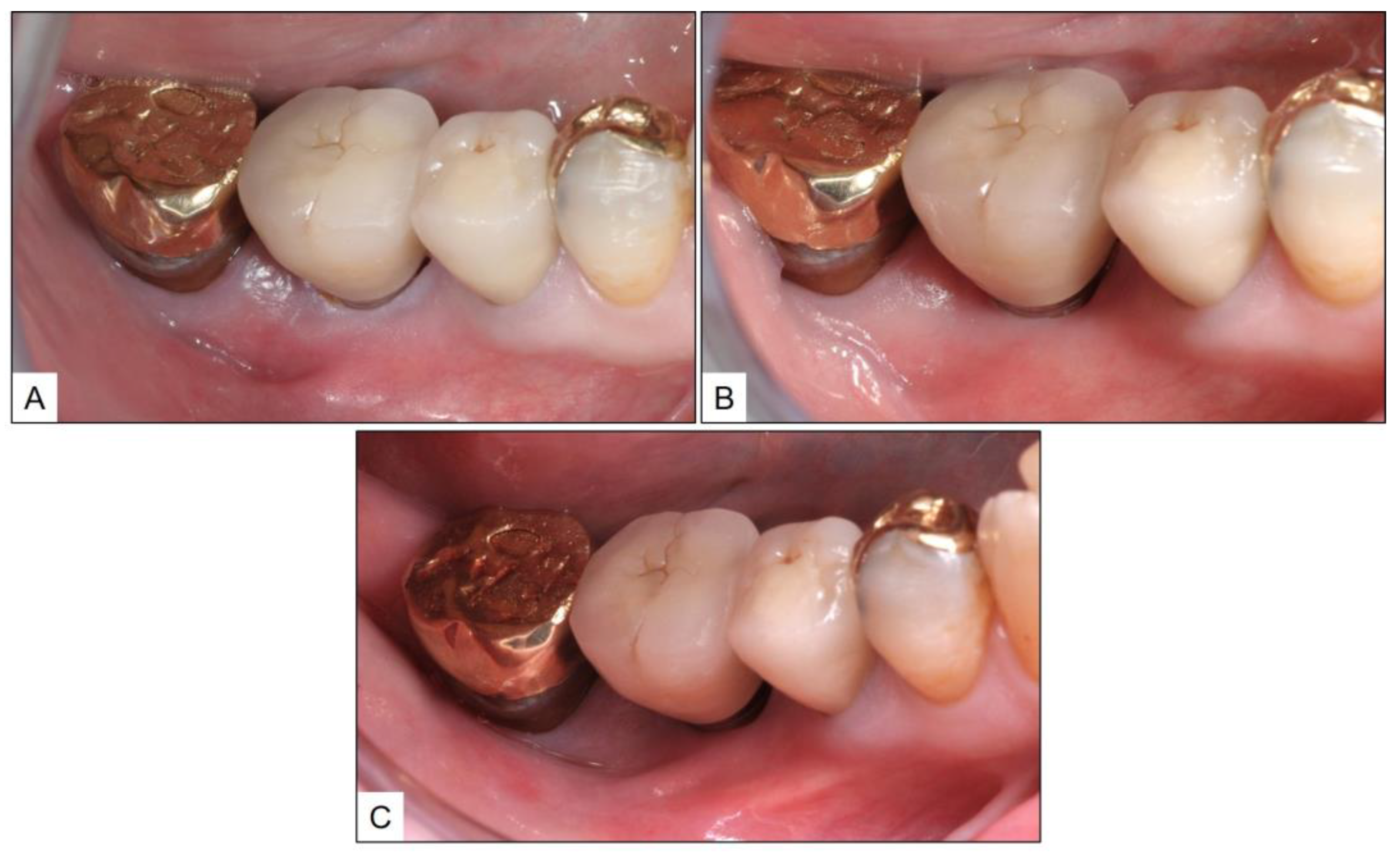
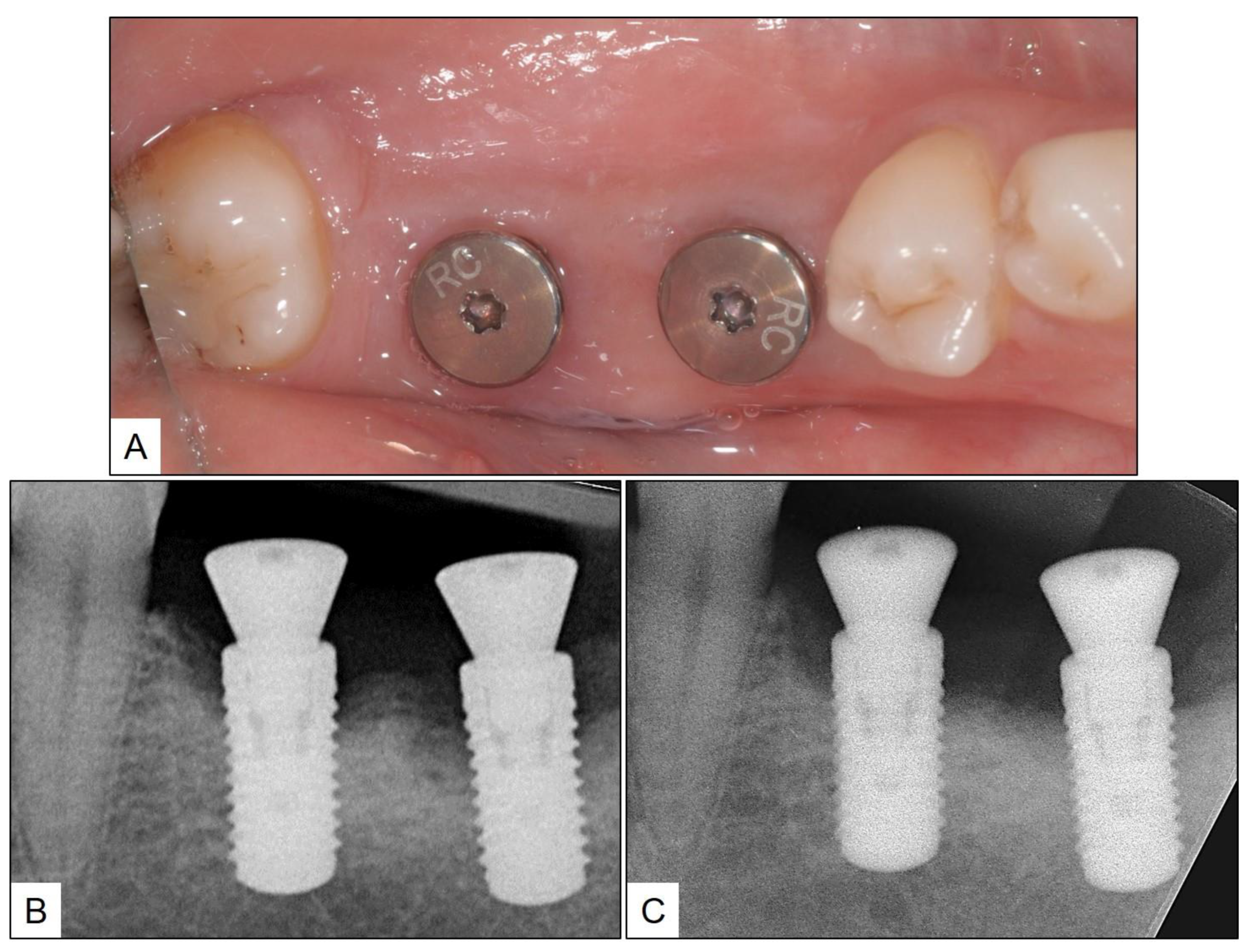
2. Clinical Case Presentation of Peri-Implant Mucositis
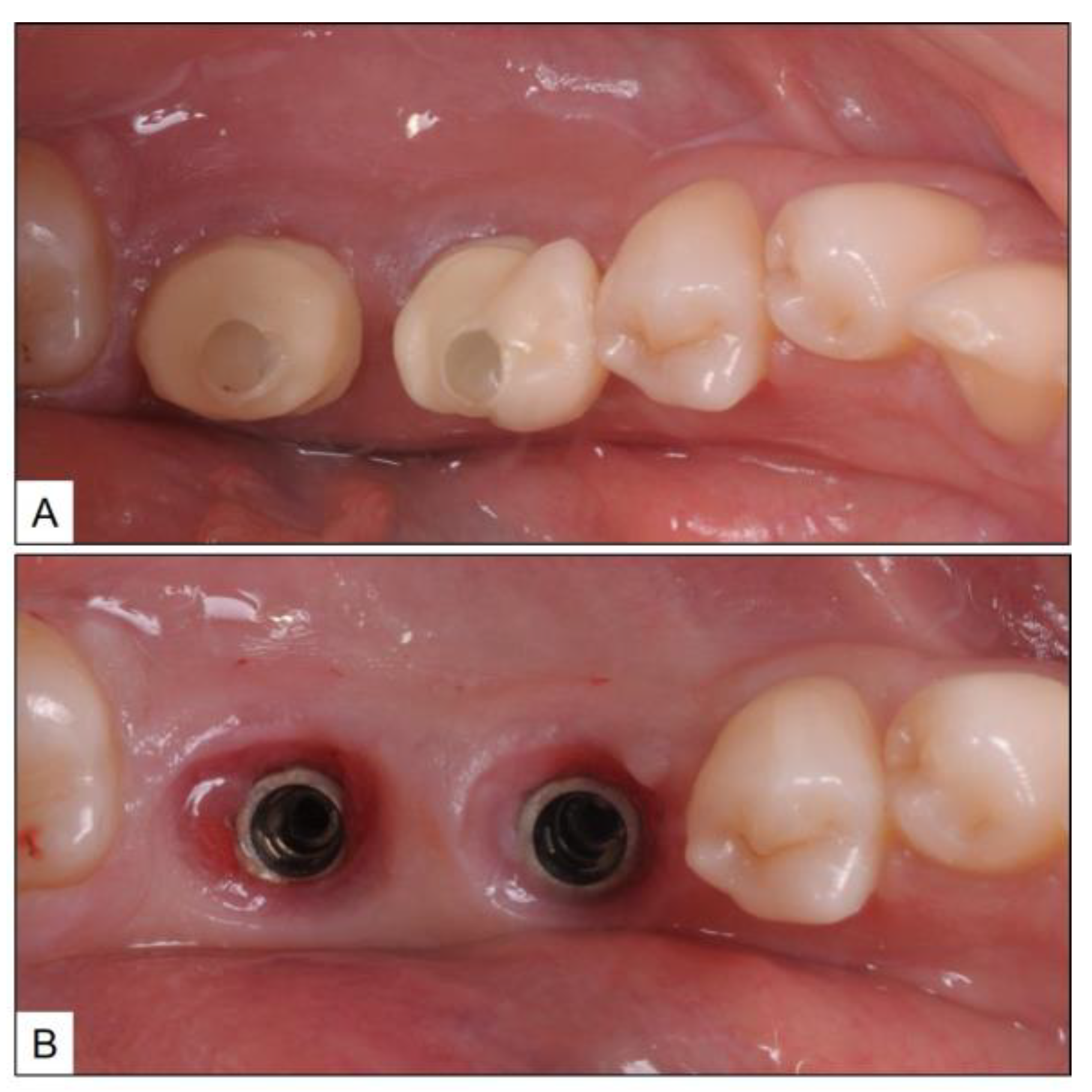
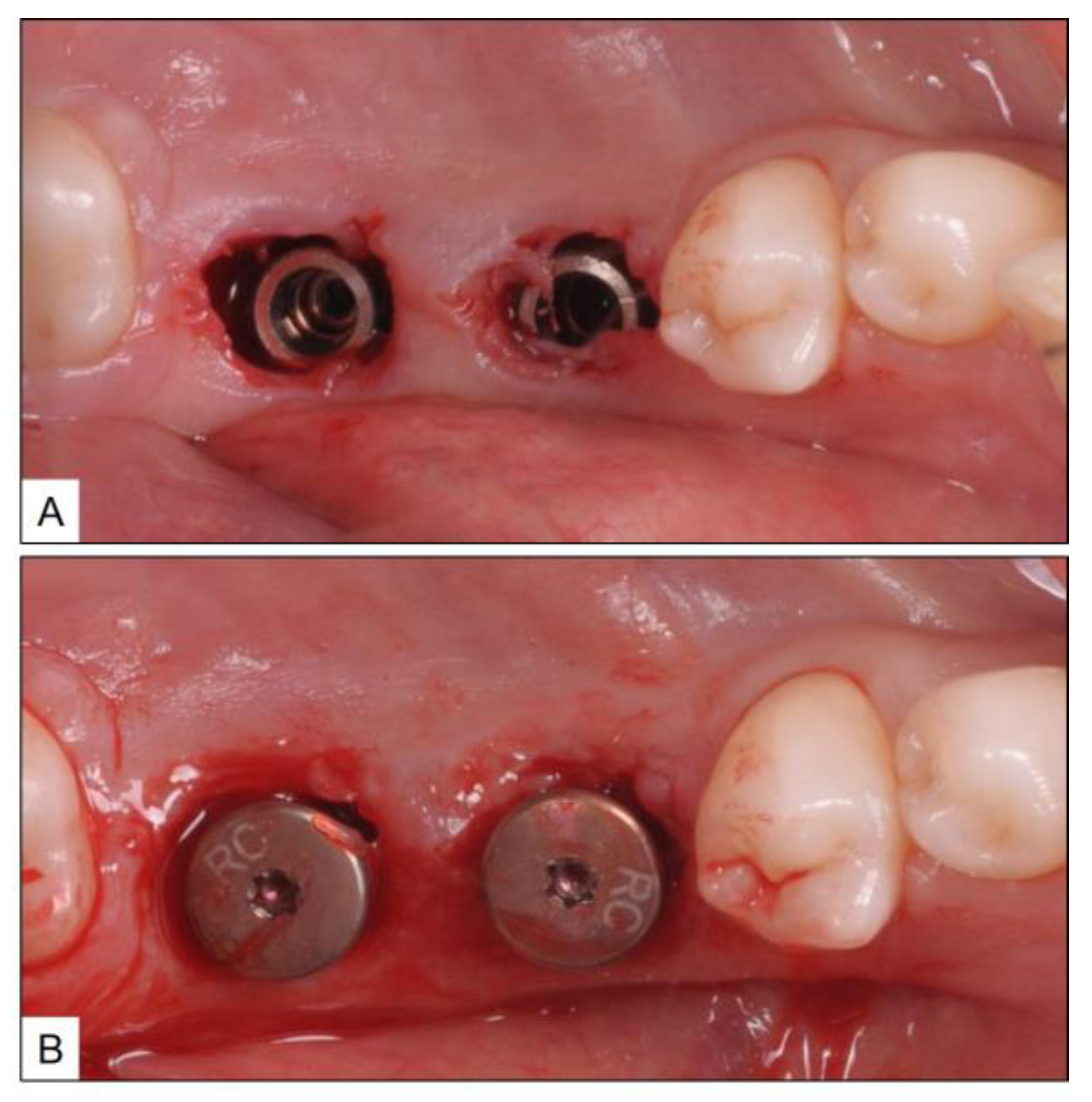
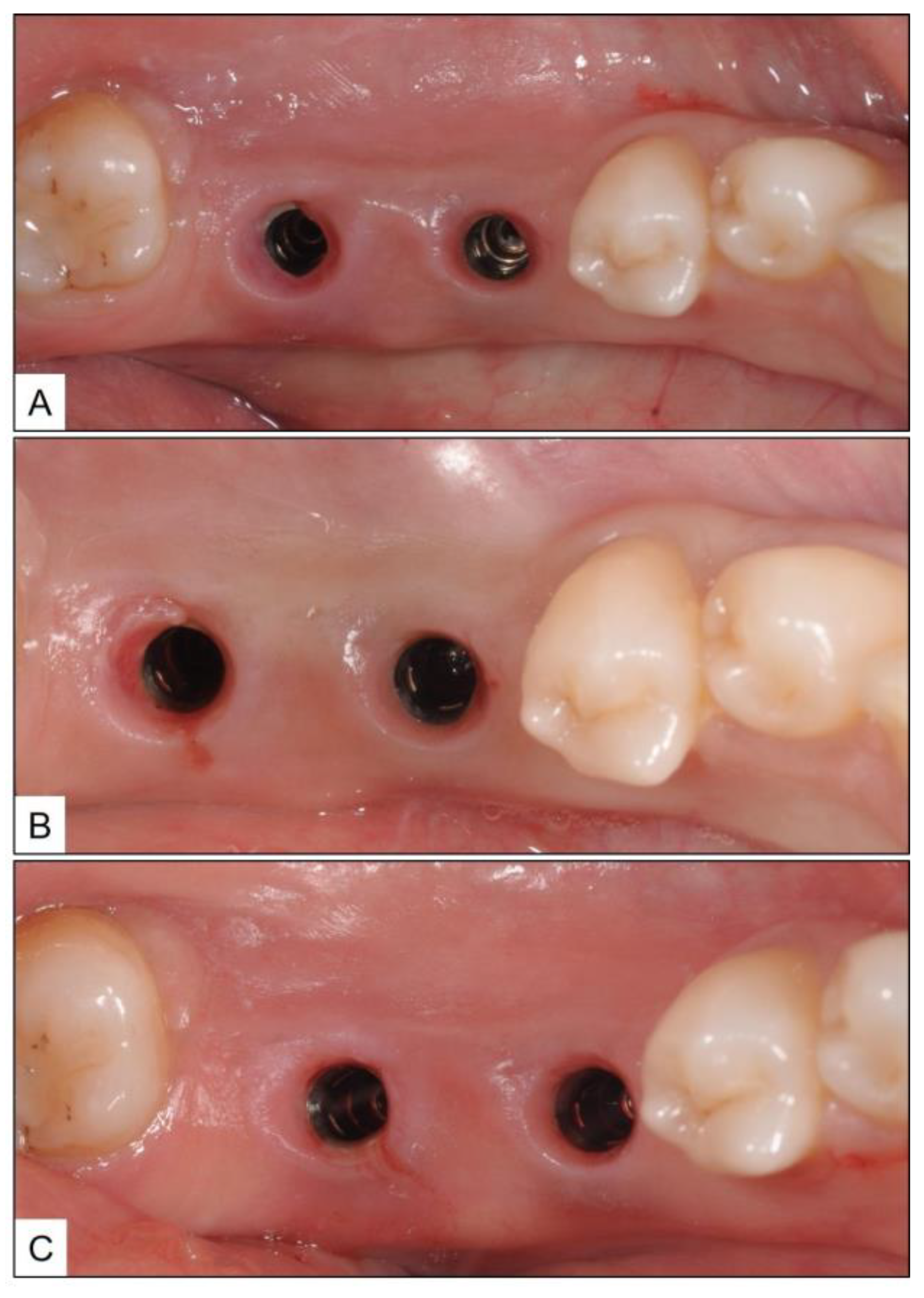
3. Clinical Cases Presentation of Peri-Implantitis
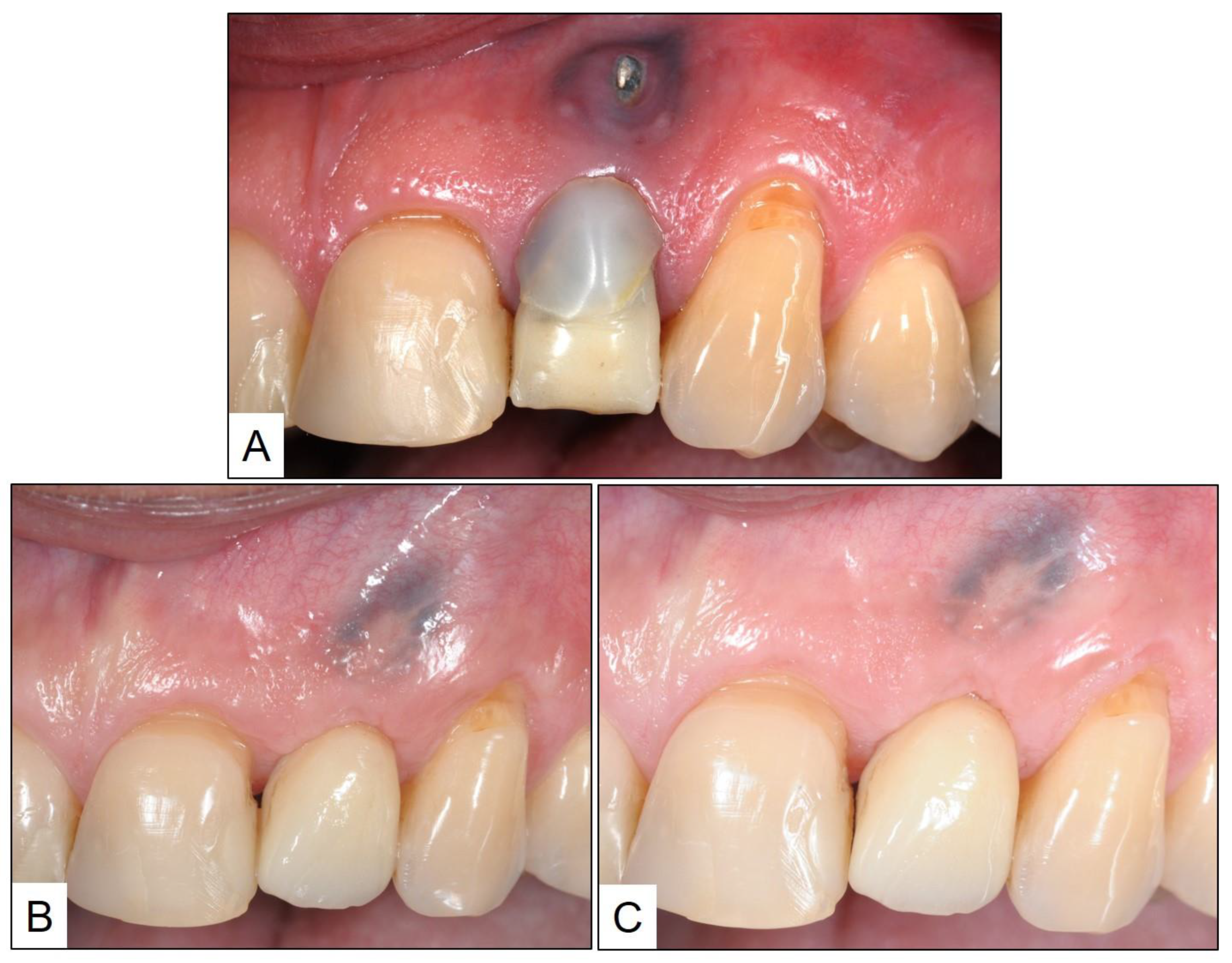
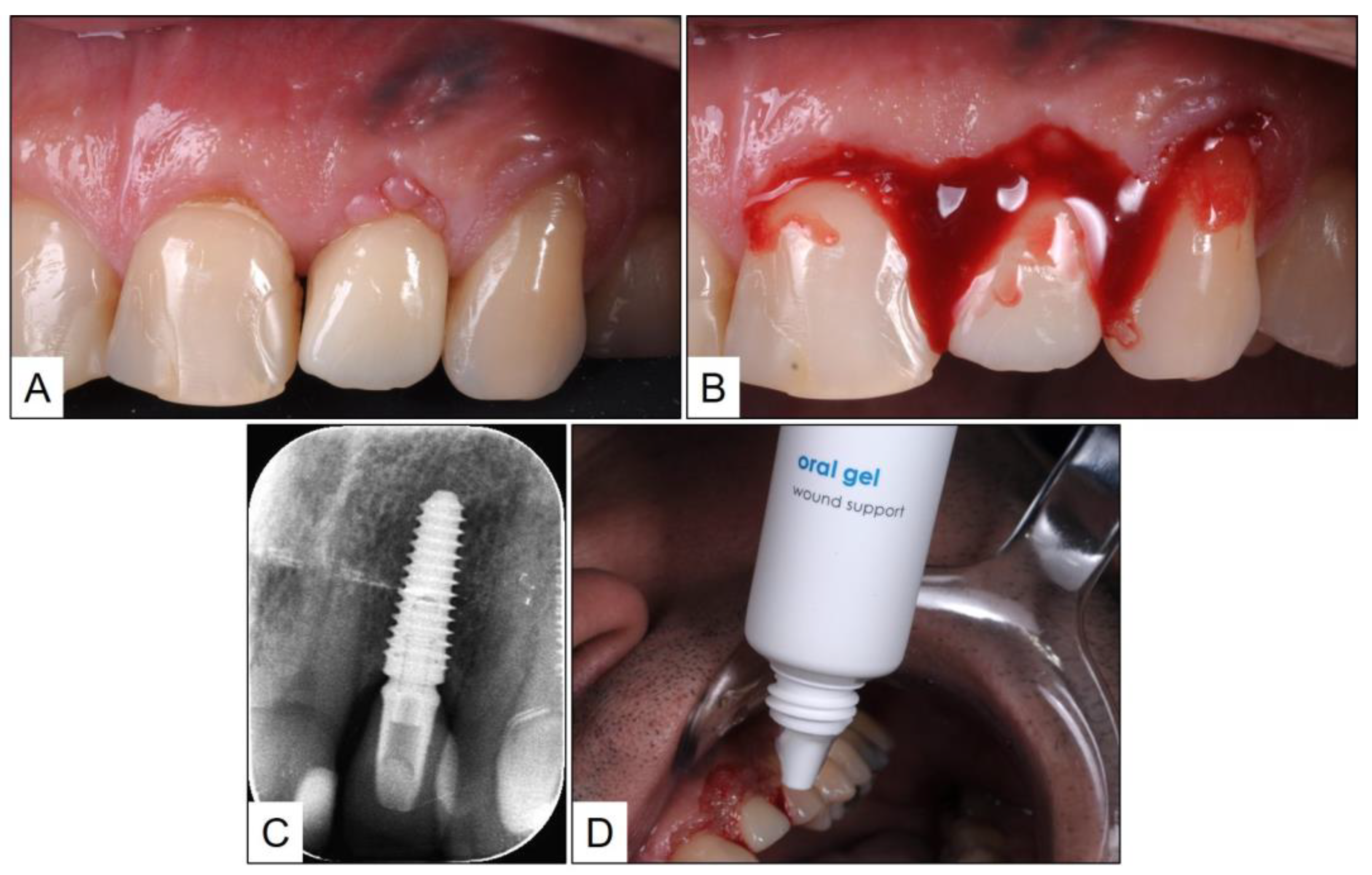
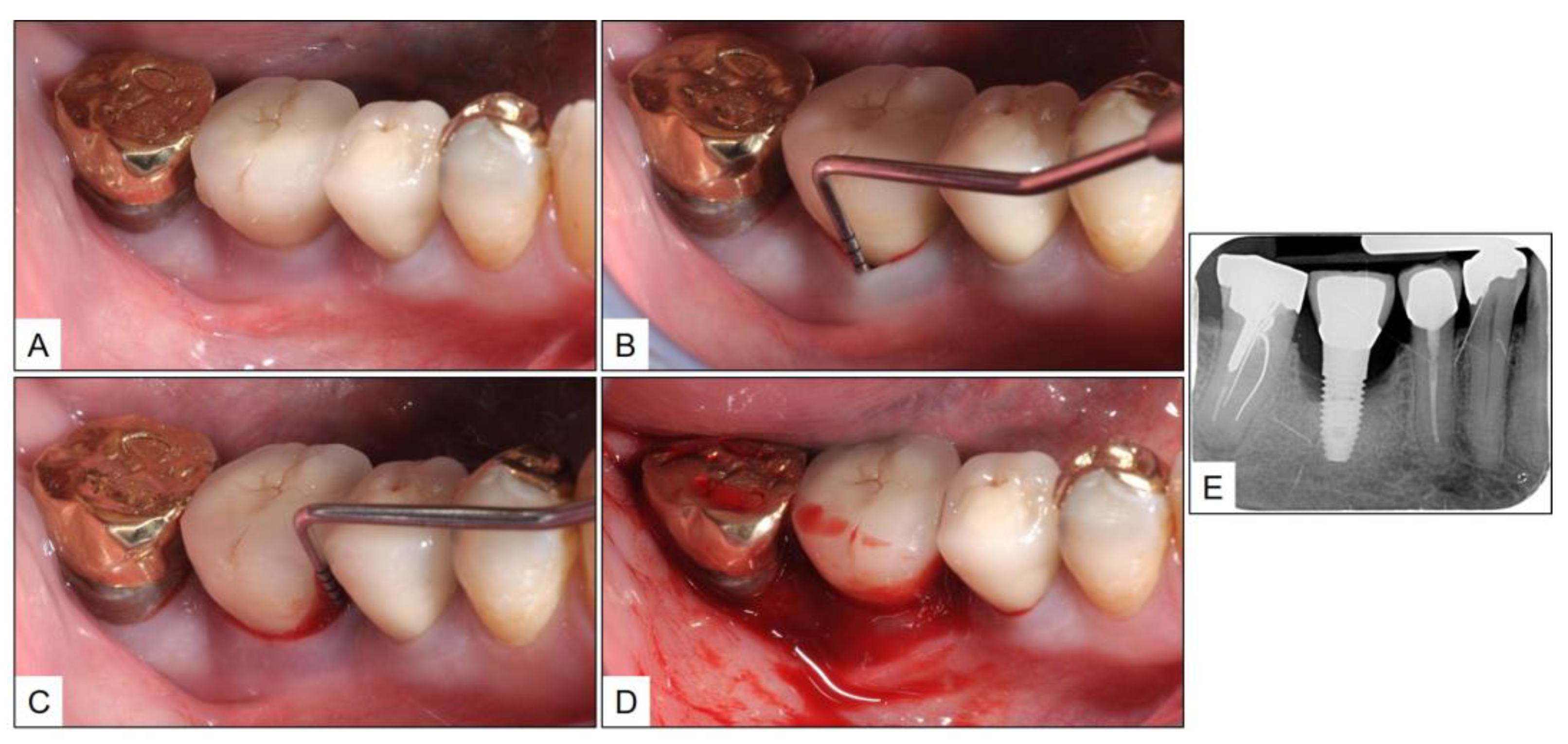
3.1. Case Report 1
3.2. Case Report 2
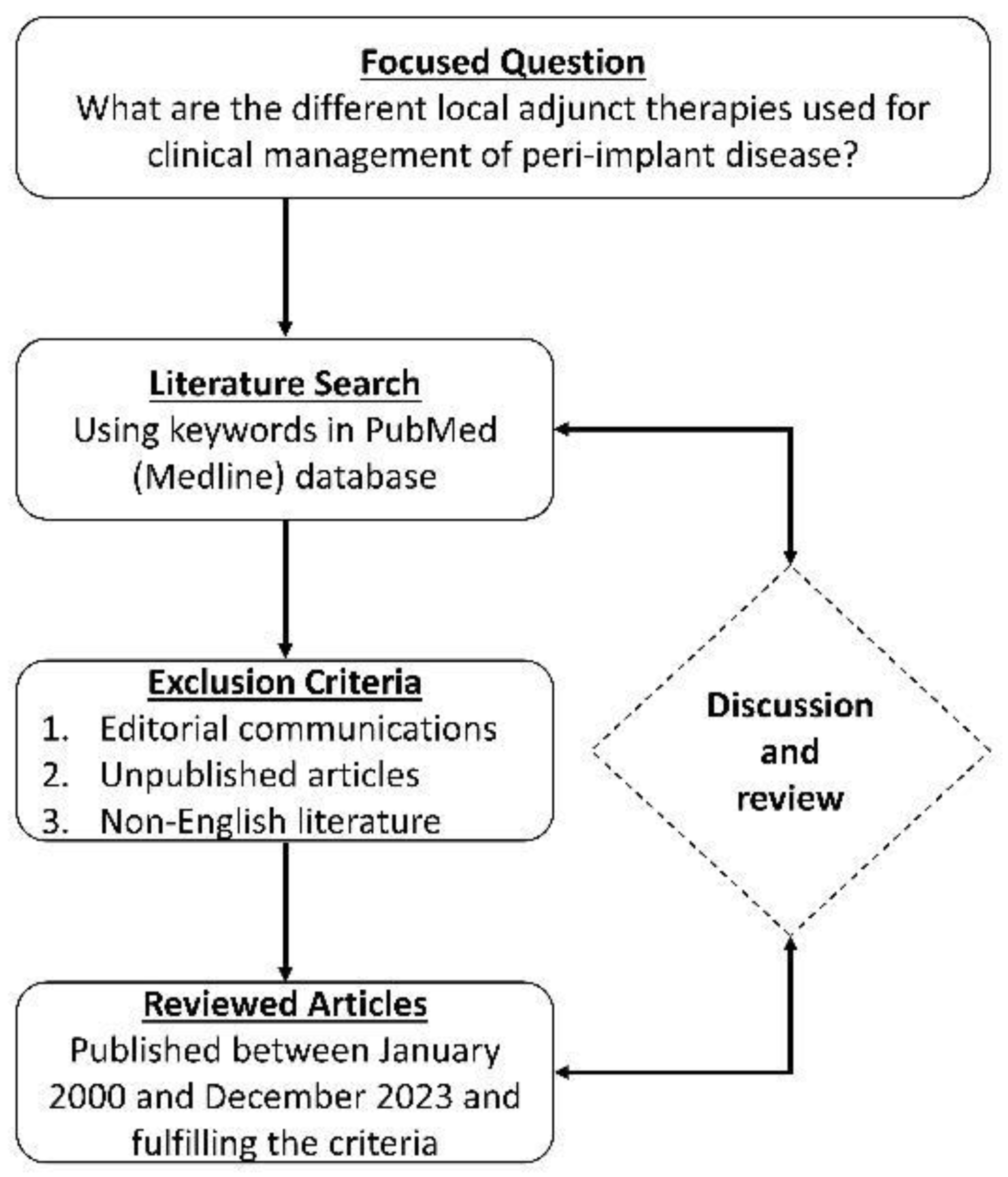
4. Systematic Review of Literature—Methodology and Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramanauskaite, A.; Fretwurst, T.; Schwarz, F. Efficacy of alternative or adjunctive measures to conventional non-surgical and surgical treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant Dent. 2021, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases-the efp s3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50 (Suppl. 26), 4–76. [Google Scholar] [CrossRef] [PubMed]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontology 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Becker, K.; Sager, M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S202–S213. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.M.; Almhöjd, U.S.; Jansson, H. Treatment of peri-implantitis: Clinical outcome of chloramine as an adjunctive to non-surgical therapy, a randomized clinical trial. Clin. Oral Implant. Res. 2017, 28, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Mayer, Y.; Ginesin, O.; Horwitz, J. A nonsurgical treatment of peri-implantitis using mechanic, antiseptic and anti-inflammatory treatment: 1 year follow-up. Clin. Exp. Dent. Res. 2020, 6, 478–485. [Google Scholar] [CrossRef]
- Santana, S.I.; Silva, P.H.F.; Salvador, S.L.; Casarin, R.C.V.; Furlaneto, F.A.C.; Messora, M.R. Adjuvant use of multispecies probiotic in the treatment of peri-implant mucositis: A randomized controlled trial. J. Clin. Periodontol. 2022, 49, 828–839. [Google Scholar] [CrossRef]
- Kashefimehr, A.; Pourabbas, R.; Faramarzi, M.; Zarandi, A.; Moradi, A.; Tenenbaum, H.C.; Azarpazhooh, A. Effects of enamel matrix derivative on non-surgical management of peri-implant mucositis: A double-blind randomized clinical trial. Clin. Oral Investig. 2017, 21, 2379–2388. [Google Scholar] [CrossRef]
- Laleman, I.; Pauwels, M.; Quirynen, M.; Teughels, W. The usage of a lactobacilli probiotic in the non-surgical therapy of peri-implantitis: A randomized pilot study. Clin. Oral Implant. Res. 2020, 31, 84–92. [Google Scholar] [CrossRef]
- Mensi, M.; Scotti, E.; Calza, S.; Pilloni, A.; Grusovin, M.G.; Mongardini, C. A new multiple anti-infective non-surgical therapy in the treatment of peri-implantitis: A case series. Minerva Stomatol. 2017, 66, 255–266. [Google Scholar] [CrossRef]
