Diagnostic Value of Galectin-3 in Exacerbations of Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Measurement of Serum Galectin-3 Levels
2.3. Statistical Analysis
3. Results
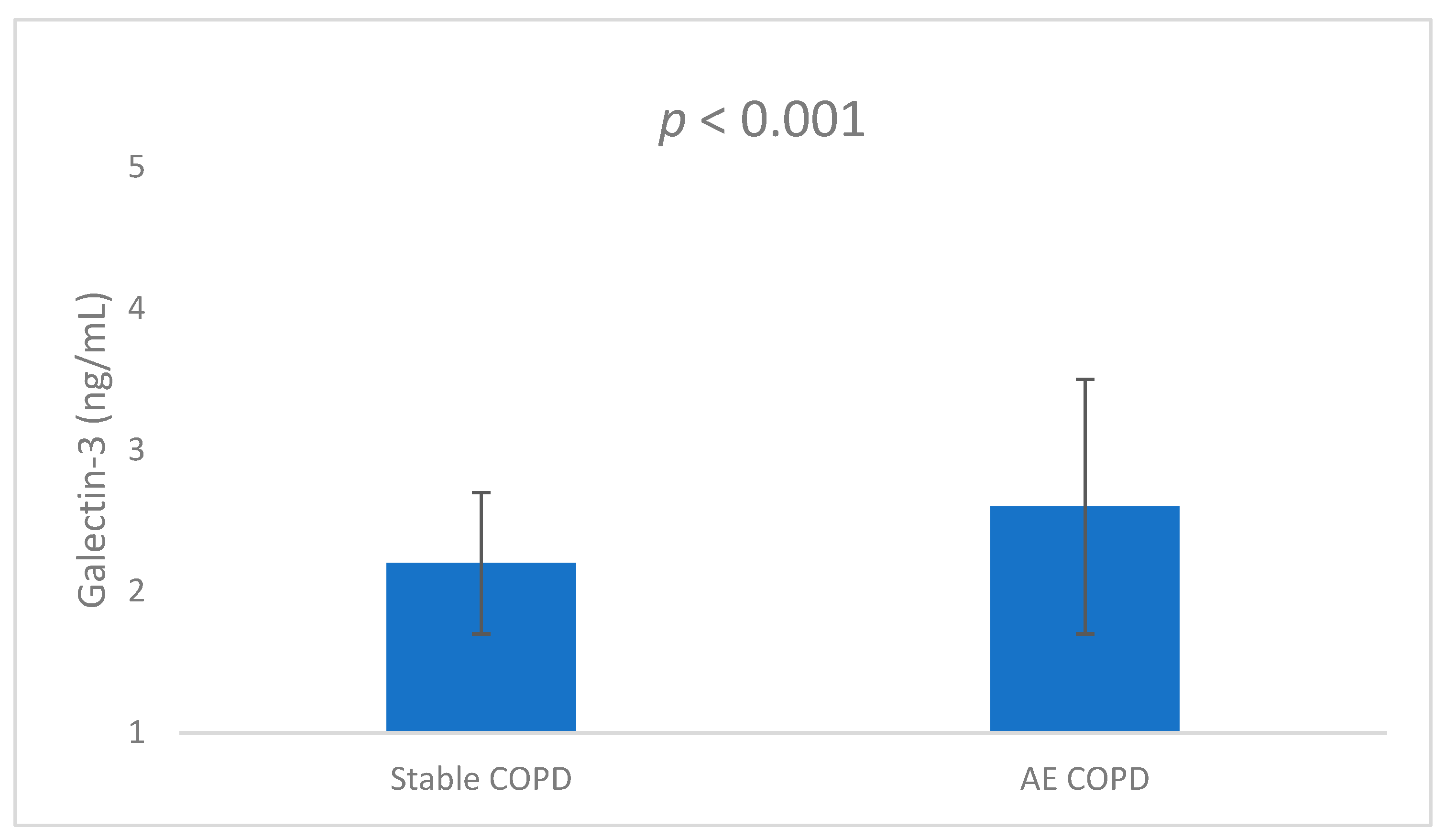
3.1. Comparison of Groups
3.2. ROC Curve Analysis
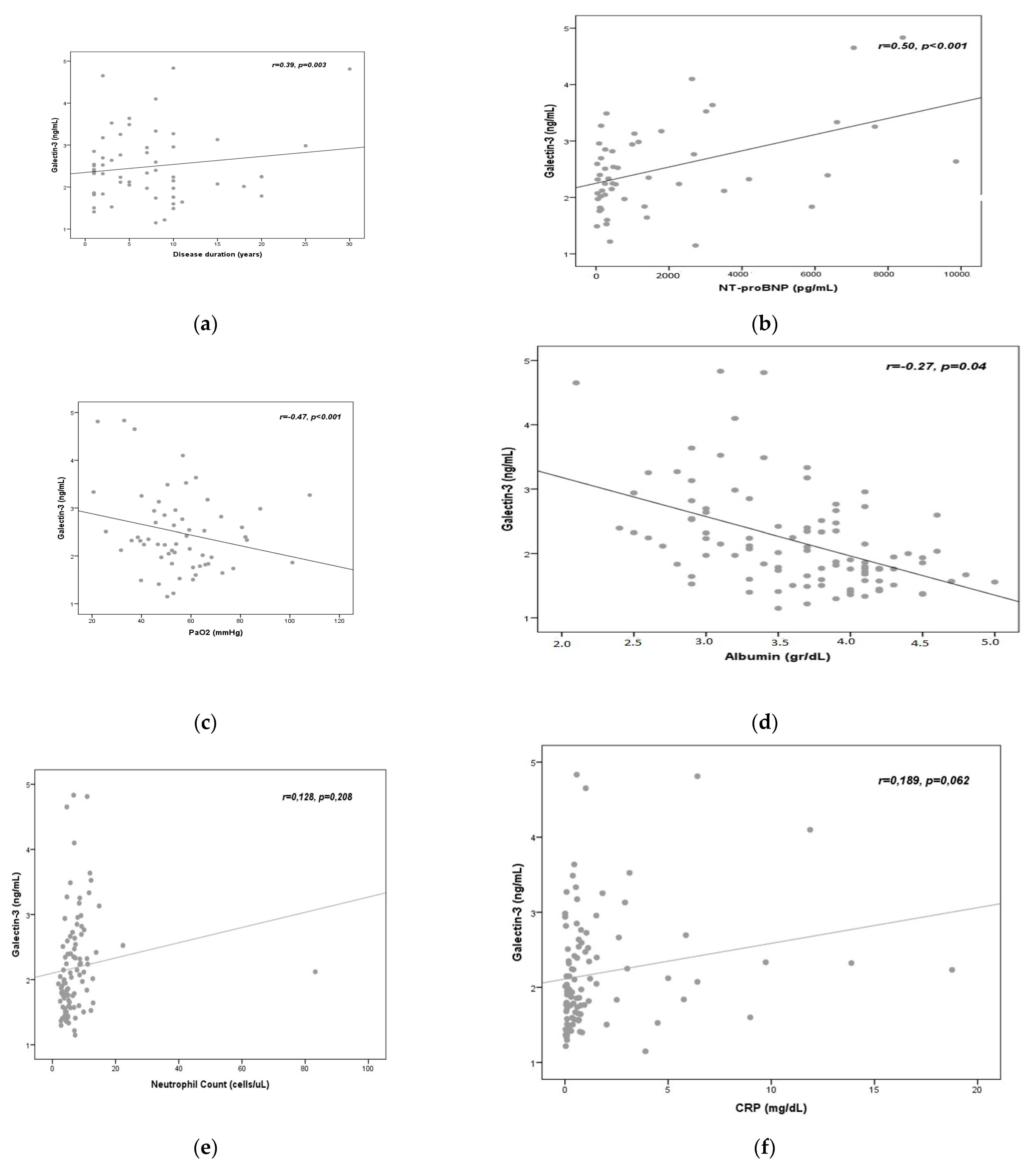
3.3. Correlation Analysis
3.4. Logistic Regression Analysis
3.5. Summary of the Results of the Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halpin, M.G.D.; Criner, G.J.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.F. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36. [Google Scholar] [CrossRef]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Nussbaumer-Ochsner, Y.; Rabe, K.F. Systemic manifestations of COPD. Chest 2011, 139, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Huang, X.; Lin, G.; Xie, C. Endothelial progenitor cell dysfunction in acute exacerbation of chronic obstructive pulmonary disease. Mol. Med. Rep. 2017, 16, 5294–5302. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Celli, B.R.; Owen, C.A. COPD as an endothelial disorder: Endothelial injury linking lesions in the lungs and other organs? Pulm. Circ. 2018, 8, 2045894018758528. [Google Scholar] [CrossRef]
- Cervantes-Alvarez, E.; la Rosa, N.L.-D.; la Mora, M.S.-D.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and fibrosis in severe COVID-19 patients: Galectin-3, a target molecule to consider. Front. Immunol. 2020, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Portacci, A.; Diaferia, F.; Santomasi, C.; Dragonieri, S.; Boniello, E.; Di Serio, F.; Carpagnano, G.E. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021, 187, 106556. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Asuthkar, S.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Research 2020, 9, 1078. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Tartaro, D.L.