Evaluating the Clinical Utility of Brachial Plexus Block for Reducing Opioid Exposure in Pediatric Elbow Fracture Surgery: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
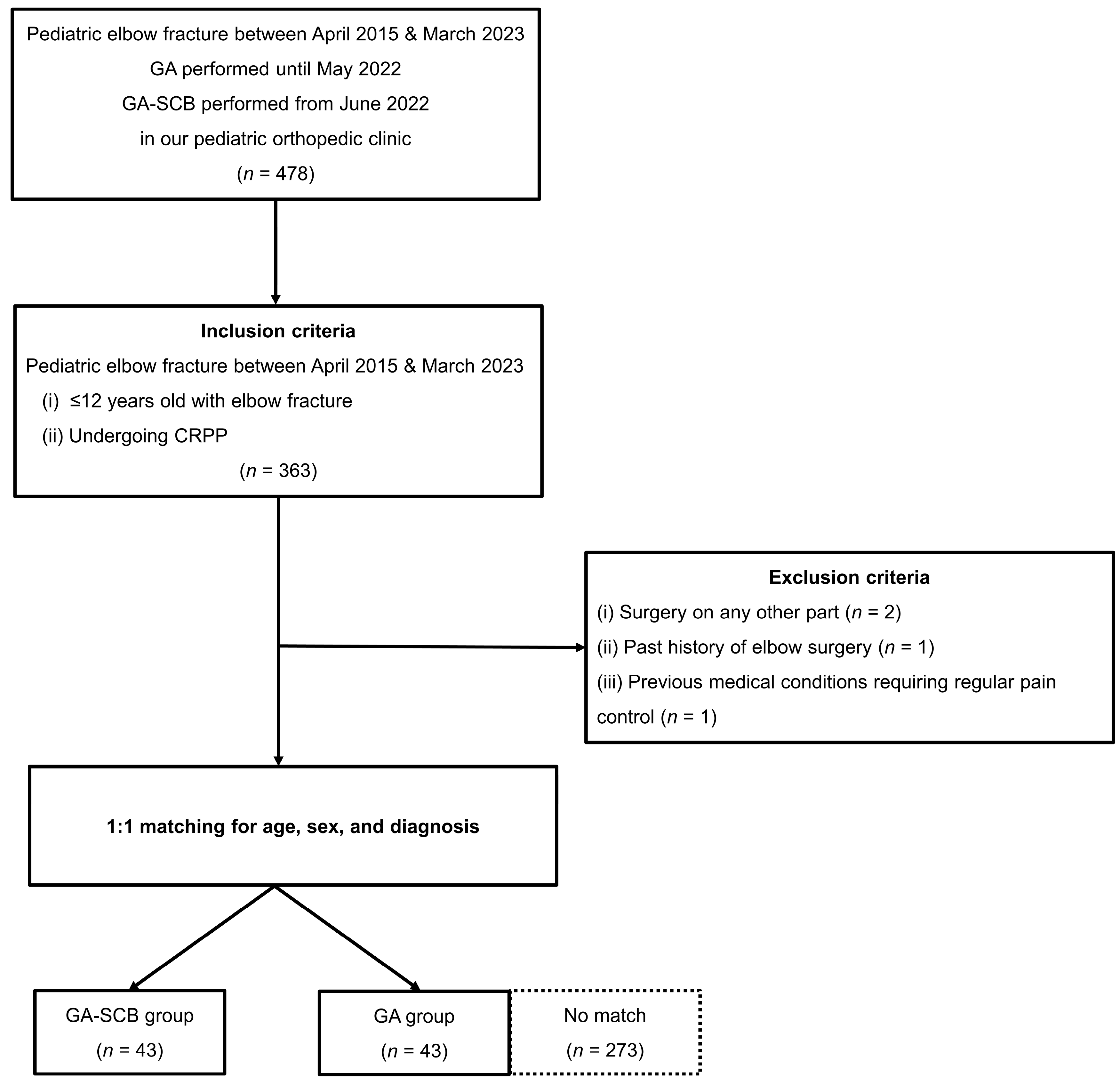
2.1. Study Design and Patients
2.2. Anesthesia Technique
2.3. Postoperative Care and Pain Management of Elbow Fracture
2.4. Investigated Variables
2.5. Statistical Analysis
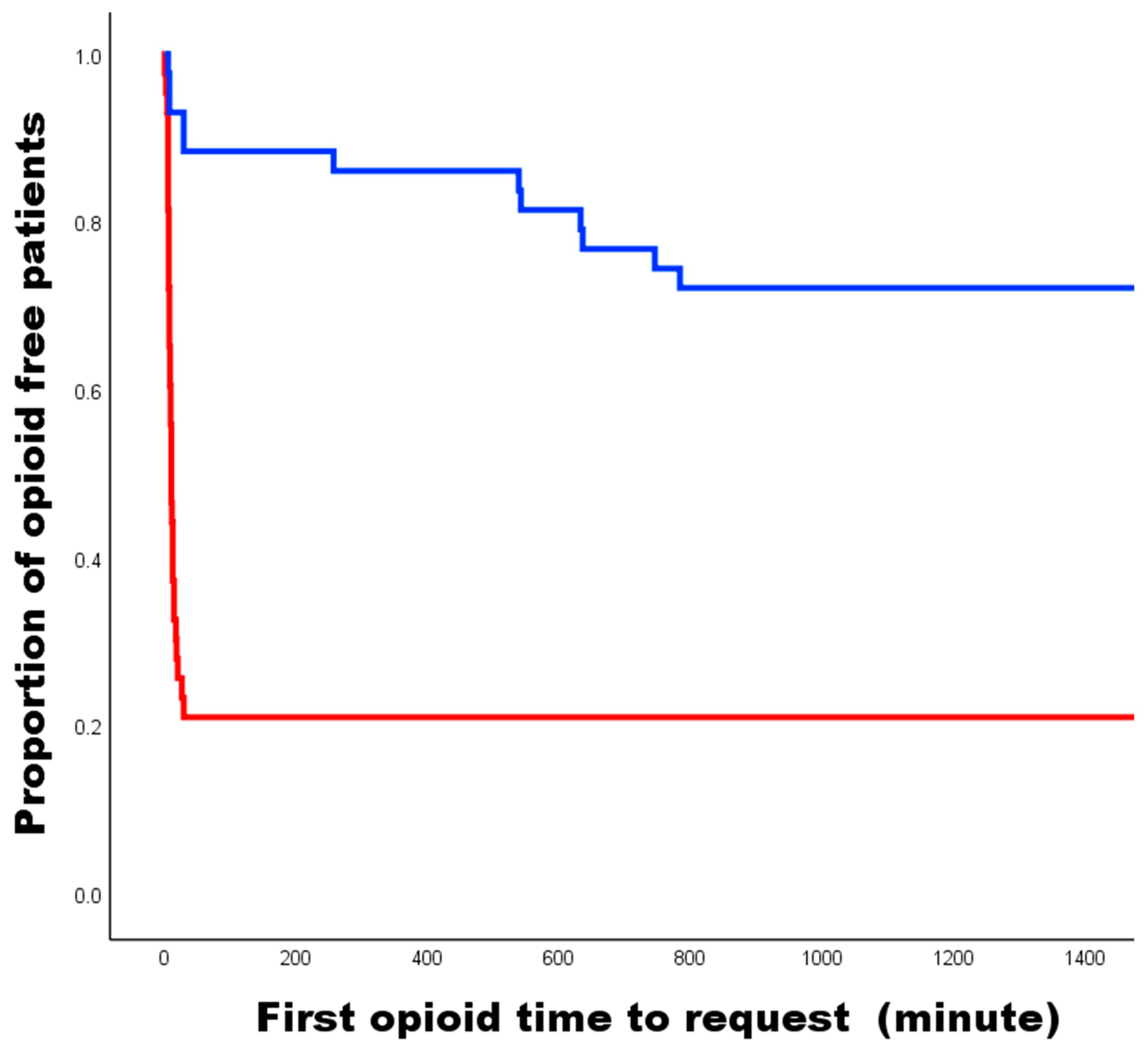
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Park, S.-S. Predisposing effect of elbow alignment on the elbow fracture type in children. J. Orthop. Trauma 2015, 29, e253–e258. [Google Scholar] [CrossRef]
- Mubarak, F.S.; Anzar, M.A.M.; Kanagratnam, K. Descriptive Study on Epidemiology, Clinical Presentation, Treatment, and Outcome of Supracondylar Fractures Treated in a Base Hospital of Sri Lanka: A Single-Center Study. Cureus 2023, 15, e40494. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, J.R.; Sung, K.H.; Moon, Y.J.; Lee, S.C.; Wang, S.I. Comparison of Functional and Cosmetic Outcomes According to Fracture Level in Gartland Type III Pediatric Supracondylar Humerus Fractures. Clin. Orthop. Surg. 2023, 15, 668. [Google Scholar] [CrossRef]
- Stillwagon, M.R.; Feinstein, S.; Nichols, B.; Andrews, P.N.; Vergun, A.D. Pain control and medication use in children following closed reduction and percutaneous pinning of supracondylar humerus fractures: Are we still overprescribing opioids? J. Pediatr. Orthop. 2020, 40, 543–548. [Google Scholar] [CrossRef]
- Abzug, J.M.; Herman, M.J. Management of supracondylar humerus fractures in children: Current concepts. JAAOS-J. Am. Acad. Orthop. Surg. 2012, 20, 69–77. [Google Scholar] [CrossRef]
