Hepatic Hemangioma: Review of Imaging and Therapeutic Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Quality Assessment
2.5. Synthesis of Results
3. Clinical Manifestations of Hepatic Hemangiomas
4. Diagnostic Approaches for Hepatic Hemangiomas
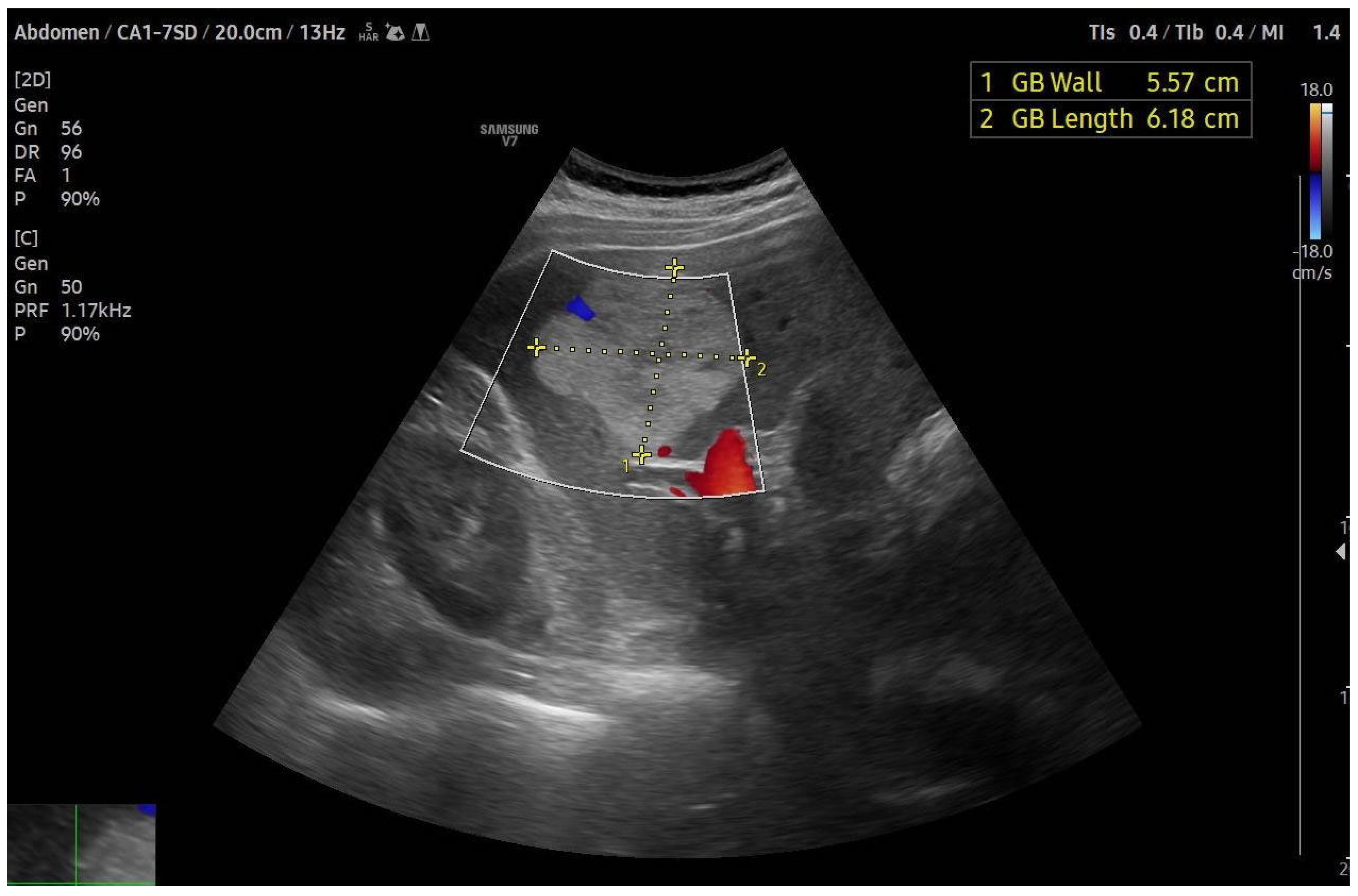
4.1. Ultrasound (US)
4.2. Contrast-Enhanced Ultrasound (CEUS)
4.3. Endoscopic Ultrasound (EUS)
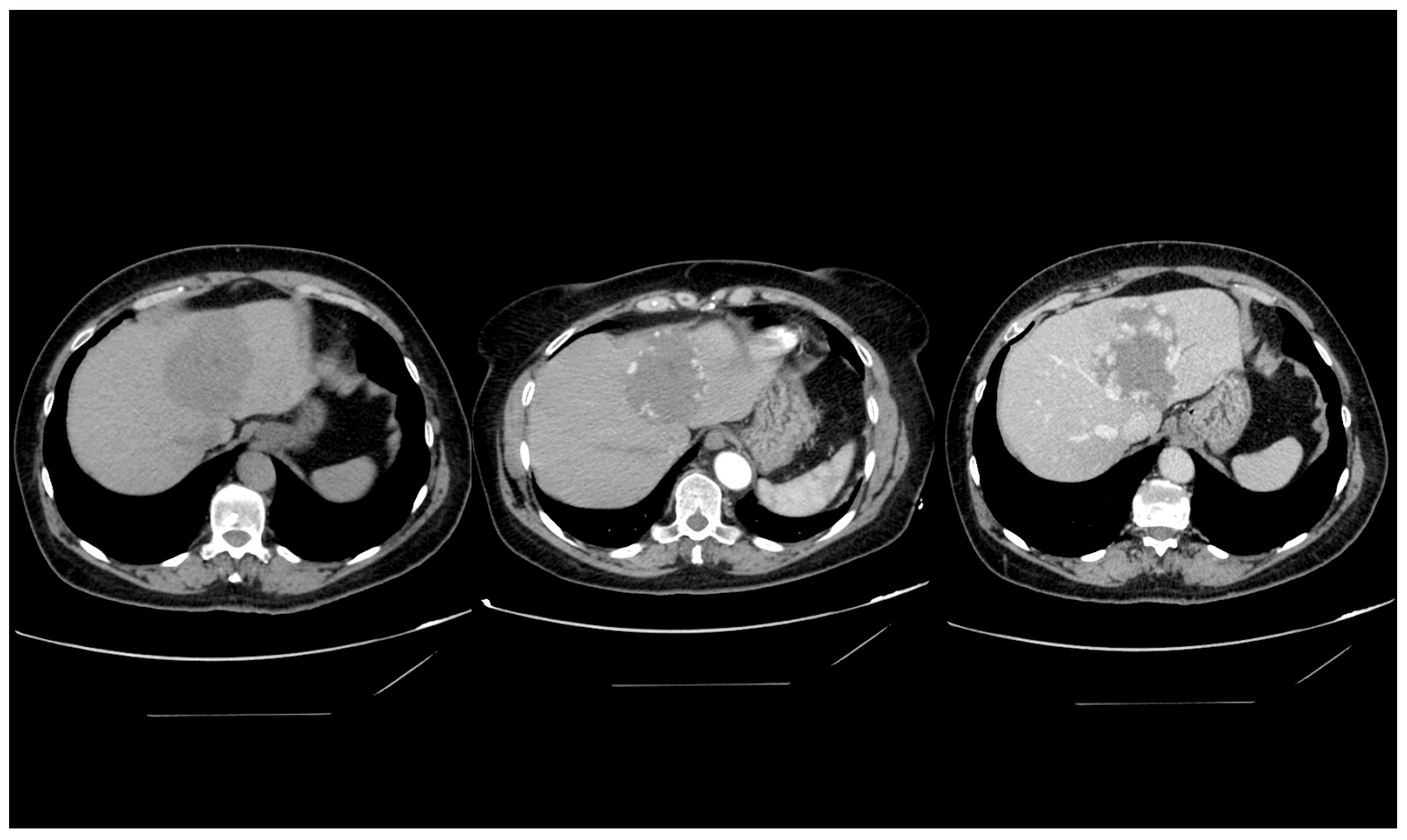
4.4. Computed Tomography
4.5. Magnetic Resonance Imaging
4.6. Technetium-99m-Labeled Red Blood Cell Imaging
5. Imaging Characterization of Hemangioma Subtypes
5.1. Cavernous Hemangioma
5.2. Capillary Hemangioma
5.3. Sclerosing Hemangioma
6. Atypical Hepatic Hemangiomas in Imaging
6.1. Giant Haemangioma
6.2. Hemangioma with Arterioportal Shunt
6.3. Hemangiomatosis
6.4. Pedunculated Haemangioma
6.5. Hepatic Steatosis
6.6. Liver Cirrhosis
7. Histology Sampling
8. Treatment
8.1. Surgical Approach
8.2. Radiofrequency Ablation
8.3. Transarterial Embolization and Chemoembolization
8.4. Liver Transplantation
9. Future Prospects
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadick, M.; Müller-Wille, R.; Wildgruber, M.; Wohlgemuth, W.A. Vascular anomalies (part I): Classification and diagnostics of vascular anomalies. Rofo 2018, 190, 825–835. [Google Scholar] [CrossRef]
- Belghiti, J.; Cauchy, F.; Paradis, V.; Vilgrain, V. Diagnosis and management of solid benign liver lesions. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 737–749. [Google Scholar] [CrossRef]
- Maruyama, M.; Isokawa, O.; Hoshiyama, K.; Hoshiyama, A.; Hoshiyama, M.; Hoshiyama, Y. Diagnosis and management of giant hepatic hemangioma: The usefulness of contrast-enhanced ultrasonography. Int. J. Hepatol. 2013, 2013, 802180. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.B.; Miscall, B.G. Giant hemangiomas of the liver. Surg. Gynecol. Obstet. 1978, 147, 783–787. [Google Scholar] [PubMed]
- Karhunen, P.J. Benign hepatic tumours and tumour like conditions in men. J. Clin. Pathol. 1986, 39, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, Y.; Li, S.; Wang, W.; Wang, Z.; Wang, Y.; Liu, B.; Wang, W.; Chang, H.; Li, Z. Transarterial Chemoembolization of Giant Liver Haemangioma: A Multi-center Study with 836 Cases. Cell Biochem. Biophys. 2015, 73, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.; Chavez, L.; Surani, S. Hepatic hemangioma: What internists need to know. World J. Gastroenterol. 2020, 26, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dockerty, M.B.; Gray, H.K.; Henson, S.W. Benign tumors of the liver. II. Hemangiomas. Surg. Gynecol. Obstet. 1956, 103, 327–331. [Google Scholar] [PubMed]
- Aziz, H.; Brown, Z.J.; Baghdadi, A.; Kamel, I.R.; Pawlik, T.M. A comprehensive review of hepatic hemangioma management. J. Gastrointest. Surg. 2022, 26, 1998–2007. [Google Scholar] [CrossRef]
- Oldhafer, K.J.; Habbel, V.; Horling, K.; Makridis, G.; Wagner, K.C. Benign Liver Tumors. Visc. Med. 2020, 36, 292–303. [Google Scholar] [CrossRef]
- Farhat, W.; Ammar, H.; Said, M.A.; Mizouni, A.; Ghabry, L.; Hammami, E.; Gupta, R.; Habiba Ben Hamada; Mabrouk, M.B.; Ali, A.B. Surgical management of giant hepatic hemangioma: A 10-year single center experience. Ann. Med. Surg. 2021, 69, 102542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Tan, H.; Xu, L.; Sun, Y.; Si, S.; Liu, L.; Zhou, W.; Huang, J. Giant liver hemangioma with adult Kasabach-Merritt syndrome: Case report and literature review. Medicine 2017, 96, e7688. [Google Scholar] [CrossRef]
- Erdogan, D.; Busch, O.R.C.; van Delden, O.M.; Bennink, R.J.; ten Kate, F.J.W.; Gouma, D.J.; van Gulik, T.M. Management of liver hemangiomas according to size and symptoms. J. Gastroenterol. Hepatol. 2007, 22, 1953–1958. [Google Scholar] [CrossRef]