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Huang, Y.; Mao, P.; Wu, S.; Yang, B.; Yang, Y.; Chen, K.; Liu, X.; Li, Y. The predictive value of plasma galectin-3 for ards severity and clinical outcome. Shock 2017, 47, 331–336. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Molec. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Pilette, C.; Colinet, B.; Kiss, R.; Andre, S.; Kaltner, H.; Gabius, H.-J.; Delos, M.; Vaerman, J.-P.; Decramer, M.; Sibille, Y. Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur. Respir. J. 2007, 29, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wu, X.; Li, S.; Zhai, C.; Wang, J.; Shi, W.; Li, M. Association of Serum Galectin-3 with the Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Med. Sci. Monit. 2017, 23, 4612–4618. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, S.; Leipsic, J.; Paul Man, S.F.; Sin, D.D. The relationship between lung inflammation and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2012, 186, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Donaldson, G.C. Exacerbations of chronic obstructive pulmonary disease. Respir. Care 2003, 48, 1204–1213. [Google Scholar] [PubMed]
- Koutsokera, A.; Stolz, D.; Loukides, S.; Kostikas, K. Systemic biomarkers in exacerbations of COPD: The evolving clinical challenge. Chest 2012, 141, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; MacCallum, P.K.; Paul, E.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Meade, T.W.; Wedzicha, J.A. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb. Haemost. 2000, 84, 210–215. [Google Scholar] [CrossRef]
- Dentener, M.A.; Creutzberg, E.C.; Schols, A.M.; Mantovanib, A.; van‘t Veera, C.; Buurmana, W.A.; Woutersa, E.F.M. Systemic anti-inflammatory mediators in COPD: Increase in soluble interleukin 1 receptor II during treatment of exacerbations. Thorax 2001, 56, 721–726. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Chen, H.; Wang, D.; Bai, C.; Wang, X. Proteomics-based biomarkers in chronic obstructive pulmonary disease. J. Proteome Res. 2010, 9, 2798–2808. [Google Scholar] [CrossRef]