- Adams, A.J.; Buczek, M.J.; Flynn, J.M.; Shah, A.S. Perioperative ketorolac for supracondylar humerus fracture in children decreases postoperative pain, opioid usage, hospitalization cost, and length-of-stay. J. Pediatr. Orthop. 2019, 39, e447–e451. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.E.; Chang, K.; Schleyer, E.; Pizzutillo, P.D.; Herman, M.J. Postoperative pain control after supracondylar humerus fracture fixation. J. Pediatr. Orthop. 2012, 32, 452–455. [Google Scholar] [CrossRef] [PubMed]
- McCabe, S.E.; Veliz, P.; Schulenberg, J.E. Adolescent context of exposure to prescription opioids and substance use disorder symptoms at age 35: A national longitudinal study. Pain 2016, 157, 2173. [Google Scholar] [CrossRef]
- Polaner, D.M.; Taenzer, A.H.; Walker, B.J.; Bosenberg, A.; Krane, E.J.; Suresh, S.; Wolf, C.; Martin, L.D. Pediatric Regional Anesthesia Network (PRAN): A multi-institutional study of the use and incidence of complications of pediatric regional anesthesia. Anesth. Analg. 2012, 115, 1353–1364. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Zadrazil, M.; Opfermann, P.; Marhofer, P.; Westerlund, A.I.; Haider, T. Brachial plexus block with ultrasound guidance for upper-limb trauma surgery in children: A retrospective cohort study of 565 cases. Br. J. Anaesth. 2020, 125, 104–109. [Google Scholar] [CrossRef]
- Emery, K.H.; Zingula, S.N.; Anton, C.G.; Salisbury, S.R.; Tamai, J. Pediatric elbow fractures: A new angle on an old topic. Pediatr. Radiol. 2016, 46, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Nakasone, M.; Kinjo, M.; Onaka, K.; Futenma, C.; Kanaya, F. Epidemiology of paediatric elbow fractures: A retrospective multi-centre study of 488 fractures. J. Child. Orthop. 2019, 13, 516–521. [Google Scholar] [CrossRef]
- Houshian, S.; Mehdi, B.; Larsen, M.S. The epidemiology of elbow fracture in children: Analysis of 355 fractures, with special reference to supracondylar humerus fractures. J. Orthop. Sci. 2001, 6, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.S.; Dartnell, J.; Lim, A.K.S.; Hui, J.H. Paediatric lateral condyle fractures: A systematic review. Arch. Orthop. Trauma Surg. 2018, 138, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Guan, X.; Jiang, F.; Lv, P. Closed reduction and percutaneous pinning for treatment of unstable lateral condyle fractures of the humerus in children. Front. Pediatr. 2023, 11, 1223615. [Google Scholar] [CrossRef]