- Gandolfi, L.; Leo, P.; Solmi, L.; Vitelli, E.; Verros, G.; Colecchia, A. Natural history of hepatic haemangiomas: Clinical and ultrasound study. Gut 1991, 32, 677–680. [Google Scholar] [CrossRef]
- Sun, J.-H.; Nie, C.-H.; Zhang, Y.-L.; Zhou, G.-H.; Ai, J.; Zhou, T.-Y.; Zhu, T.-Y.; Zhang, A.-B.; Wang, W.-L.; Zheng, S.-S. Transcatheter arterial embolization alone for giant hepatic hemangioma. PLoS ONE 2015, 10, e0135158. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Aggarwal, A.; Singla, R.; Kalra, N.; Chawla, Y.K. Giant hemangioma causing budd-Chiari syndrome. J. Clin. Exp. Hepatol. 2014, 4, 380–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakanuma, Y. Non-neoplastic nodular lesions in the liver. Pathol. Int. 1995, 45, 703–714. [Google Scholar] [CrossRef]
- Bajenaru, N.; Balaban, V.; Săvulescu, F.; Campeanu, I.; Patrascu, T. Hepatic hemangioma—Review. J. Med. Life 2015, 8, 4–11. [Google Scholar]
- Oak, C.Y.; Jun, C.H.; Cho, E.A.; Lee, D.H.; Cho, S.B.; Park, C.H.; Joo, Y.E.; Kim, H.S.; Rew, J.S.; Choi, S.K. Hepatic Hemangioma with Kasabach-Merritt Syndrome in an Adult Patient. Korean J. Gastroenterol. 2016, 67, 220–223. [Google Scholar] [CrossRef][Green Version]
- Aslan, A.; Meyer Zu Vilsendorf, A.; Kleine, M.; Bredt, M.; Bektas, H. Adult Kasabach-Merritt Syndrome due to Hepatic Giant Hemangioma. Case Rep. Gastroenterol. 2009, 3, 306–312. [Google Scholar] [CrossRef]
- Hall, G.W. Kasabach-Merritt syndrome: Pathogenesis and management. Br. J. Haematol. 2001, 112, 851–862. [Google Scholar] [CrossRef]
- Toro, A.; Mahfouz, A.-E.; Ardiri, A.; Malaguarnera, M.; Malaguarnera, G.; Loria, F.; Bertino, G.; Di Carlo, I. What is changing in indications and treatment of hepatic hemangiomas. Ann. Rev. Ann. Hepatol. 2014, 13, 327–339. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, Q.; Chen, F.; You, Q.; Jiang, T. Atypical appearance of hepatic hemangiomas with contrast-enhanced ultrasound. Oncotarget 2018, 9, 12662–12670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moody, A.R.; Wilson, S.R. Atypical hepatic hemangioma: A suggestive sonographic morphology. Radiology 1993, 188, 413–417. [Google Scholar] [CrossRef]
- Bree, R.L.; Schwab, R.E.; Glazer, G.M.; Fink-Bennett, D. The varied appearances of hepatic cavernous hemangiomas with sonography, computed tomography, magnetic resonance imaging and scintigraphy. Radiographics 1987, 7, 1153–1175. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, T.K.; Han, J.K.; Kim, A.Y.; Lee, H.J.; Park, S.H.; Kim, Y.H.; Choi, B.I. Hepatic hemangiomas: Spectrum of US appearances on gray-scale, power Doppler, and contrast-enhanced US. Korean J. Radiol. 2000, 1, 191–197. [Google Scholar] [CrossRef][Green Version]
- Kim, T.K.; Han, J.K.; Kim, A.Y.; Park, S.J.; Choi, B.I. Signal from hepatic hemangiomas on power Doppler US: Real or artefactual? Ultrasound Med. Biol. 1999, 25, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, J.; Kakhki, V.R.D. Accuracy of Gray-Scale and Color Doppler Sonography in Diagnosis of Hepatic Hemangioma, Hepatocellular Carcinoma and Liver Metastasis. Iran. J. Radiol. 2008, 5, 129–134. [Google Scholar]
- Bartolotta, T.V.; Taibbi, A.; Galia, M.; Lo Re, G.; La Grutta, L.; Grassi, R.; Midiri, M. Centrifugal (inside-out) enhancement of liver hemangiomas: A possible atypical appearance on contrast-enhanced US. Eur. J. Radiol. 2007, 64, 447–455. [Google Scholar] [CrossRef]
- Kim, S.; Chung, J.J.; Kim, M.J.; Park, S.; Lee, J.T.; Yoo, H.S. Atypical inside-out pattern of hepatic hemangiomas. Am. J. Roentgenol. 2000, 174, 1571–1574. [Google Scholar] [CrossRef]
- Westwood, M.; Joore, M.; Grutters, J.; Redekop, K.; Armstrong, N.; Lee, K.; Gloy, V.; Raatz, H.; Misso, K.; Severens, J.; et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2013, 17, 1–243. [Google Scholar] [CrossRef]
