Exploring a New Pathophysiological Association in Acne Vulgaris and Metabolic Syndrome: The Role of Biogenic Amines and Glutathione Peroxidase
Abstract
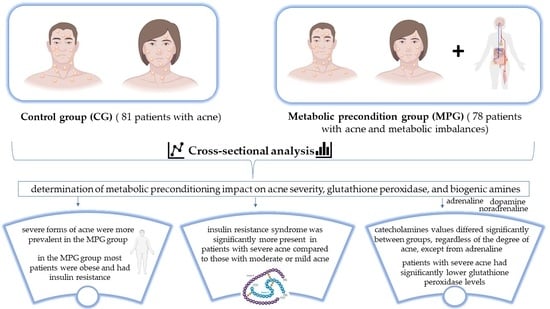
:1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Statistical Design
3. Results
| Characteristics | CG (n = 81) | MPG (n = 78) | p 1 | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Gender | |||||
| Women | 63 | 77.78 | 57 | 73.08 | 0.491 |
| Men | 18 | 22.22 | 21 | 26.92 | |
| Environment | |||||
| Urban | 70 | 86.4 | 57 | 73.1 | 0.036 * |
| Rural | 11 | 13.6 | 21 | 26.9 | |
| Age (years) | |||||
| 14–25 | 77 | 4.9 | 52 | 33.3 | <0.001 * |
| >25 | 4 | 95.1 | 26 | 66.7 | |
| Average (mean ± SD) | 21.05 ± 3.35 | 23.81 ± 4.84 | 0.002 2,* | ||
| Acne stage | |||||
| Mild acne | 26 | 32.1 | 5 | 6.41 | <0.001 * |
| Moderate acne | 34 | 42.0 | 25 | 32.05 | |
| Severe acne | 21 | 25.9 | 48 | 61.54 | |
| Body mass index (kg/m2) | 24.72 | 1.09 | 32.40 | 4.79 | <0.001 2,* |
| Paraclinical parameters | Mean | SD | Mean | SD | p 2 |
| Fasting blood glucose (mg/dL) | 84.43 | 3.96 | 87.03 | 8.49 | 0.007 * |
| HOMA-IR (insulin (µU/mL) × glucose level (mg/dL))/405 | 1.22 | 0.63 | 2.55 | 2.00 | <0.001 * |
| LDL cholesterol (mg/dL) | 123.53 | 20.78 | 123.37 | 24.14 | 0.573 |
| HDL cholesterol (mg/dL) | 57.77 | 7.55 | 47.73 | 4.86 | <0.001 * |
| Triglycerides (mg/dL) | 133.96 | 9.58 | 139.03 | 14.12 | 0.669 |
| Blood pressure (mm Hg) | 107/68 | 0.3/0.04 | 110/72 | 0.6/0.23 | 0.239 |
| Glutathione peroxidase (U/L4) | 6015.33 | 1345.49 | 5680.37 | 1167.02 | 0.114 |
| Dopamine (µg/g creatinine) | 323.81 | 163.03 | 549.67 | 183.20 | <0.001 * |
| Noradrenaline (µg/g creatinine) | 38.12 | 13.39 | 61.55 | 12.82 | |
| Adrenaline (µg/g creatinine) | 4.79 | 3.78 | 11.42 | 1.88 | |
| NADR/ADR | 7.96 | 6.45 | 5.39 | 3.41 | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swarup, S.; Goyal, A.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459248/ (accessed on 24 January 2024).