- Wilkins, K.E. Residuals of elbow trauma in children. Orthop. Clin. N. Am. 1990, 21, 291–314. [Google Scholar] [CrossRef]
- Meng, C.; Meng, Z.; Huang, X.; Zhao, F.; Yang, Q. A meta-analysis of closed reduction percutaneous pinning and open reduction with pin fixation of pediatric humeral lateral condylar fracture. Front. Pediatr. 2023, 11, 1205755. [Google Scholar] [CrossRef]
- Sapienza, M.; Testa, G.; Vescio, A.; Panvini, F.M.C.; Caldaci, A.; Parisi, S.C.; Pavone, V.; Canavese, F. The role of patient position in the surgical treatment of supracondylar fractures of the humerus: Comparison of prone and supine position. Medicina 2023, 59, 374. [Google Scholar] [CrossRef]
- Eccleston, C.; Fisher, E.; Howard, R.F.; Slater, R.; Forgeron, P.; Palermo, T.M.; Birnie, K.A.; Anderson, B.J.; Chambers, C.T.; Crombez, G. Delivering transformative action in paediatric pain: A Lancet Child & Adolescent Health Commission. Lancet Child Adolesc. Health 2021, 5, 47–87. [Google Scholar]
- Bosenberg, A. Benefits of regional anesthesia in children. Pediatr. Anesth. 2012, 22, 10–18. [Google Scholar] [CrossRef]
- Brindle, M.E.; McDiarmid, C.; Short, K.; Miller, K.; MacRobie, A.; Lam, J.Y.; Brockel, M.; Raval, M.V.; Howlett, A.; Lee, K.-S. Consensus guidelines for perioperative care in neonatal intestinal surgery: Enhanced recovery after surgery (ERAS®) society recommendations. World J. Surg. 2020, 44, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Raney, E.M.; van Bosse, H.J.; Shea, K.G.; Abzug, J.M.; Schwend, R.M. Current state of the opioid epidemic as it pertains to pediatric orthopaedics from the Advocacy Committee of the Pediatric Orthopaedic Society of North America. J. Pediatr. Orthop. 