Metaphedrone (3-Methylmethcathinone): Pharmacological, Clinical, and Toxicological Profile
Abstract
:1. Introduction
2. Methodology
3. Key Results and Discussion
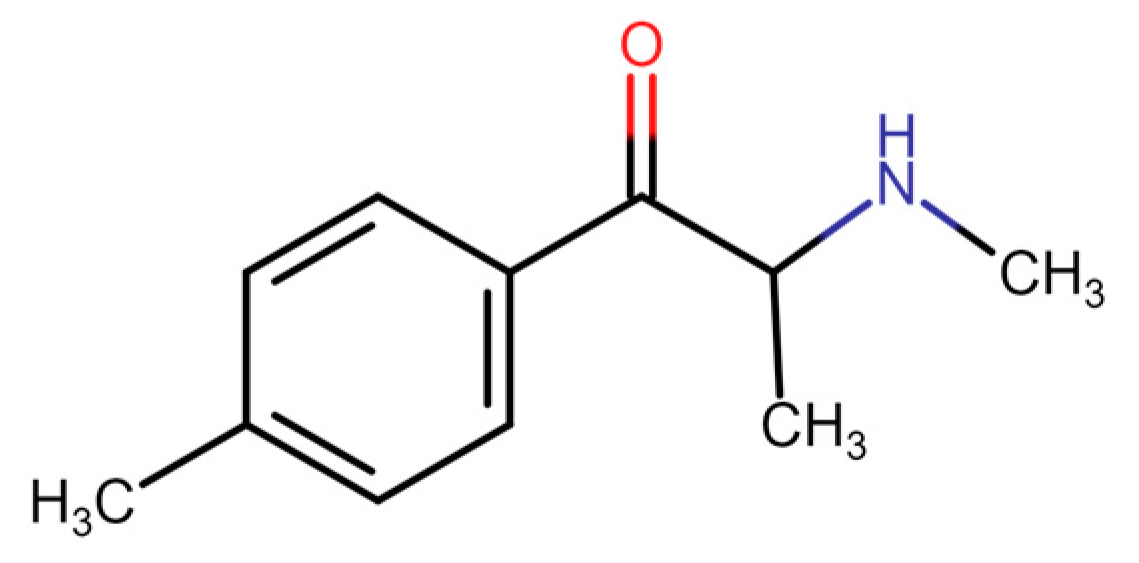
3.1. Metaphedrone (3-Methylmethcathinone, 3-MMC)
3.2. Pharmacological Profile
3.3. Clinical Profile
3.4. Toxicological Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, B.; Dias da Silva, D.; Carvalho, F.; de Lourdes Bastos, M.; Carmo, H. The novel psychoactive substance 3-methylmethcathinone (3-MMC or metaphedrone): A Review. Forensic Sci. Int. 2019, 295, 54–63. [Google Scholar] [CrossRef]
- Zuba, D.; Adamowicz, P. Distinction of constitutional isomers of mephedrone by chromatographic and spectrometric methods. Aust. J. Forensic Sci. 2016, 49, 637–649. [Google Scholar] [CrossRef]
- Nugteren-van Lonkhuyzen, J.J.; Essink, S.; Rietjens, S.J.; Ohana, D.; de Lange, D.W.; van Riel, A.J.H.P.; Hondebrink, L. 3-Methylmethcathinone (3-MMC) poisonings: Acute clinical toxicity and time trend between 2013 and 2021 in the Netherlands. Ann. Emerg. Med. 2022, 80, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Al-Maweri, S.A.; Warnakulasuriya, S.; Samran, A. Khat (Catha edulis) and its oral health effects: An updated review. J. Investig. Clin. Dent. 2017, 9, e12288. [Google Scholar] [CrossRef]
- Szendrei, K. The chemistry of khat. Bull. Narc. 1980, 32, 5–35. [Google Scholar] [PubMed]
- Coppola, M.; Mondola, R. Synthetic cathinones: Chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”. Toxicol. Lett. 2012, 211, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.F.; Browning, E.; Adams, R. Synthetic homologs of d,l-ephedrine. J. Am. Chem. Soc. 1928, 50, 2287–2292. [Google Scholar] [CrossRef]
- Sanchez, S. Sur un homologue de l’éphédrine. Bull. Soc. Chim. Fr. 1929, 45, 284–286. [Google Scholar]
- Zawilska, J.B. Mephedrone and other cathinones. Curr. Opin. Psychiatry 2014, 27, 256–262. [Google Scholar] [CrossRef]
- Bank RPD. RCSB PDB: Chemical Sketch Tool [Internet]. Available online: https://www.rcsb.org/chemical-sketch (accessed on 28 February 2024).