- Bungau, S.G.; Tit, D.M.; Vesa, C.M.; Abid, A.; Szilagyi, D.V.; Radu, A.F.; Bungau, A.F.; Tarce, A.G.; Behl, T.; Stoicescu, M.; et al. Non-Conventional Therapeutical Approaches to Acne Vulgaris Related to Its Association with Metabolic Disorders. Eur. J. Pharmacol. 2022, 923, 174936. [Google Scholar] [CrossRef] [PubMed]
- Bekkouche, L.; Bouchenak, M.; Malaisse, W.J.; Yahia, D.A. The Mediterranean Diet Adoption Improves Metabolic, Oxidative, and Inflammatory Abnormalities in Algerian Metabolic Syndrome Patients. Horm. Metab. Res. 2014, 46, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kardeh, S.; Moein, S.A.; Namazi, M.R.; Kardeh, B. Evidence for the Important Role of Oxidative Stress in the Pathogenesis of Acne. Galen Med. J. 2019, 8, e1291. [Google Scholar] [CrossRef]
- Tran, J.T.; Diaz, M.J.; Rodriguez, D.; Kleinberg, G.; Aflatooni, S.; Palreddy, S.; Abdi, P.; Taneja, K.; Batchu, S.; Forouzandeh, M. Evidence-Based Utility of Adjunct Antioxidant Supplementation for the Prevention and Treatment of Dermatologic Diseases: A Comprehensive Systematic Review. Antioxidants 2023, 12, 1503. [Google Scholar] [CrossRef] [PubMed]
- Md Jaffri, J. Reactive Oxygen Species and Antioxidant System in Selected Skin Disorders. Malays. J. Med. Sci. 2023, 30, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Moftah, N.H.; Hamad, W.A.M.; Abd Al Salam, F.M.; Marzouk, S.A.; Said, M. Glutathione Peroxidase and Malondialdehyde in Skin Lesions of Acne Vulgaris. J. Egypt. Women’s Dermatol. Soc. 2011, 8, 25–29. [Google Scholar] [CrossRef]
- Perihan, O.; Ergul, K.B.; Neslihan, D.; Filiz, A. The Activity of Adenosine Deaminase and Oxidative Stress Biomarkers in Scraping Samples of Acne Lesions. J. Cosmet. Dermatol. 2012, 11, 323–328. [Google Scholar] [CrossRef]
- Baek, J.; Lee, M.-G. Oxidative Stress and Antioxidant Strategies in Dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Popa, G.L.; Mitran, C.I.; Mitran, M.I.; Tampa, M.; Matei, C.; Popa, M.I.; Georgescu, S.R. Markers of Oxidative Stress in Patients with Acne: A Literature Review. Life 2023, 13, 1433. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Rao, A.; Douglas, S.C.; Hall, J.M. Endocrine Disrupting Chemicals, Hormone Receptors, and Acne Vulgaris: A Connecting Hypothesis. Cells 2021, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Bungau, A.F.; Radu, A.F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Endres, L.M. Oxidative Stress and Metabolic Syndrome in Acne Vulgaris: Pathogenetic Connections and Potential Role of Dietary Supplements and Phytochemicals. Biomed. Pharmacother. 2023, 164, 115003. [Google Scholar] [CrossRef]
- Matiș, L.; Alexandru, B.A.; Ghitea, T.C. Catecholamine Variations in Pediatric Gastrointestinal Disorders and Their Neuropsychiatric Expression. Biomedicines 2023, 11, 2600. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Kirschbaum, C. Biological Bases of the Stress Response. In Stress and Addiction. Biological and Psychological Mechanism; Al’Absi, M., Ed.; Academic Press: Burlington, MA, USA, 2007; pp. 3–19. ISBN 978-0-12-370632-4. [Google Scholar]