2018, 38, e238–e244. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Soneji, N.; Ko, D.T.; Yun, L.; Wijeysundera, D.N. Rates and risk factors for prolonged opioid use after major surgery: Population based cohort study. BMJ 2014, 348, g1251. [Google Scholar] [CrossRef]
- Hill, M.V.; McMahon, M.L.; Stucke, R.S.; Barth Jr, R.J. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann. Surg. 2017, 265, 709–714. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, C.S.; Kim, S.D.; Lee, J.-R. Fascia iliaca compartment block reduces emergence agitation by providing effective analgesic properties in children. J. Clin. Anesth. 2011, 23, 119–123. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Halpern, S.H.; Aoyama, K.; Brull, R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth. Analg. 2015, 120, 1114–1129. [Google Scholar] [CrossRef]
- Barry, G.S.; Bailey, J.G.; Sardinha, J.; Brousseau, P.; Uppal, V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br. J. Anaesth. 2021, 126, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Lavand’homme, P. Rebound pain after regional anesthesia in the ambulatory patient. Curr. Opin. Anesthesiol. 2018, 31, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Leyva, F.; Cubillos, J.; Chin, K.J. Managing rebound pain after regional anesthesia. Korean J. Anesthesiol. 2020, 73, 372–383. [Google Scholar] [CrossRef]
- Reiss, W.; Kurapati, S.; Shariat, A.; Hadzic, A. Nerve injury complicating ultrasound/electrostimulation-guided supraclavicular brachial plexus block. Reg. Anesth. Pain Med. 2010, 35, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Kakazu, C.; Tokhner, V.; Li, J.; Ou, R.; Simmons, E. In the new era of ultrasound guidance: Is pneumothorax from supraclavicular block a rare complication of the past? Br. J. Anaesth. 2014, 113, 190–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lönnqvist, P.