- Pulcini, A.; Bollaín, J.; Sanz-Sánchez, I.; Figuero, E.; Alonso, B.; Sanz, M.; Herrera, D. Clinical effects of the adjunctive use of a 0.03% chlorhexidine and 0.05% cetylpyridinium chloride mouth rinse in the management of peri-implant diseases: A randomized clinical trial. J. Clin. Periodontol. 2019, 46, 342–353. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J. Antibiotics in the treatment of peri-implantitis. Eur. J. Oral Implantol. 2012, 5, S43–S50. [Google Scholar]
- Wilensky, A.; Shapira, L.; Limones, A.; Martin, C. The efficacy of implant surface decontamination using chemicals during surgical treatment of peri-implantitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 336–358. [Google Scholar] [CrossRef]
- Alhumaidan, A.A.; Alrabiah, M.; Al-Aali, K.A.; Javed, F.; Vohra, F.; Abduljabbar, T. Efficacy of adjunct subgingival minocycline delivery for treatment of peri-implantitis in moderate cigarette smokers. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5698–5705. [Google Scholar]
- Renvert, S.; Lessem, J.; Dahlén, G.; Lindahl, C.; Svensson, M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: A randomized clinical trial. J. Clin. Periodontol. 2006, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.; Alshaikh, M.; Mehmood, A.; Alqhtani, N.; Alkhtani, F.; Alenazi, A. Efficacy of antibiotic versus probiotics as adjuncts to mechanical debridement for the treatment of peri-implant mucositis. J. Oral Implantol. 2022, 48, 99–104. [Google Scholar] [CrossRef] [PubMed]
- De Siena, F.; Francetti, L.; Corbella, S.; Taschieri, S.; Del Fabbro, M. Topical application of 1% chlorhexidine gel versus 0.2% mouthwash in the treatment of peri-implant mucositis. An observational study. Int. J. Dent. Hyg. 2013, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dommisch, H.; Hoedke, D.; Valles, C.; Vilarrasa, J.; Jepsen, S.; Pascual La Rocca, A. Efficacy of professionally administered chemical agents as an adjunctive treatment to sub-marginal instrumentation during the therapy of peri-implant mucositis. J. Clin. Periodontol. 2023, 50 (Suppl. 26), 146–160. [Google Scholar] [CrossRef]
- Philip, J.; Laine, M.L.; Wismeijer, D. Adjunctive effect of mouthrinse on treatment of peri-implant mucositis using mechanical debridement: A randomized clinical trial. J. Clin. Periodontol. 2020, 47, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Bonez, P.C.; Dos Santos Alves, C.F.; Dalmolin, T.V.; Agertt, V.A.; Mizdal, C.R.; Flores Vda, C.; Marques, J.B.; Santos, R.C.; Anraku de Campos, M.M. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control 2013, 41, e119–e122. [Google Scholar] [CrossRef]