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of synthetic cathinones. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 113–142. [Google Scholar]
- WHO. Critical Review Report: 3-Methylmethcathinone (3-MMC) for 45th ECDD Meeting. [Internet]; World Health Organization: Geneva, Switzerland, 2022. Available online: https://researchonline.ljmu.ac.uk/id/eprint/18334/1/3-mmc_draft%20%28accepted_uncorrected%29.pdf (accessed on 14 August 2023).
- Shimshoni, J.A.; Britzi, M.; Sobol, E.; Willenz, U.; Nutt, D.; Edery, N. 3-Methyl-methcathinone: Pharmacokinetic profile evaluation in pigs in relation to Pharmacodynamics. J. Psychopharmacol. 2015, 29, 734–743. [Google Scholar] [CrossRef]
- Dargan, P.I.; Sedefov, R.; Gallegos, A.; Wood, D.M. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test. Anal. 2011, 3, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Ricciardi, A.; Corazza, O.; Deluca, P.; Davey, Z.; Rafanelli, C.; Gruppo di Ricerca “Psychonaut Web Mapping”. New drugs of abuse on the Web: The role of the Psychonaut Web Mapping Project. Riv. Psichiatr. 2010, 45, 88–93. [Google Scholar] [PubMed]
- Sande, M. Characteristics of the use of 3-MMC and other new psychoactive drugs in Slovenia, and the perceived problems experienced by users. Int. J. Drug Policy 2016, 27, 65–73. [Google Scholar] [CrossRef]
- Adamowicz, P.; Zuba, D.; Byrska, B. Fatal intoxication with 3-methyl-N-methylcathinone (3-MMC) and 5-(2-aminopropyl)benzofuran (5-APB). Forensic Sci. Int. 2014, 245, 126–132. [Google Scholar] [CrossRef]
- Spiller, H.A.; Ryan, M.L.; Weston, R.G.; Jansen, J. Clinical experience with and analytical confirmation of “Bath salts” and “Legal highs” (synthetic cathinones) in the United States. Clin. Toxicol. 2011, 49, 499–505. [Google Scholar] [CrossRef]
- The Council of the European Union. Council Decision of 2 December 2010 on Submitting 4-Methylmethcathinone (Mephedrone) to Control Measures; OJEU: Luxembourg, 2010; Available online: https://www.emcdda.europa.eu/drugs-library/2010759eu-council-decision-2-december-2010-submitting-4-methylmethcathinone-mephedrone-control-measures_en (accessed on 14 August 2023).
- Aknouche, F.; Ameline, A.; Gheddar, L.; Maruejouls, C.; Kintz, P. Fatal rectal injection of 3-MMC in a sexual context: Toxicological investigations including metabolites identification using LC–HRMS. J. Anal. Toxicol. 2022, 46, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Ameline, A.; Dumestre-Toulet, V.; Raul, J.-S.; Kintz, P. Determination of a threshold fatal 3-MMC concentration in human: Mission impossible. Psychopharmacology 2018, 236, 865–867. [Google Scholar] [CrossRef]
- Luethi, D.; Kolaczynska, K.E.; Docci, L.; Krähenbühl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 2018, 134, 4–12. [Google Scholar] [CrossRef]
- Bäckberg, M.; Lindeman, E.; Beck, O.; Helander, A. Characteristics of analytically confirmed 3-MMC-related intoxications from the Swedish strida project. Clin. Toxicol. 2014, 53, 46–53. [Google Scholar] [CrossRef]
- Adamowicz, P.; Gieroń, J.; Gil, D.; Lechowicz, W.; Skulska, A.; Tokarczyk, B. 3-methylmethcathinone—Interpretation of blood concentrations based on analysis of 95 cases. J. Anal. Toxicol. 2016, 40, 272–276. [Google Scholar] [CrossRef]
- Adamowicz, P.; Gieroń, J.; Gil, D.; Lechowicz, W.; Skulska, A.; Tokarczyk, B. The prevalence of new psychoactive substances in biological material—A three-year review of casework in Poland. Drug Test. Anal. 2015, 8, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Drevin, G.; Rossi, L.H.; Férec, S.; Briet, M.; Abbara, C. Chemsex/slamsex-related intoxications: A case report involving gamma-hydroxybutyrate (GHB) and 3-methylmethcathinone (3-MMC) and a review of the literature. Forensic Sci. Int. 2021, 321, 110743. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic: An Update from the EU Early Warning System. Publications Office of the European Union; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2020. [Google Scholar]