-A.; Ecoffey, C.; Bosenberg, A.; Suresh, S.; Ivani, G. The European society of regional anesthesia and pain therapy and the American society of regional anesthesia and pain medicine joint committee practice advisory on controversial topics in pediatric regional anesthesia I and II: What do they tell us? Curr. Opin. Anaesthesiol. 2017, 30, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Long, J.B.; Sathyamoorthy, M.; Birstler, J.; Wolf, C.; Bosenberg, A.T.; Flack, S.H.; Krane, E.J.; Sethna, N.F.; Suresh, S. Complications in pediatric regional anesthesia: An analysis of more than 100,000 blocks from the pediatric regional anesthesia network. Anesthesiology 2018, 129, 721–732. [Google Scholar] [CrossRef]
- DeVera, H.V.; Furukawa, K.T.; Matson, M.D.; Scavone, J.A.; James, M.A. Regional techniques as an adjunct to general anesthesia for pediatric extremity and spine surgery. J. Pediatr. Orthop. 2006, 26, 801–804. [Google Scholar] [CrossRef][Green Version]
| Characteristics | GA (n = 43) | GA-SCB (n = 43) | p-Value |
|---|---|---|---|
| Age, years | 6.9 ± 2.4 | 6.8 ± 2.4 | 0.634 |
| BMI, kg/m2 | 17.0 ± 2.6 | 16.9 ± 3.2 | 0.795 |
| Gender, Male | 19 (44.1) | 19 (44.1) | 1.000 |
| Diagnosis | 1.000 | ||
| SCHF | 32 (74.4) | 32 (74.4) | |
| LCF | 6 (14.0) | 6 (14.0) | |
| RNF | 5 (11.6) | 5 (11.6) |
| Characteristics | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 0.892 | 0.638–1.247 | 0.505 |
| Sex: Male | 2.745 | 0.697–10.814 | 0.149 |
| Diagnosis | 0.560 | ||
| SCHF (reference) | 0.911 | ||
| LCF | 0.878 | 0.090–8.599 | 0.430 |
| RNF | 0.319 | 0.019–5.438 | 0.125 |
| BMI | 0.797 | 0.596–1.065 | 0.126 |
| Anesthetic methods: GA-SCB | 0.013 | 0.002–0.077 | <0.001 |
| Characteristics | GA (n = 43) | GA-SCB (n = 43) | p-Value |
|---|---|---|---|
| Opioid consumption | |||
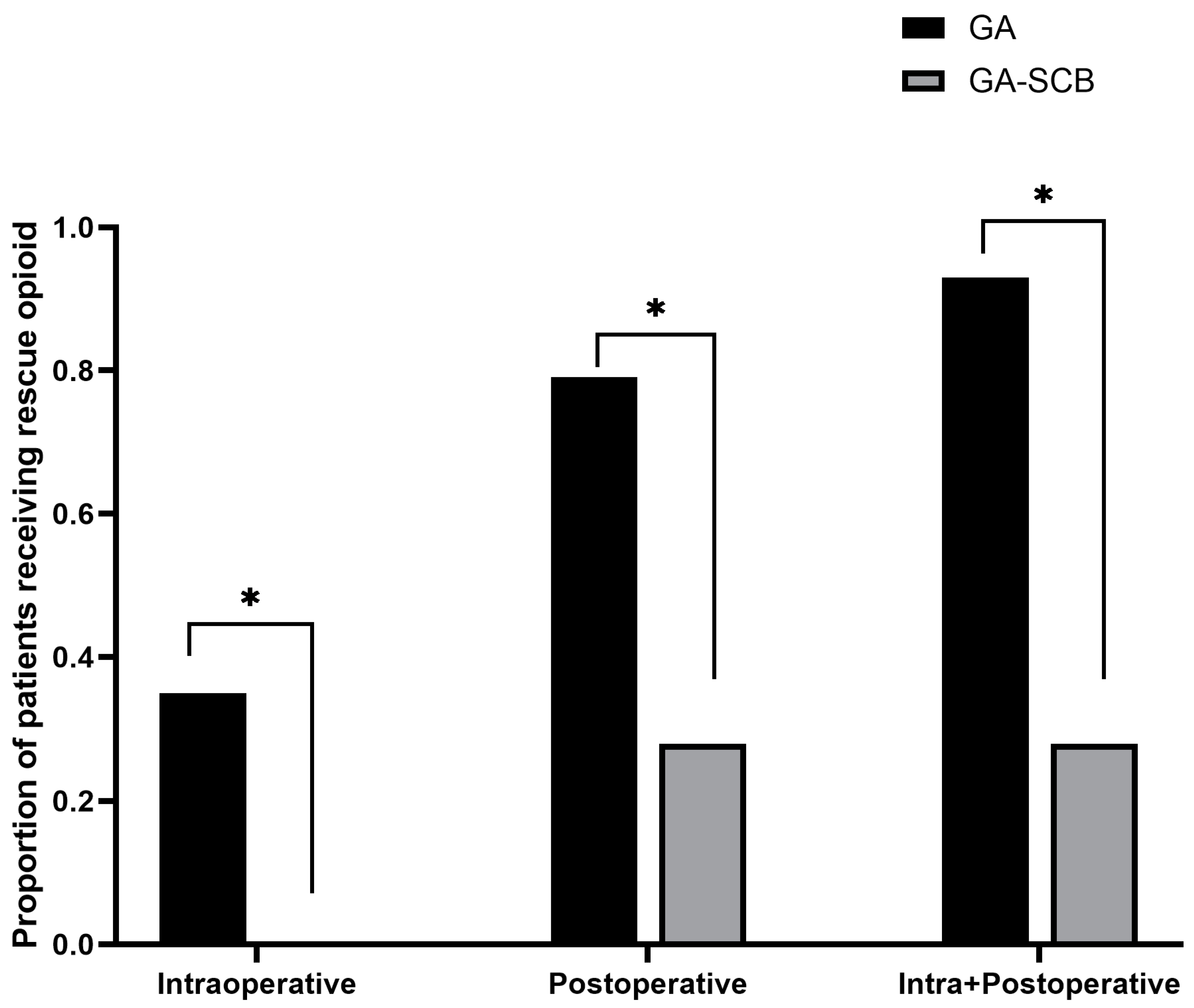
| Intraoperative a | 0.8 ± 1.3 | 0 | <0.001 |
| During postoperative 24 h a | 3.2 ± 3.0 | 0.9 ± 1.8 | <0.001 |