- Medina, A.M.; Marteles, M.S.; Sáiz, E.B.; Martínez, S.S.; Laiglesia, F.R.; Rodríguez, J.A.N.; Pérez-Calvo, J.I. Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur. J. Intern. Med. 2011, 22, 167–171. [Google Scholar] [CrossRef]
- Tian, F.; Song, W.; Wang, L.; Zeng, Q.; Zhao, Z.; Feng, N.; Fan, J.; Wang, Y.; Wang, J.; Ma, X. NT-pro BNP in AECOPD-PH: Old biomarker, new insights-based on a large retrospective case-controlled study. Respir. Res. 2021, 22, 321. [Google Scholar] [CrossRef]
- Chin, K.M.; Rubin, L.J.; Channick, R.; Di Scala, L.; Gaine, S.; Galiè, N.; Ghofrani, H.-A.; Hoeper, M.M.; Lang, I.M.; McLaughlin, V.V.; et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation 2019, 139, 2440–2450. [Google Scholar] [CrossRef]
- Wrobel, J.P.; Thompson, B.R.; Williams, T.J. Mechanisms of pulmonary hypertension in chronic obstructive pulmonary disease: A pathophysiologic review. J. Heart Lung Transplant. 2012, 31, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Turner, A.M. The role of the endothelium in asthma and chronic obstructive pulmonary disease (COPD). Respir. Res. 2017, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, E.; Fois, A.G.; Sotgiu, E.; Mellino, S.; Mangoni, A.A.; Carru, C.; Zinellu, A.; Pirina, P. Serum Albumin Concentrations in Stable Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbu-minemia: Pathogenesis and clinical significance. J. Parenter Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, A.; Kumar, R.; Fang, Y.; Chen, G.; Zhu, Y.; Lin, S. Hypo-albuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020, 92, 2152–2158. [Google Scholar] [CrossRef]
- Huang, W.; Li, C.; Wang, Z.; Wang, H.; Zhou, N.; Jiang, J.; Ni, L.; Zhang, X.A.; Wang, D.-W. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: Hepatic injury analysis from 2623 hospitalized cases. Sci. China Life Sci. 2020, 63, 1678–1687. [Google Scholar] [CrossRef] [PubMed]

| Variable | COPD Group | Mean | Std. Deviation | p |