- Srinivasan, I.; Tang, S.-J.; Vilmann, A.S.; Menachery, J.; Vilmann, P. Hepatic applications of endoscopic ultrasound: Current status and future directions. World J. Gastroenterol. 2015, 21, 12544–12557. [Google Scholar] [CrossRef]
- Alvarez-Sánchez, M.V.; Jenssen, C.; Faiss, S.; Napoléon, B. Interventional endoscopic ultrasonography: An overview of safety and complications. Surg. Endosc. 2014, 28, 712–734. [Google Scholar] [CrossRef]
- ASGE Technology Committee; Kaul, V.; Adler, D.G.; Conway, J.D.; Farraye, F.A.; Kantsevoy, S.V.; Kethu, S.R.; Kwon, R.S.; Mamula, P.; Pedrosa, M.C.; et al. Interventional EUS. Gastrointest. Endosc. 2010, 72, 1–4. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, M.Y.; Jeong, S.W.; Kim, T.Y.; Kim, S.U.; Lee, S.H.; Suk, K.T.; Park, S.Y.; Woo, H.Y.; Kim, S.G.; et al. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin. Mol. Hepatol. 2013, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McFarland, E.G.; Mayo-Smith, W.W.; Saini, S.; Hahn, P.F.; Goldberg, M.A.; Lee, M.J. Hepatic hemangiomas and malignant tumors: Improved differentiation with heavily T2-weighted conventional spin-echo MR imaging. Radiology 1994, 193, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Lee, S.F.; Yu, S.C.H.; Lai, P.; Ching, A.S.C. Hepatic malignant tumour versus cavernous haemangioma: Differentiation on multiple breath-hold turbo spin-echo MRI sequences with different T2-weighting and T2-relaxation time measurements on a single slice multi-echo sequence. Clin. Radiol. 2002, 57, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Tateyama, A.; Fukukura, Y.; Takumi, K.; Shindo, T.; Kumagae, Y.; Kamimura, K.; Nakajo, M. Gd-EOB-DTPA-enhanced magnetic resonance imaging features of hepatic hemangioma compared with enhanced computed tomography. World J. Gastroenterol. 2012, 18, 6269–6276. [Google Scholar] [CrossRef] [PubMed]
- Ziessman, H.A.; Silverman, P.M.; Patterson, J.; Harkness, B.; Fahey, F.H.; Zeman, R.K.; Keyes, J.W. Improved detection of small cavernous hemangiomas of the liver with high-resolution three-headed SPECT. J. Nucl. Med. 1991, 32, 2086–2091. [Google Scholar] [PubMed]
- Birnbaum, B.A.; Weinreb, J.C.; Megibow, A.J.; Sanger, J.J.; Lubat, E.; Kanamuller, H.; Noz, M.E.; Bosniak, M.A. Definitive diagnosis of hepatic hemangiomas: MR imaging versus Tc-99m-labeled red blood cell SPECT. Radiology 1990, 176, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Danieli, R.; Manni, C.; Capoccetti, F.; Simonetti, G. Technetium-99m-labelled red blood cell imaging in the diagnosis of hepatic haemangiomas: The role of SPECT/CT with a hybrid camera. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1011–1015. [Google Scholar] [CrossRef]
- Klotz, T.; Montoriol, P.F.; Da Ines, D.; Petitcolin, V.; Joubert-Zakeyh, J.; Garcier, J.M. Hepatic haemangioma: Common and uncommon imaging features. Diagn. Interv. Imaging 2013, 94, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Mamone, G.; Di Piazza, A.; Carollo, V.; Cannataci, C.; Cortis, K.; Bartolotta, T.V.; Miraglia, R. Imaging of hepatic hemangioma: From A to Z. Abdom. Radiol. 2020, 45, 672–691. [Google Scholar] [CrossRef] [PubMed]
- Caseiro-Alves, F.; Brito, J.; Araujo, A.E.; Belo-Soares, P.; Rodrigues, H.; Cipriano, A.; Sousa, D.; Mathieu, D. Liver haemangioma: Common and uncommon findings and how to improve the differential diagnosis. Eur. Radiol. 2007, 17, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Mamone, G.; Miraglia, R. The “light bulb sign” in liver hemangioma. Abdom. Radiol. 2019, 44, 2327–2328. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Boulos, L.; Vullierme, M.P.; Denys, A.; Terris, B.; Menu, Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics 2000, 20, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Valls, C.; Reñe, M.; Gil, M.; Sanchez, A.; Narvaez, J.A.; Hidalgo, F. Giant cavernous hemangioma of the liver: Atypical CT and MR findings. Eur. Radiol. 1996, 6, 448–450. [Google Scholar] [CrossRef]