| FLACC scale b | |||
| Arrival in PACU (postoperative 0–1 h) | 4.9 ± 2.3 | 0.9 ±1.5 | <0.001 |
| Discharge from PACU (postoperative 1–2 h) | 3.1 ± 2.5 | 0.4 ± 0.8 | <0.001 |
| First pain check in the ward (postoperative 4 ± 2 h) | 2.2 ± 1.8 | 1.0 ± 1.4 | 0.017 |
| Pain check at postoperative 12 ± 2 h | 1.5 ± 1.5 | 3.8 ± 2.4 | <0.001 |
| POD 1 day (postoperative 24 ± 2 h) | 1.0 ± 1.5 | 0.8 ± 0.9 | 0.36 |
| Worst pain until POD 1 | 5.4 ± 2.0 | 4.7 ± 2.1 | 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, K.; Kim, Y.J.; Lim, H.W.; Kang, M.S.; Kim, H.-J.; Koh, W.U.; Ro, Y.-j.; Cho, J.; Kim, H.J.; Park, S.-S.; et al. Evaluating the Clinical Utility of Brachial Plexus Block for Reducing Opioid Exposure in Pediatric Elbow Fracture Surgery: A Retrospective Cohort Study. Medicina 2024, 60, 483. https://doi.org/10.3390/medicina60030483
Bae K, Kim YJ, Lim HW, Kang MS, Kim H-J, Koh WU, Ro Y-j, Cho J, Kim HJ, Park S-S, et al. Evaluating the Clinical Utility of Brachial Plexus Block for Reducing Opioid Exposure in Pediatric Elbow Fracture Surgery: A Retrospective Cohort Study. Medicina. 2024; 60(3):483. https://doi.org/10.3390/medicina60030483
Chicago/Turabian StyleBae, Kunhyung, Yeon Ju Kim, Hyo Won Lim, Michael Seougcheol Kang, Ha-Jung Kim, Won Uk Koh, Young-jin Ro, Jooyeon Cho, Hwa Jung Kim, Soo-Sung Park, and et al. 2024. "Evaluating the Clinical Utility of Brachial Plexus Block for Reducing Opioid Exposure in Pediatric Elbow Fracture Surgery: A Retrospective Cohort Study" Medicina 60, no. 3: 483. https://doi.org/10.3390/medicina60030483
APA StyleBae, K., Kim, Y. J., Lim, H. W., Kang, M. S., Kim, H.-J., Koh, W. U., Ro, Y.-j., Cho, J., Kim, H. J., Park, S.-S., Kwak, Y. H., & Kim, H. (2024). Evaluating the Clinical Utility of Brachial Plexus Block for Reducing Opioid Exposure in Pediatric Elbow Fracture Surgery: A Retrospective Cohort Study. Medicina, 60(3), 483. https://doi.org/10.3390/medicina60030483