- Güner, R.; Hasanoğlu, İ.; Aktaş, F. COVID-19: Prevention and control measures in community. Turk. J. Med. Sci. 2020, 50, 571–577. [Google Scholar] [CrossRef]
- Vincenti, F.; Gregori, A.; Flammini, M.; Di Rosa, F.; Salomone, A. Seizures of New Psychoactive Substances on the Italian territory during the COVID-19 pandemic. Forensic Sci. Int. 2021, 326, 110904. [Google Scholar] [CrossRef] [PubMed]
- Jamey, C.; Kintz, P.; Martrille, L.; Raul, J.S. Fatal Combination with 3-Methylmethcathinone (3-MMC) and Gamma-Hydroxybutyric Acid (GHB). J. Anal. Toxicol. 2016, 40, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Institóris, L.; Árok, Z.; Seprenyi, K.; Varga, T.; Sára-Klausz, G.; Keller, E.; Tóth, R.A.; Sala, L.; Kereszty, É.; Róna, K. Frequency and structure of stimulant designer drug consumption among suspected drug users in Budapest and South-East Hungary in 2012–2013. Forensic Sci. Int. 2015, 248, 181–186. [Google Scholar] [CrossRef]
- Giorgetti, R.; Tagliabracci, A.; Schifano, F.; Zaami, S.; Marinelli, E.; Busardò, F.P. When “Chems” Meet Sex: A rising phenomenon called “ChemSex”. Curr. Neuropharmacol. 2017, 15, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Ding, Z.; Wu, X.; Wang, G.; Shi, J. Effects of 3-methylmethcathinone on conditioned place preference and anxiety-like behavior: Comparison with methamphetamine. Front. Mol. Neurosci. 2022, 15, 975820. [Google Scholar] [CrossRef]
- Romanek, K.; Stenzel, J.; Schmoll, S.; Schrettl, V.; Geith, S.; Eyer, F.; Rabe, C. Synthetic cathinones in Southern Germany—Characteristics of users, substance-patterns, co-ingestions, and complications. Clin. Toxicol. 2017, 55, 573–578. [Google Scholar] [CrossRef]
- Ledberg, A. The interest in eight new psychoactive substances before and after scheduling. Drug Alcohol Depend. 2015, 152, 73–78. [Google Scholar] [CrossRef]
- Martínez-Clemente, J.; López-Arnau, R.; Carbó, M.; Piccoli, D.A.; Camarasa, J.; Escubedo, E. Mephedrone pharmacokinetics after intravenous and oral administration in rats: Relation to pharmacodynamics. Psychopharmacology 2013, 229, 295–306. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.G.; Breimer, D.D.; Mattie, H.; Pronk, J.; Gubbens-Stibbe, J.M. Rectal bioavailability of lidocaine in man: Partial avoidance of “first-pass” metabolism. Clin. Pharmacol. Ther. 1979, 26, 701–709. [Google Scholar] [CrossRef]
- Frison, G.; Frasson, S.; Zancanaro, F.; Tedeschi, G.; Zamengo, L. Detection of 3-methylmethcathinone and its metabolites 3-methylephedrine and 3-methylnorephedrine in pubic hair samples by liquid chromatography–high resolution/high accuracy Orbitrap mass spectrometry. Forensic Sci. Int. 2016, 265, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Magalhães, T.; Dinis-Oliveira, R. Mephedrone, the new designer drug of abuse: Pharmacokinetics, pharmacodynamics and clinical and forensic issues. Acta Med. Port. 2012, 25, 111–117. [Google Scholar]
- Vaux, S.; Chevaliez, S.; Saboni, L.; Sauvage, C.; Sommen, C.; Barin, F.; Alexandre, A.; Jauffret-Roustide, M.; Lot, F.; Annie Velter for the ANRS-Prevagay Group. Prevalence of hepatitis C infection, screening and associated factors among men who have sex with men attending gay venues: A cross-sectional survey (PREVAGAY), France, 2015. BMC Infect. Dis. 2019, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Margasińska-Olejak, J.; Skowronek, R.; Fischer, A.; Stojko, J. A fatal case of poisoning of a 19-year-old after taking 3-MMC. Forensic Sci. Int. 2019, 300, e34–e37. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, C.; Cartiser, N.; Gaillard, Y.; Boyer, B.; Bévalot, F. A fatal case of 3-methylmethcathinone (3-MMC) poisoning. Toxicol. Anal. Clin. 2017, 29, 123–129. [Google Scholar] [CrossRef]