- Pałka, Ł.; Nowakowska-Toporowska, A.; Dalewski, B. Is chlorhexidine in dentistry an ally or a foe? A narrative review. Healthcare 2022, 10, 764. [Google Scholar] [CrossRef]
- Juliana, H.; Tarek, S. Comparative study of the effect of bluem active oxygen gel and coe-pack dressing on postoperative surgical depigmentation healing. Saudi Dent. J. 2022, 34, 328–334. [Google Scholar] [CrossRef]
- Mattei, B.M.; Imanishi, S.A.W.; de Oliveira Ramos, G.; de Campos, P.S.; Weiss, S.G.; Deliberador, T.M. Mouthwash with active oxygen (blue®m) reduces postoperative inflammation and pain. Case Rep. Dent. 2021, 2021, 5535807. [Google Scholar] [CrossRef]
- Sindhusha, V.B.; Rajasekar, A. Efficacy of oxygen-enriched mouthwash as a pre-procedural mouth rinse against oral microbes produced during ultrasonic scaling. Cureus 2023, 15, e49164. [Google Scholar] [CrossRef]
- González Regueiro, I.; Martínez Rodriguez, N.; Barona Dorado, C.; Sanz-Sánchez, I.; Montero, E.; Ata-Ali, J.; Duarte, F.; Martínez-González, J.M. Surgical approach combining implantoplasty and reconstructive therapy with locally delivered antibiotic in the treatment of peri-implantitis: A prospective clinical case series. Clin. Implant Dent. Relat. Res. 2021, 23, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Porras, R.; Anderson, G.B.; Caffesse, R.; Narendran, S.; Trejo, P.M. Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J. Periodontol. 2002, 73, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Salvi, G.E.; Botticelli, D.; Mombelli, A.; Faddy, M.; Lang, N.P.; Implant Complication Research Group. Anti-infective treatment of peri-implant mucositis: A randomised controlled clinical trial. Clin. Oral Implant. Res. 2011, 22, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Blasi, A.; Stratul, S.I.; Ramaglia, L.; Sculean, A.; Salvi, G.E.; Rusu, D. Anti-infective therapy of peri-implant mucositis with adjunctive delivery of a sodium hypochlorite gel: A 6-month randomized triple-blind controlled clinical trial. Clin. Oral Investig. 2020, 24, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Feloutzis, A.; Brägger, U.; Lang, N.P. Treatment of peri-implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. Clin. Oral Implant. Res. 2001, 12, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Frankenthal, S.; Joseph, L.; Rozitsky, D.; Levi, G.; Machtei, E.E. Water jet with adjunct chlorhexidine gel for nonsurgical treatment of peri-implantitis. Quintessence Int. 2015, 46, 133–137. [Google Scholar] [PubMed]