- Kokka, I.; Chrousos, G.P.; Darviri, C.; Bacopoulou, F. Measuring Adolescent Chronic Stress: A Review of Established Biomarkers and Psychometric Instruments. Horm. Res. Paediatr. 2023, 96, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lyga, J. Brain-Skin Connection: Stress, Inflammation and Skin Aging. Inflamm. Allergy Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Basoglu, O.K.; Sarac, F.; Sarac, S.; Uluer, H.; Yilmaz, C. Metabolic Syndrome, Insulin Resistance, Fibrinogen, Homocysteine, Leptin, and C-Reactive Protein in Obese Patients with Obstructive Sleep Apnea Syndrome. Ann. Thorac. Med. 2011, 6, 120–125. [Google Scholar] [CrossRef]
- Dréno, B.; Poli, F.; Pawin, H.; Beylot, C.; Faure, M.; Chivot, M.; Auffret, N.; Moyse, D.; Ballanger, F.; Revuz, J. Development and Evaluation of a Global Acne Severity Scale (GEA Scale) Suitable for France and Europe. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 43–48. [Google Scholar] [CrossRef]
- Bungau, A.F.; Tit, D.M.; Bungau, S.G.; Vesa, C.M.; Radu, A.-F.; Marin, R.C.; Endres, L.M.; Moleriu, L.-C. Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity. Int. J. Mol. Sci. 2024, 25, 721. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Tomé, M.A.; Botana, M.A.; Cadarso-Suárez, C.; Rego-Iraeta, A.; Fernández-Mariño, A.; Mato, J.A.; Solache, I.; Perez-Fernandez, R. Prevalence of Metabolic Syndrome in Galicia (NW Spain) on Four Alternative Definitions and Association with Insulin Resistance. J. Endocrinol. Investig. 2009, 32, 505–511. [Google Scholar]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Winpenny, E.M.; van Sluijs, E.M.F.; White, M.; Klepp, K.-I.; Wold, B.; Lien, N. Changes in Diet through Adolescence and Early Adulthood: Longitudinal Trajectories and Association with Key Life Transitions. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Habdous, M.; Herbeth, B.; Vincent-Viry, M.; Lamont, J.V.; Fitzgerald, P.S.; Visvikis, S.; Siest, G. Serum Total Antioxidant Status, Erythrocyte Superoxide Dismutase and Whole-Blood Glutathione Peroxidase Activities in the Stanislas Cohort: Influencing Factors and Reference Intervals. Clin. Chem. Lab. Med. 2003, 41, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Nandy, P.; Shrivastava, T. Exploring the Multifaceted Impact of Acne on Quality of Life and Well-Being. Cureus 2024, 16, e52727. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Giorgino, F.; Benigno, R.; Guida, P.; Giorgino, R. Independent Influence of Insulin, Catecholamines, and Thyroid Hormones on Metabolic Syndrome. Obesity 2008, 16, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Bagatin, E.; Blume-Peytavi, U.; Rocha, M.; Gollnick, H. Female Type of Adult Acne: Physiological and Psychological Considerations and Management. J. Dtsch. Dermatol. Ges. 2018, 16, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Morshed, A.S.M.; Noor, T.; Uddin Ahmed, M.A.; Mili, F.S.; Ikram, S.; Rahman, M.; Ahmed, S.; Uddin, M.B. Understanding the Impact of Acne Vulgaris and Associated Psychological Distress on Self-Esteem and Quality of Life via Regression Modeling with CADI, DLQI, and WHOQoL. Sci. Rep. 2023, 13, 21084. [Google Scholar] [CrossRef]
- Baik, J.-H. Stress and the Dopaminergic Reward System. Exp. Mol. Med. 2020, 52, 1879–1890. [Google Scholar] [CrossRef]
- Bloomfield, M.A.; McCutcheon, R.A.; Kempton, M.; Freeman, T.P.; Howes, O. The Effects of Psychosocial Stress on Dopaminergic Function and the Acute Stress Response. eLife 2019, 8, e46797. [Google Scholar] [CrossRef]
- Jia, L.; Wang, F.; Zhang, K.; Wang, D.; Wang, X.; Li, X.; Zhang, J. L-Theanine Inhibits (−)-Epigallocatechin-3-Gallate Oxidation via Chelating Copper. J. Agric. Food Chem. 2022, 70, 7751–7761. [Google Scholar] [CrossRef]