- Rojek, S.; Kula, K.; Maciow-Glab, M.; Klys, M. Determination of 3-MMC and identification of its metabolites by GC-EI-MS-MS and GC-EI/PCI-MS in postmortem biological material. In Proceedings of the 53rd TIAFT Meeting, Firenze, Italy, 30 August–4 September 2015. [Google Scholar]
- Bottinelli, C.; Gaillard, Y.; Fanton, L.; Bévalot, F. À propos de deux décès par intoxication impliquant la 3-MMC. Toxicol. Anal. Clin. 2016, 28, S25. [Google Scholar] [CrossRef]
- Karinen, R.; Tuv, S.S.; Rogde, S.; Peres, M.D.; Johansen, U.; Frost, J.; Vindenes, V.; Øiestad, M.L. Lethal poisonings with AH-7921 in combination with other substances. Forensic Sci. Int. 2014, 244, e21–e24. [Google Scholar] [CrossRef]
- Valente, M.J.; De Pinho, P.G.; De Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2013, 88, 15–45. [Google Scholar] [CrossRef]
- Henry, J.; Jeffreys, K.J.; Dawling, S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (“ecstasy”). Lancet 1992, 340, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, W.C.; Hull, A.R.; Knochel, J.P. Rhabdomyolysis and shock after intravenous amphetamine administration. Ann. Intern. Med. 1977, 86, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Paillet-Loilier, M.; Cesbron, A.; Boisselier, R.L.; Bourgine, J.; Debruyne, D. Emerging drugs of abuse: Current perspectives on substituted cathinones. Subst. Abus. Rehabil. 2014, 5, 37–52. [Google Scholar]
- Hanna, J.; Danel, V.; Saviuc, P. Hyperthermie du syndrome malin des neuroleptiques, du syndrome sérotoninergique, ou liée à lecstasy: Approche thérapeutique. Réanimation 2001, 10, 412–417. [Google Scholar] [CrossRef]
- Daziani, G.; Lo Faro, A.F.; Montana, V.; Goteri, G.; Pesaresi, M.; Bambagiotti, G.; Montanari, E.; Giorgetti, R.; Montana, A. Synthetic cathinones and Neurotoxicity Risks: A Systematic review. Int. J. Mol. Sci. 2023, 24, 6230. [Google Scholar] [CrossRef]
- Da Silva, D.D.; Ferreira, B.G.A.; Bravo, R.R.; Rebelo, R.; De Almeida, T.D.; Valente, M.J.; Silva, J.P.; Carvalho, F.; Bastos, M.d.L.; Carmo, H. The new psychoactive substance 3-methylmethcathinone (3-MMC or metaphedrone) induces oxidative stress, apoptosis, and autophagy in primary rat hepatocytes at human-relevant concentrations. Arch. Toxicol. 2019, 93, 2617–2634. [Google Scholar] [CrossRef]
- Ahmed, S.; Zhou, Z.; Zhou, J.; Chen, S. Pharmacogenomics of Drug metabolizing Enzymes and Transporters: Relevance to Precision Medicine. Genom. Proteom. Bioinform. 2016, 14, 298–313. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelečević, I.; Vejnović, A.-M.; Javorac, J.; Gvozdenović, N.; Janjić, N.; Mijatović Jovin, V. Metaphedrone (3-Methylmethcathinone): Pharmacological, Clinical, and Toxicological Profile. Medicina 2024, 60, 466. https://doi.org/10.3390/medicina60030466
Kelečević I, Vejnović A-M, Javorac J, Gvozdenović N, Janjić N, Mijatović Jovin V. Metaphedrone (3-Methylmethcathinone): Pharmacological, Clinical, and Toxicological Profile. Medicina. 2024; 60(3):466. https://doi.org/10.3390/medicina60030466
Chicago/Turabian StyleKelečević, Igor, Ana-Marija Vejnović, Jovan Javorac, Nemanja Gvozdenović, Nataša Janjić, and Vesna Mijatović Jovin. 2024. "Metaphedrone (3-Methylmethcathinone): Pharmacological, Clinical, and Toxicological Profile" Medicina 60, no. 3: 466. https://doi.org/10.3390/medicina60030466
APA StyleKelečević, I., Vejnović, A.-M., Javorac, J., Gvozdenović, N., Janjić, N., & Mijatović Jovin, V. (2024). Metaphedrone (3-Methylmethcathinone): Pharmacological, Clinical, and Toxicological Profile. Medicina, 60(3), 466. https://doi.org/10.3390/medicina60030466