- Machtei, E.E.; Romanos, G.; Kang, P.; Travan, S.; Schmidt, S.; Papathanasiou, E.; Tatarakis, N.; Tandlich, M.; Liberman, L.H.; Horwitz, J.; et al. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: A multicenter, randomized, comparative clinical trial. J. Periodontol. 2021, 92, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Song, Y.W.; Cha, J.K.; Lee, J.S.; Kim, Y.T.; Shin, H.S.; Lee, D.W.; Lee, J.H.; Kim, C.S. Adjunctive use of metronidazole-minocycline ointment in the nonsurgical treatment of peri-implantitis: A multicenter randomized controlled trial. Clin. Implant Dent. Relat. Res. 2021, 23, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, P.C.; Netti, A.; Lopez, M.A.; Giaquinto, E.F.; De Rosa, G.; Aureli, G.; Bodnarenko, A.; Papi, P.; Starzyńska, A.; Pompa, G.; et al. Local/topical antibiotics for peri-implantitis treatment: A systematic review. Antibiotics 2021, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pu, R.; Qian, Y.; Shi, J.; Si, M. Antimicrobial photodynamic therapy versus antibiotics as an adjunct in the treatment of periodontitis and peri-implantitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2021, 34, 102231. [Google Scholar] [CrossRef]
- Gaggl, A.J.; Rainer, H.; Grund, E.; Chiari, F.M. Local oxygen therapy for treating acute necrotizing periodontal disease in smokers. J. Periodontol. 2006, 77, 31–38. [Google Scholar] [CrossRef]
- Sundar, C.; Ramalingam, S.; Mohan, V.; Pradeepa, R.; Ramakrishnan, M.J. Periodontal therapy as an adjunctive modality for hba1c reduction in type-2 diabetic patients. J. Educ. Health Promot. 2018, 7, 152. [Google Scholar]
- Ramalingam, S.; Sundar, C.; Jansen, J.A.; Alghamdi, H. Chapter 1—Alveolar bone science: Structural characteristics and pathological changes. In Dental Implants and Bone Grafts; Alghamdi, H., Jansen, J., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 1–22. [Google Scholar]
- Ramalingam, S.; Al-Hindi, M.; Al-Eid, R.A.; Nooh, N. Clinical evaluation of implant survival based on size and site of placement: A retrospective study of immediate implants at single rooted teeth sites. Saudi Dent. J. 2015, 27, 105–111. [Google Scholar] [CrossRef]

| Author | Nature of Non-Surgical Therapy | Local Adjunct Therapy | Compared with | Conclusions |
|---|---|---|---|---|
| Porras et al. [27] | Scaling with plastic scaler + mechanical cleansing with rubber cup and polishing paste | Chlorhexidine (0.12%) gel + rinse (Test) | Placebo (Control) | After 3 months follow-up; use of chlorhexidine (0.12%) gel + rinse as a local adjunct to mechanical therapy for peri-implant mucositis resulted in resolution of inflammation and a significant reduction in PPD. |