- Choi, B.I.; Han, M.C.; Park, J.H.; Kim, S.H.; Han, M.H.; Kim, C.W. Giant cavernous hemangioma of the liver: CT and MR imaging in 10 cases. Am. J. Roentgenol. 1989, 152, 1221–1226. [Google Scholar] [CrossRef]
- Danet, I.M.; Semelka, R.C.; Braga, L.; Armao, D.; Woosley, J.T. Giant hemangioma of the liver: MR imaging characteristics in 24 patients. Magn. Reson. Imaging 2003, 21, 95–101. [Google Scholar] [CrossRef]
- Yu, J.S.; Kim, M.J.; Kim, K.W.; Chang, J.C.; Jo, B.J.; Kim, T.H.; Lee, J.T.; Yoo, H.S. Hepatic cavernous hemangioma: Sonographic patterns and speed of contrast enhancement on multiphase dynamic MR imaging. Am. J. Roentgenol. 1998, 171, 1021–1025. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, T.K.; Han, J.K.; Kim, A.Y.; Lee, H.J.; Choi, B.I. Hepatic hemangiomas with arterioportal shunt: Findings at two-phase CT. Radiology 2001, 219, 707–711. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, A.Y.; Kim, T.K.; Kim, S.Y.; Kim, M.-J.; Park, M.-S.; Park, S.H.; Lee, K.H.; Kim, J.K.; Kim, P.-N.; et al. Hepatic hemangiomas with arterioportal shunt: Sonographic appearances with CT and MRI correlation. Am. J. Roentgenol. 2006, 187, W406–W414. [Google Scholar] [CrossRef]
- Shimada, M.; Matsumata, T.; Ikeda, Y.; Urata, K.; Hayashi, H.; Shimizu, M.; Sugimachi, K. Multiple hepatic hemangiomas with significant arterioportal venous shunting. Cancer 1994, 73, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Winograd, J.; Palubinskas, A.J. Arterial-portal venous shunting in cavernous hemangioma of the liver. Radiology 1977, 122, 331–332. [Google Scholar] [CrossRef]
- Sousa, M.S.C.; Ramalho, M.; Herédia, V.; Matos, A.P.; Palas, J.; Jeon, Y.H.; Afonso, D.; Semelka, R.C. Perilesional enhancement of liver cavernous hemangiomas in magnetic resonance imaging. Abdom. Imaging 2014, 39, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Infante, A.; Rinninella, E.; Spinelli, I.; Mazziotti, M.A.; De Gaetano, A.M.; Pompili, M.; Bonomo, L. A peculiar case of diffuse hemangiomatosis of the left hepatic lobe in an asymptomatic adult patient: Case report and literature review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1593–1597. [Google Scholar] [PubMed]
- Liu, M.C.; Little, E.C. Isolated hepatic hemangiomatosis in 2 septuagenarians. Radiol. Case Rep. 2018, 13, 1097–1103. [Google Scholar] [CrossRef]
- Blondet, A.; Ridereau-Zins, C.; Michalak, S.; Pessaux, P.; Aubertin, A.; Aubé, C. Multiple pedunculated liver hemangiomas presenting with volvulus. J. Radiol. 2007, 88, 891–894. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kim, T.K.; Lim, H.K.; Park, S.J.; Sim, J.S.; Kim, H.Y.; Lee, J.-H. Hepatic hemangioma: Atypical appearances on CT, MR imaging, and sonography. Am. J. Roentgenol. 2003, 180, 135–141. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, M.J.; Lee, S.S.; Kim, H.J.; Shin, Y.M.; Kim, P.-N.; Lee, M.-G. Sparing of fatty infiltration around focal hepatic lesions in patients with hepatic steatosis: Sonographic appearance with CT and MRI correlation. Am. J. Roentgenol. 2008, 190, 1018–1027. [Google Scholar] [CrossRef]
- Marsh, J.I.; Gibney, R.G.; Li, D.K. Hepatic hemangioma in the presence of fatty infiltration: An atypical sonographic appearance. Gastrointest. Radiol. 1989, 14, 262–264. [Google Scholar] [CrossRef]