- Stanwood, G.D. Dopamine and Stress. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 105–114. ISBN 978-0-12-813146-6. [Google Scholar]
- Chandak, S.; Singh, A.; Madke, B.; Jawade, S.; Khandelwal, R. Acne Vulgaris and Metabolic Syndrome: A Possible Association. Cureus 2022, 14, e24750. [Google Scholar] [CrossRef]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The Role of Glutathione Peroxidase-1 in Health and Disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Tapbergenov, S.O.; Sovetov, B.S.; Smailova, Z.K. Adrenergic Receptors in the Mechanism of Regulation of Mitochondrial and Cytoplasmic Enzymes of Cardiomyocytes by Catecholamines. Bull. Exp. Biol. Med. 2022, 173, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R. The Glutathione Peroxidases. Cell. Mol. Life Sci. C 2001, 57, 1825–1835. [Google Scholar] [CrossRef]


| Parameters | EGs | n | Mean | SD | p 2 | AGs | Mean | SD | p 2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI | U | 127 | 28.543 | 5.177 | 0.726 | >25 | 30 | 28.907 | 4.738 | 0.329 |
| R | 32 | 28.577 | 5.165 | <25 | 129 | 28.213 | 5.266 | |||
| Fasting blood glucose (mg/dL) | U | 127 | 85.819 | 6.807 | 0.453 | >25 | 30 | 85.677 | 4.367 | 0.500 |
| R | 32 | 85.641 | 6.278 | <25 | 129 | 85.783 | 7.133 | |||
| HOMA-IR 1 | U | 127 | 1.783 | 1.573 | 0.185 | >25 | 30 | 1.947 | 1.469 | 0.443 |
| R | 32 | 1.987 | 1.735 | <25 | 129 | 1.823 | 1.644 | |||
| LDL cholesterol (mg/dL) | U | 127 | 123.866 | 23.286 | 0.753 | >25 | 30 | 123.867 | 20.257 | 0.654 |
| R | 32 | 123.034 | 18.798 | <25 | 129 | 123.033 | 22.960 | |||
| HDL cholesterol (mg/dL) | U | 127 | 51.688 | 11.894 | 0.474 | >25 | 30 | 50.892 | 11.521 | 0.470 |
| R | 32 | 53.812 | 11.931 | <25 | 129 | 54.608 | 11.857 | |||
| Triglycerides (mg/dL) | U | 127 | 138.228 | 12.231 | 0.471 | >25 | 30 | 138.085 | 12.894 | 0.224 |
| R | 32 | 134.732 | 10.010 | <25 | 129 | 134.905 | 8.794 | |||
| Glutathione peroxidase (U/L4) | U | 127 | 5753.472 | 1205.422 | 0.054 | >25 | 30 | 5697.809 | 1218.556 | 0.233 |
| R | 32 | 5942.228 | 1448.781 | <25 | 129 | 5997.891 | 1301.329 | |||
| Dopamine (µg/g creatinine) | U | 127 | 434.355 | 205.990 | 0.811 | >25 | 30 | 440.000 | 226.457 | 0.841 |
| R | 32 | 439.125 | 211.776 | <25 | 129 | 433.480 | 202.509 | |||
| Noradrenaline (µg/g creatinine) | U | 127 | 50.014 | 18.236 | 0.498 | >25 | 30 | 51.05 | 24.308 | 0.416 |
| R | 32 | 49.656 | 14.910 | <25 | 129 | 48.620 | 16.052 | |||
| Adrenalin (µg/g creatinine) | U | 127 | 7.847 | 4.568 | 0.638 | >25 | 30 | 8.110 | 2.319 | 0.322 |
| R | 32 | 8.363 | 4.035 | <25 | 129 | 8.100 | 4.418 | |||
| NADR/ADR | U | 127 | 6.373 | 3.984 | 0.663 | >25 | 30 | 6.295 | 3.241 | 0.056 |
| R | 32 | 5.938 | 3.024 | <25 | 129 | 6.002 | 4.328 |
| Correlation of Age with the Studied Variables | Pearson’s r Coefficient | p |
|---|---|---|
| BMI | −0.014 | 0.864 |
| Fasting blood glucose | 0.022 | 0.788 |
| HOMA-IR | −0.105 | 0.187 |
| LDL cholesterol | 0.056 | 0.484 |
| HDL cholesterol | −0.068 | 0.392 |
| Triglycerides | 0.159 | 0.055 |
| Blood pressure | 0.019 | 0.866 |
| Glutathione peroxidase | −0.012 | 0.882 |
| Dopamine | 0.051 | 0.521 |
| Noradrenaline | −0.011 | 0.889 |
| Adrenaline | 0.044 | 0.583 |
| NADR/ADR | −0.166 | 0.056 |
| Patients MPG (n = 78) | Mild (n = 5) | Moderate (n = 25) | Severe (n = 48) | p 1 | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| High glucose | 0 | 0 | 1 | 4 | 2 | 4.2 | 0.898 |