| Heitz-Mayfield et al. [28] | Scaling with plastic scaler + mechanical cleansing with rubber cup and polishing paste | Chlorhexidine (0.5%) gel to be brushed around the implant; twice a day for 4 weeks (Test) | Placebo gel (Control) | After 3 months follow-up; use of chlorhexidine (0.5%) gel as a local adjunct to mechanical therapy did not significantly enhance clinical outcomes in peri-implant mucositis. Implants with restoration margins placed supra-gingivally showed better treatment response than implants with sub-mucosal restoration margins. |
| De Siena et al. [18] | Professional oral prophylaxis administered by dental hygienist | Chlorhexidine (0.2%) mouthwash 10 mL—rinsed twice a day for 10 days | Chlorhexidine (1%) gel 1 mL placed sub-mucosally twice a day for 10 days | After 3 months follow-up; use of chlorhexidine rinse (0.2%) or gel (1%) as a local adjunct to treat peri-implant mucositis gave better clinical outcomes. Nevertheless; there was no difference in outcomes between the two formulations. |
| Pulcini et al. [12] | Ultrasonic scaling with plastic tip + erythritol-based air powder polishing | Chlorhexidine (0.03%) + CPC (0.05%) mouthwash (Test) | Mouthwash without chlorhexidine or CPC (Control) | After 12 months follow-up; use of chlorhexidine (0.03%) + CPC (0.05%) mouthwash as a local adjunct in peri-implant mucositis resulted in better clinical outcomes than with mouthwash without the above active ingredients. However, the formulation did not result in complete resolution of peri-implant disease. |
| Iorio-Siciliano et al. [29] | Ultrasonic scaling with plastic tips + mechanical cleansing with rubber cup and polishing paste | Amino acid buffered sodium hypochlorite gel—applied 5 times in the peri-implant tissues immediately after non-surgical therapy (Test) | Placebo gel—applied in the same way as test group (Control) | After 6 months follow-up; use of sodium hypochlorite gel as a local adjunct to non-surgical therapy of peri-implant mucositis resulted in a significant reduction in PPD and number of implants with BOP, which was better than that with placebo gel, but not significantly. Neither modality resulted in complete peri-implant disease resolution. |
| Philip et al. [20] | Ultrasonic scaling with plastic tips + mechanical cleansing with rubber cup and polishing paste | Delmopinol hydrochloride (0.2%) mouthwash twice daily until follow-up (Test) | Chlorhexidine (0.2%) mouthwash twice daily until follow-up (Positive Control)/Placebo mouthwash twice daily until follow-up (Negative Control) | After 3 months follow-up; use of delmopinol hydrochloride mouthwash as an adjunct to non-surgical therapy of peri-implant mucositis resulted in a significant improvement in clinical parameters, than with the use of chlorhexidine mouthwash. There was 87% disease resolution among patients who used delmopinol mouthwash; in comparison to 60% and 71% in those who used chlorhexidine and placebo mouthwashes, respectively. |