- Brancatelli, G.; Federle, M.P.; Blachar, A.; Grazioli, L. Hemangioma in the cirrhotic liver: Diagnosis and natural history. Radiology 2001, 219, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, M.; Kanematsu, M.; Leonardou, P.; Braga, L.; Woosley, J.T.; Semelka, R.C. Cavernous hemangiomas in patients with chronic liver disease: MR imaging findings. Magn. Reson. Imaging 2004, 22, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Vernuccio, F.; Ronot, M.; Dioguardi Burgio, M.; Lebigot, J.; Allaham, W.; Aubé, C.; Brancatelli, G.; Vilgrain, V. Uncommon evolutions and complications of common benign liver lesions. Abdom. Radiol. 2018, 43, 2075–2096. [Google Scholar] [CrossRef] [PubMed]
- Davies, R. Haemorrhage after fine-needle aspiration biopsy of an hepatic haemangioma. Med. J. Aust. 1993, 158, 364. [Google Scholar] [CrossRef] [PubMed]
- Taavitsainen, M.; Airaksinen, T.; Kreula, J.; Päivänsalo, M. Fine-needle aspiration biopsy of liver hemangioma. Acta Radiol. 1990, 31, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Glinkova, V.; Shevah, O.; Boaz, M.; Levine, A.; Shirin, H. Hepatic haemangiomas: Possible association with female sex hormones. Gut 2004, 53, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Mungovan, J.A.; Cronan, J.J.; Vacarro, J. Hepatic cavernous hemangiomas: Lack of enlargement over time. Radiology 1994, 191, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, L.T.; Bieze, M.; Erdogan, D.; Roelofs, J.J.T.H.; Beuers, U.H.W.; van Gulik, T.M. Management of giant liver hemangiomas: An update. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 263–268. [Google Scholar] [CrossRef]
- Abdel Wahab, M.; El Nakeeb, A.; Ali, M.A.; Mahdy, Y.; Shehta, A.; Abdulrazek, M.; El Desoky, M.; Abdel Wahab, R. Surgical Management of Giant Hepatic Hemangioma: Single Center’s Experience with 144 Patients. J. Gastrointest. Surg. 2018, 22, 849–858. [Google Scholar] [CrossRef]
- Xie, Q.-S.; Chen, Z.-X.; Zhao, Y.-J.; Gu, H.; Geng, X.-P.; Liu, F.-B. Outcomes of surgery for giant hepatic hemangioma. BMC Surg. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Qiu, B.; Xu, H.; He, L. Invasive management of symptomatic hepatic hemangioma. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1079–1084. [Google Scholar] [CrossRef]
- Fu, X.-H.; Lai, E.C.H.; Yao, X.-P.; Chu, K.-J.; Cheng, S.-Q.; Shen, F.; Wu, M.-C.; Lau, W.Y. Enucleation of liver hemangiomas: Is there a difference in surgical outcomes for centrally or peripherally located lesions? Am. J. Surg. 2009, 198, 184–187. [Google Scholar] [CrossRef]
- Torkian, P.; Li, J.; Kaufman, J.A.; Jahangiri, Y. Effectiveness of Transarterial Embolization in Treatment of Symptomatic Hepatic Hemangiomas: Systematic Review and Meta-analysis. Cardiovasc. Intervent. Radiol. 2021, 44, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, I.; Toro, A. Limiting the surgical indications for liver hemangiomas may help surgeons and patients. J. Am. Coll. Surg. 2011, 212, 1098–1099. [Google Scholar] [CrossRef]
- Pietrabissa, A.; Giulianotti, P.; Campatelli, A.; Di Candio, G.; Farina, F.; Signori, S.; Mosca, F. Management and follow-up of 78 giant haemangiomas of the liver. Br. J. Surg. 1996, 83, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Tak, W.Y.; Jung, M.K.; Jeon, S.W.; Cho, C.M.; Kweon, Y.O.; Kim, K.C. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J. Hepatol. 2011, 54, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.E.; Dodd, G.D. Percutaneous radiofrequency ablation of symptomatic giant hepatic cavernous hemangiomas: Report of two cases and review of literature. J. Vasc. Interv. Radiol. 2012, 23, 971–975. [Google Scholar] [CrossRef]
- Kong, J.; Gao, R.; Wu, S.; Shi, Y.; Yin, T.; Guo, S.; Xin, Z.; Li, A.; Kong, X.; Ma, D.; et al. Safety and efficacy of microwave versus radiofrequency ablation for large hepatic hemangioma: A multicenter retrospective study with propensity score matching. Eur. Radiol. 2022, 32, 3309–3318. [Google Scholar] [CrossRef]