| Insulin resistance syndrome | 1 | 20 | 5 | 20 | 22 | 48.83 | 0.039 * |
| Diabetes | 0 | 0 | 0 | 0 | 2 | 4.2 | 0.527 |
| Dyslipidemia | 0 | 0 | 2 | 8 | 6 | 12.5 | 0.615 |
| High Blood Pressure | 0 | 0 | 0 | 0 | 1 | 2.1 | 0.729 |
| Overweight | 5 | 100 | 11 | 44 | 25 | 52.1 | 0.072 |
| Obesity | 0 | 0 | 14 | 56 | 23 | 47.92 | 0.079 |
| Characteristics | CG (n = 81) | MPG (n = 78) | p 1 | ||
|---|---|---|---|---|---|
| Glutathione peroxidase (U/L4) | |||||
| Mild acne | 5548.79 | 1688.27 | 4855.38 | 118.36 | 0.081 |
| Moderate acne | 6089.29 | 760.39 | 5874.26 | 1348.97 | 0.154 |
| Severe acne | 6855.50 | 1085.19 | 5583.60 | 620.32 | 0.015 |
| p 2 | p = 0.001 | p = 0.675 | - | ||
| Catecholamines (µg/g creatinine) | |||||
| Dopamine | |||||
| Mild acne | 250.10 | 162.89 | 545.19 | 2.68 | 0.001 |
| Moderate acne | 328.96 | 163.95 | 535.71 | 178.63 | 0.001 |
| Severe acne | 365.41 | 141.81 | 673.80 | 194.01 | 0.001 |
| p = 0.003 | p = 0.005 | ||||
| Noradrenaline | |||||
| Mild acne | 33.05 | 13.12 | 52.60 | 0.55 | 0.007 |
| Moderate acne | 38.53 | 14.42 | 55.94 | 13.14 | 0.001 |
| Severe acne | 41.69 | 10.70 | 66.76 | 10.99 | 0.001 |
| p 2 | p = 0.098 | p = 0.001 | |||
| Adrenaline | |||||
| Mild acne | 3.34 | 4.49 | 11.00 | 0.00 | 0.059 |
| Moderate acne | 5.36 | 3.93 | 10.14 | 2.16 | 0.001 |
| Severe acne | 5.22 | 1.85 | 12.41 | .98 | 0.001 |
| p 2 | p = 0.129 | p < 0.001 | |||
| NADR/ADR | |||||
| Mild acne | 9.70 | 5.60 | 4.77 | 0.05 | 0.001 |
| Moderate acne | 7.18 | 6.06 | 5.81 | 1.80 | 0.001 |
| Severe acne | 7.98 | 7.82 | 5.5 | 4.26 | 0.015 |
| p 2 | p = 0.064 | p = 0.019 | |||
| Studied Variables | MPG | CG | ||
|---|---|---|---|---|
| Spearman Coefficient | p * | Spearman Coefficient | p * | |
| Glutathione peroxidase | −0.186 | 0.103 | −0.562 * | <0.001 * |
| Adrenaline | 0.326 * | 0.004 * | 0.180 | 0.108 |
| Noradrenaline | 0.365 * | 0.001 * | 0.244 * | 0.028 * |
| NADR/ADR | −0.101 | 0.378 | −0.062 | 0.583 |
| Dopamine | −0.159 | 0.165 | −0.169 | 0.132 |
| HOMA index | 0.344 * | 0.002 * | 0.368 * | <0.001 * |
| Body Mass Index | 0.019 | 0.866 | 0.496 * | <0.001 * |
| LDL values | 0.091 | 0.426 | 0.004 | 0.970 |
| Fasting blood glucose | 0.275 * | 0.015 * | 0.451 * | <0.001 * |
| HDL values | −0.020 | 0.863 | 0.171 | 0.127 |
| TG values | 0.051 | 0.655 | 0.212 | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungau, A.F.; Tit, D.M.; Stoicescu, M.; Moleriu, L.-C.; Muresan, M.; Radu, A.; Brisc, M.C.; Ghitea, T.C. Exploring a New Pathophysiological Association in Acne Vulgaris and Metabolic Syndrome: The Role of Biogenic Amines and Glutathione Peroxidase. Medicina 2024, 60, 513. https://doi.org/10.3390/medicina60030513
Bungau AF, Tit DM, Stoicescu M, Moleriu L-C, Muresan M, Radu A, Brisc MC, Ghitea TC. Exploring a New Pathophysiological Association in Acne Vulgaris and Metabolic Syndrome: The Role of Biogenic Amines and Glutathione Peroxidase. Medicina. 2024; 60(3):513. https://doi.org/10.3390/medicina60030513
Chicago/Turabian StyleBungau, Alexa Florina, Delia Mirela Tit, Manuela Stoicescu, Lavinia-Cristina Moleriu, Mariana Muresan, Ada Radu, Mihaela Cristina Brisc, and Timea Claudia Ghitea. 2024. "Exploring a New Pathophysiological Association in Acne Vulgaris and Metabolic Syndrome: The Role of Biogenic Amines and Glutathione Peroxidase" Medicina 60, no. 3: 513. https://doi.org/10.3390/medicina60030513