| Alqahtani et al. [17] | Ultrasonic scaling with plastic tips + mechanical cleansing with rubber cup and polishing paste | Probiotic lozenge containing Lactobacillus reuteri; chewed orally twice a day after brushing; for 21 days (Test) | Amoxycillin 500 mg administered orally; three times a day for 7 days (Positive control)/Non-surgical therapy only (Negative control) | After 3 months follow-up; use of probiotic therapy as a topical adjunct to non-surgical therapy of peri-implant mucositis was more effective than adjunct antibiotic therapy in terms of significantly improved clinical outcomes. |
| Santana et al. [8] | Ultrasonic scaling with Teflon-coated tips + mechanical cleansing with rubber cup and polishing paste | Topically applied carboxymethyl cellulose gel containing a probiotic formulation of Bifidobacterium lactis, Lactobacillus rhamnosus, and Lactobacillus paracasei (Test) | Non-surgical therapy only (Control) | After 6 months follow-up; use of probiotic therapy as a topical adjunct to non-surgical therapy of peri-implant mucositis in edentulous patients resulted in significantly improved clinical outcomes and immunological benefits. |
| Author | Nature of Non-Surgical Therapy | Local Adjunct Therapy | Compared with | Conclusions |
|---|---|---|---|---|
| Mombelli et al. [30] | Scaling with plastic scaler + mechanical cleansing with rubber cup and polishing paste | Tetracycline fibers were placed in pocket for 10 days | - | After 6 months follow-up; use of tetracycline as a local adjunct to non-surgical therapy of peri-implantitis resulted in a significant improvement in clinical parameters and reduction in microbial colonies. |
| Renvert et al. [16] | Scaling with plastic scaler + mechanical cleansing with rubber cup and polishing paste | Minocycline microspheres (1 mg) placed sub-mucosally (Test) | Chlorhexidine (1%) gel 1 mL placed sub-mucosally (Control) | After 12 months follow-up; use of minocycline as a local adjunct to mechanical therapy for peri-implantitis resulted in a greater sustained reduction in PPD over 12 months, than with the use of chlorhexidine. |
| Levin et al. [31] | Ultrasonic scaling and surface debridement with specialized instruments | Water jet irrigation with chlorhexidine gel 5 mL (Test) | Only water jet irrigation (Control) | After 3 months follow-up; use of local chlorhexidine gel delivered through water jet irrigation as an adjunct to mechanical therapy for peri-implantitis significantly decreased BOP and PPD, than when using water jet alone. There was no significant improvement in RBL in both groups. |