- Gao, J.; Ke, S.; Ding, X.; Zhou, Y.; Qian, X.; Sun, W. Radiofrequency ablation for large hepatic hemangiomas: Initial experience and lessons. Surgery 2013, 153, 78–85. [Google Scholar] [CrossRef]
- Wu, S.; Gao, R.; Yin, T.; Zhu, R.; Guo, S.; Xin, Z.; Li, A.; Kong, X.; Gao, J.; Sun, W. Complications of radiofrequency ablation for hepatic hemangioma: A multicenter retrospective analysis on 291 cases. Front. Oncol. 2021, 11, 706619. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, M.; Yang, X.; Xu, L.; Ke, S.; Ding, X.; Sun, W.; Gao, J. Endothelial pyroptosis underlies systemic inflammatory response following radiofrequency ablation of hepatic hemangiomas. Scand. J. Clin. Lab. Investig. 2019, 79, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Furumaya, A.; van Rosmalen, B.V.; Takkenberg, R.B.; van Delden, O.M.; Dejong, C.H.C.; Verheij, J.; van Gulik, T.M. Transarterial (Chemo-)Embolization and Lipiodolization for Hepatic Haemangioma. Cardiovasc. Intervent. Radiol. 2019, 42, 800–811. [Google Scholar] [CrossRef]
- Özgür, Ö.; Sindel, H.T. Giant hepatic hemangioma treatment with transcatheter arterial embolisation and transcatheter arterial chemoembolisation; Comparative results. Turk. J. Med. Sci. 2021, 51, 2943–2950. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Tan, H.; Huang, J.; Xu, L.; Liu, L.; Si, S.; Sun, Y. Long-term result of transcatheter arterial embolization for liver hemangioma. Medicine 2017, 96, e9029. [Google Scholar] [CrossRef]
- Özden, İ.; Poyanlı, A.; Önal, Y.; Demir, A.A.; Hoş, G.; Acunaş, B. Superselective transarterial chemoembolization as an alternative to surgery in symptomatic/enlarging liver hemangiomas. World J. Surg. 2017, 41, 2796–2803. [Google Scholar] [CrossRef]
- Della Corte, A.; Marino, R.; Ratti, F.; Palumbo, D.; Guazzarotti, G.; Gusmini, S.; Augello, L.; Cipriani, F.; Fiorentini, G.; Venturini, M.; et al. The Two-Step Treatment for Giant Hepatic Hemangiomas. J. Clin. Med. 2021, 10, 4381. [Google Scholar] [CrossRef]
- Kacała, A.; Dorochowicz, M.; Korbecki, A.; Sobański, M.; Puła, M.; Patrzałek, D.; Janczak, D.; Guziński, M. Transarterial Bleomycin-Lipiodol Chemoembolization for the Treatment of Giant Hepatic Hemangiomas: An Assessment of Effectiveness. Cancers 2024, 16, 380. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.-L.; Duan, F.; Wang, M.-Q. Medium and Long-Term Outcome of Superselective Transcatheter Arterial Embolization with Lipiodol-Bleomycin Emulsion for Giant Hepatic Hemangiomas: Results in 241 Patients. J. Clin. Med. 2022, 11, 4762. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Ouyang, X.H.; Gu, S.B. DSA examination and diagnosis of adult hepatic cavernous hemangioma with arteriovenous short circuit. Zhonghua Fang She Xian Yi Xue Za Zhi 2000, 34, 523–527. [Google Scholar]
- Qingle, Z.; Yong, C.; Jianbo, Z.; Kewei, Z.; Yanhao, L.I. Intra-arterial embolization with pingyangmycin-lipiodol emulsion for the treatment of hepatic cavernous hemangioma: An analysis of factors affecting therapeutic results. J. Interv. Radiol. 2009, 12, 656–660. [Google Scholar]
- Sato, M.; Tateishi, R.; Yasunaga, H.; Horiguchi, H.; Yoshida, H.; Matsuda, S.; Koike, K. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: A national survey of 54,145 patients. J. Gastroenterol. 2012, 47, 1125–1133. [Google Scholar] [CrossRef]
- Kacała, A.; Dorochowicz, M.; Patrzałek, D.; Janczak, D.; Guziński, M. Safety and Feasibility of Transarterial Bleomycin-Lipiodol Embolization in Patients with Giant Hepatic Hemangiomas. Medicina 2023, 59, 1358. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Carrafiello, G.; Ierardi, A.M.; Tsetis, D.; Brountzos, E. Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc. Intervent. Radiol. 2012, 35, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Longeville, J.H.; de la Hall, P.; Dolan, P.; Holt, A.W.; Lillie, P.E.; Williams, J.A.; Padbury, R.T. Treatment of a giant haemangioma of the liver with Kasabach-Merritt syndrome by orthotopic liver transplant a case report. HPB Surg. 1997, 10, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Francis, F.; Aquino, A.; Xu, R.; Morgan, G.; Teperman, L. Liver transplant for mixed capillary-cavernous hemangioma masquerading as hepatocellular carcinoma in a patient with hepatocellular carcinoma. Exp. Clin. Transplant. 2011, 9, 344–348. [Google Scholar] [PubMed]
- Yamashita, Y.; Ogata, I.; Urata, J.; Takahashi, M. Cavernous hemangioma of the liver: Pathologic correlation with dynamic CT findings. Radiology 1997, 203, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, M.S.; Garden, O.J. Giant haemangioma of the liver: Observation or resection? Dig. Surg. 2010, 27, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Thung, S.N.; Tsui, W.M.S.; Ferrell, L.D. Hepatic cavernous hemangioma: Underrecognized associated histologic features. Liver Int. 2006, 26, 334–338. [Google Scholar] [CrossRef]
- Ribeiro, M.A.; Papaiordanou, F.; Gonçalves, J.M.; Chaib, E. Spontaneous rupture of hepatic hemangiomas: A review of the literature. World J. Hepatol. 2010, 2, 428–433. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawarada, Y.; Yano, T.; Noguchi, T.; Mizumoto, R. Spontaneous rupture of hemangioma of the liver: Treatment with transcatheter hepatic arterial embolization. Am. J. Gastroenterol. 1991, 86, 1645–1649. [Google Scholar]
- Chen, Z.-Y.; Qi, Q.-H.; Dong, Z.-L. Etiology and management of hemmorrhage in spontaneous liver rupture: A report of 70 cases. World J. Gastroenterol. 2002, 8, 1063–1066. [Google Scholar] [CrossRef]
- Jain, V.; Ramachandran, V.; Garg, R.; Pal, S.; Gamanagatti, S.R.; Srivastava, D.N. Spontaneous rupture of a giant hepatic hemangioma—Sequential management with transcatheter arterial embolization and resection. Saudi J. Gastroenterol. 2010, 16, 116–119. [Google Scholar] [CrossRef]
- Moinuddin, M.; Allison, J.R.; Montgomery, J.H.; Rockett, J.F.; McMurray, J.M. Scintigraphic diagnosis of hepatic hemangioma: Its role in the management of hepatic mass lesions. Am. J. Roentgenol. 1985, 145, 223–228. [Google Scholar] [CrossRef]
- Seitz, K.; Bernatik, T.; Strobel, D.; Blank, W.; Friedrich-Rust, M.; Strunk, H.; Greis, C.; Kratzer, W.; Schuler, A. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM Multicenter Trial): CEUS vs. MRI—A prospective comparison in 269 patients. Ultraschall Med. 2010, 31, 492–499. [Google Scholar] [CrossRef]
- Ginting, K.; Tailor, A.; Braverman, T.; Agarwal, A.; Allamaneni, S. Imaging Characteristics and Management of Infected Hepatic Hemangioma: Case-in Discussion. J. Gastrointest. Abdom. Radiol. 2021, 4, 236–239. [Google Scholar] [CrossRef]
- Bailey, J.; Di Carlo, S.; Blackwell, J.; Gomez, D. Same day arterial embolisation followed by hepatic resection for treatment of giant haemangioma. BMJ Case Rep. 2016, 2016, bcr2015213259. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, B.-H.; Qiu, S.-J.; Ren, Z.-G.; Zhou, J.; Chen, X.-H.; Zhou, Y.; Fan, J. Combined hepatocellular carcinoma and cholangiocarcinoma: Clinical features, treatment modalities, and prognosis. Ann. Surg. Oncol. 2012, 19, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, L.; Li, M.; Cheng, Q.; Zhang, L.; Zheng, S. Atypical hemangioma mimicking mixed hepatocellular cholangiocarcinoma: Case report. Medicine 2017, 96, e9192. [Google Scholar] [CrossRef] [PubMed]