| Roos-Jansåker et al. [6] | Ultrasonic scaling with sub-mucosal debridement using piezo-ceramic scaler tips | Sub-mucosally administered chloramine to cover all implant surfaces (Test) | Only scaling and debridement (Control) | After 3 months follow-up; use of local chloramine as an adjunct to non-surgical therapy of peri-implantitis was only as effective as conventional treatment. Irrespective of the use of chloramine or not, there was a significant improvement in clinical outcomes. |
| Kashefimehr et al. [9] | Sub-gingival scaling with plastic tips + air polishing with glycine-based powder | EMD administered sub-mucosally; 2 weeks after non-surgical therapy (Test) | Non-surgical therapy only (Control) | After 3 months follow-up; use of EMD as a local adjunct to non-surgical mechanical therapy for peri-implantitis resulted in a significant improvement in clinical outcomes, in comparison to mechanical debridement alone. There was no complete disease resolution either with or without EMD. |
| Mensi et al. [11] | Ultrasonic scaling + supra- and sub-gingival erythritol-based air powder polishing | Doxycycline administered supra- and sub-gingivally (one week after non-surgical therapy + additional peri-implant doxycycline application one week later) | - | After 12 months follow-up; use of multiple anti-infective adjunct therapy with doxycycline and eythritol air polishing along with mechanical therapy for peri-implantitis resulted in a significant improvement in clinical parameters. |
| Laleman et al. [10] | Ultrasonic scaling with specialized tips + sub-gingival debridement with titanium curettes + Air polishing | Dual strain probiotic Lactobacillus reuteri drops in peri-implant area after non-surgical therapy + lozenges (1–2 per day) containing the above probiotic strains for 12 weeks (Test) | Placebo drops and lozenges without probiotic bacteria (Control) | After 6 months follow-up; use of dual strain probiotic Lactobacillus reuteri as an adjunct for non-surgical therapy of peri-implantitis showed no clinically discernible benefits. |
| Mayer et al. [7] | Ultrasonic scaling with specialized tips + sub-gingival debridement with Teflon-coated curettes | Amino acid buffered sodium hypochlorite gel—applied 3 times in the peri-implant tissues immediately after non-surgical therapy + 1 mg minocycline (Test) | Non-surgical therapy only (Control) | After 12 months follow-up; use of sodium hypochlorite gel with minocycline as a local adjunct to non-surgical therapy of peri-implantitis resulted in a significant reduction in inflammation and better connective tissue reattachment. This formulation provided a local antiseptic and anti-inflammatory effect. |