- Yedibela, S.; Alibek, S.; Müller, V.; Aydin, U.; Langheinrich, M.; Lohmüller, C.; Hohenberger, W.; Perrakis, A. Management of hemangioma of the liver: Surgical therapy or observation? World J. Surg. 2013, 37, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Schnelldorfer, T.; Ware, A.L.; Smoot, R.; Schleck, C.D.; Harmsen, W.S.; Nagorney, D.M. Management of giant hemangioma of the liver: Resection versus observation. J. Am. Coll. Surg. 2010, 211, 724–730. [Google Scholar] [CrossRef]
- Yamagata, M.; Kanematsu, T.; Matsumata, T.; Utsunomiya, T.; Ikeda, Y.; Sugimachi, K. Management of haemangioma of the liver: Comparison of results between surgery and observation. Br. J. Surg. 1991, 78, 1223–1225. [Google Scholar] [CrossRef]
- Ho, H.-Y.; Wu, T.-H.; Yu, M.-C.; Lee, W.-C.; Chao, T.-C.; Chen, M.-F. Surgical management of giant hepatic hemangiomas: Complications and review of the literature. Chang Gung Med. J. 2012, 35, 70–78. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, X.; Zhou, J. Ultrasound-guided percutaneous sclerotherapy versus surgical resection in the treatment of large hepatic hemangiomas: A retrospective study. BMC Surg. 2022, 22, 130. [Google Scholar] [CrossRef]
- Ayoobi Yazdi, N.; Mehrabinejad, M.-M.; Dashti, H.; Pourghorban, R.; Nassiri Toosi, M.; Rokni Yazdi, H. Percutaneous Sclerotherapy with Bleomycin and Ethiodized Oil: A Promising Treatment in Symptomatic Giant Liver Hemangioma. Radiology 2021, 301, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Bozkaya, H.; Cinar, C.; Besir, F.H.; Parıldar, M.; Oran, I. Minimally invasive treatment of giant haemangiomas of the liver: Embolisation with bleomycin. Cardiovasc. Intervent. Radiol. 2014, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Hirotani, K.; Ogasawara, H.; Katayama, T.; Ashino-Fuse, H.; Shimamura, M.; Iwaguchi, T.; Nakamura, O. Inhibition of angiogenesis by bleomycin and its copper complex. Chem. Pharm. Bull. 1990, 38, 1790–1792. [Google Scholar] [CrossRef] [PubMed]

| Diagnostic Method | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Ultrasonography | 96.9 | 60.3 |
| Computed tomography | 98.3 | 55 |
| Magnetic resonance imaging | 100 | 85.7 |
| Tc-99m RBC blood pool scintigraphy | 67 | 100 |
| Type of Blood Supply | Artery Characteristics | Arterial Phase | Parenchymal Phase |
|---|---|---|---|
| Rich | Mild to moderate thickening of the arteries | Abnormal blood sinusoids | Dilatation of most blood sinusoids |
| Moderate | Mild thickening of the arteries | Abnormal blood sinusoids | Dilatation of some blood sinusoids |
| Poor | No thickening of the arteries | Very few abnormal blood sinusoids | No visible dilatation of blood sinusoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacała, A.; Dorochowicz, M.; Matus, I.; Puła, M.; Korbecki, A.; Sobański, M.; Jacków-Nowicka, J.; Patrzałek, D.; Janczak, D.; Guziński, M. Hepatic Hemangioma: Review of Imaging and Therapeutic Strategies. Medicina 2024, 60, 449. https://doi.org/10.3390/medicina60030449
Kacała A, Dorochowicz M, Matus I, Puła M, Korbecki A, Sobański M, Jacków-Nowicka J, Patrzałek D, Janczak D, Guziński M. Hepatic Hemangioma: Review of Imaging and Therapeutic Strategies. Medicina. 2024; 60(3):449. https://doi.org/10.3390/medicina60030449
Chicago/Turabian StyleKacała, Arkadiusz, Mateusz Dorochowicz, Iwona Matus, Michał Puła, Adrian Korbecki, Michał Sobański, Jagoda Jacków-Nowicka, Dariusz Patrzałek, Dariusz Janczak, and Maciej Guziński. 2024. "Hepatic Hemangioma: Review of Imaging and Therapeutic Strategies" Medicina 60, no. 3: 449. https://doi.org/10.3390/medicina60030449
APA StyleKacała, A., Dorochowicz, M., Matus, I., Puła, M., Korbecki, A., Sobański, M., Jacków-Nowicka, J., Patrzałek, D., Janczak, D., & Guziński, M. (2024). Hepatic Hemangioma: Review of Imaging and Therapeutic Strategies. Medicina, 60(3), 449. https://doi.org/10.3390/medicina60030449