| Machtei et al. [32] | Supra-gingival ultrasonic scaling + sub-gingival implant surface debridement with specialized tips | Sub-gingival chlorhexidine chips applied bi-weekly for 12 weeks (Test) | Non-surgical therapy only (Control) | After 6 months follow-up; use of chlorhexidine chips as a local adjunct to non-surgical therapy of peri-implantitis resulted in a significant improvement in clinical parameters (PPD and CAL). |
| Park et al. [33] | Ultrasonic scaling + sub-gingival mechanical debridement with specialized tips | Metronidazole + Minocycline ointment administered locally (Test 1)/Minocycline ointment administered locally (Test 2) | Non-surgical therapy only (Control) | After 3 months follow-up; use of either a combination of metronidazole and minocycline or minocycline alone as a local adjunct to non-surgical therapy of peri-implantitis resulted in significantly improved clinical treatment outcomes. However, in deep pockets (≥8 mm), the use of metronidazole and minocycline resulted in greater PPD reduction. |
| Alhumaidan et al. [15] | Ultrasonic scaling + sub-gingival mechanical debridement with specialized tips | Minocycline microspheres (1 mg) placed sub-gingivally (Test) | Non-surgical therapy only (Control) | After 6 months follow-up; use of minocycline administered sub-gingivally as a single-use adjunct to non-surgical therapy of peri-implantitis resulted in significantly improved clinical outcomes than with the use of non-surgical therapy alone. It may be assumed that only topical application of minocycline in peri-implantitis might be as effective as non-surgical therapy alone. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, M.Y.; Abas, I.; Basudan, A.M.; Alghamdi, H.S. Local Oxygen-Based Therapy (blue®m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies. Medicina 2024, 60, 447. https://doi.org/10.3390/medicina60030447
Shaheen MY, Abas I, Basudan AM, Alghamdi HS. Local Oxygen-Based Therapy (blue®m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies. Medicina. 2024; 60(3):447. https://doi.org/10.3390/medicina60030447
Chicago/Turabian StyleShaheen, Marwa Y., Irfan Abas, Amani M. Basudan, and Hamdan S. Alghamdi. 2024. "Local Oxygen-Based Therapy (blue®m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies" Medicina 60, no. 3: 447. https://doi.org/10.3390/medicina60030447
APA StyleShaheen, M. Y., Abas, I., Basudan, A. M., & Alghamdi, H. S. (2024). Local Oxygen-Based Therapy (blue®m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies. Medicina, 60(3), 447. https://doi.org/10.3390/medicina60030447