|---|---|---|---|---|
| Age (years) | Stable COPD | 66.14 | 10.03 | 0.07 |
| COPD with AE | 71.57 | 12.36 | ||
| BMI (kg/m2) | Stable COPD | 27.75 | 7.78 | 0.34 |
| COPD with AE | 26.15 | 4.12 | ||
| Disease duration (years) | Stable COPD | 5.46 | 3.24 | 0.001 |
| COPD with AE | 9.47 | 4.11 | ||
| Smoking (pack-years) | Stable COPD | 55.11 | 16.69 | 0.13 |
| COPD with AE | 46.38 | 15.72 | ||
| Leukocyte (103/L) | Stable COPD | 10.39 | 3.95 | 0.78 |
| COPD with AE | 10.13 | 3.12 | ||
| Hemoglobin (g/dL) | Stable COPD | 14.16 | 2.61 | 0.99 |
| COPD with AE | 14.15 | 2.51 | ||
| Thrombocyte (103/L) | Stable COPD | 288.50 | 65.71 | 0.09 |
| COPD with AE | 246.77 | 49.04 | ||
| Creatinine (mg/dL) | Stable COPD | 0.88 | 0.20 | 0.98 |
| COPD with AE | 0.88 | 0.24 | ||
| ALT (U/L) | Stable COPD | 30.46 | 4.36 | 0.98 |
| COPD with AE | 30.26 | 4.45 | ||
| AST (U/L) | Stable COPD | 22.11 | 13.79 | 0.66 |
| COPD with AE | 23.80 | 15.77 | ||
| Albumin (g/dL) | Stable COPD | 3.46 | 0.48 | 0.009 |
| COPD with AE | 3.13 | 0.45 | ||
| CRP (mg/L) | Stable COPD | 1.20 | 1.11 | 0.01 |
| COPD with AE | 4.53 | 3.44 | ||
| NT-proBNP (pg/mL) | Stable COPD | 606.24 | 399.18 | 0.001 |
| COPD with AE | 2187.56 | 1159.17 | ||
| FVC (%) | Stable COPD | 53.78 | 25.41 | 0.03 |
| COPD with AE | 41.50 | 17.53 | ||
| FEV1 (%) | Stable COPD | 44.93 | 22 | 0.03 |
| COPD with AE | 33.60 | 17.41 | ||
| FEV1/FVC | Stable COPD | 63.82 | 6.96 | 0.49 |
| COPD with AE | 62.47 | 8.10 | ||
| pH | Stable COPD | 7.37 | 0.06 | 0.53 |
| COPD with AE | 7.36 | 0.06 | ||
| PaO2 (mmHg) | Stable COPD | 58.27 | 9.35 | 0.008 |
| COPD with AE | 50.9 | 11.18 | ||
| SaO2 (%) | Stable COPD | 87.01 | 6.04 | 0.01 |
| COPD with AE | 80.49 | 11.72 | ||
| PaCO2 (mmHg) | Stable COPD | 46.32 | 15.31 | 0.03 |
| COPD with AE | 55.46 | 17 | ||
| Galectin (ng/mL) | Stable COPD | 2.08 | 0.52 | 0.001 |
| COPD with AE | 2.86 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berber, N.K.; Atlı, S.; Geçkil, A.A.; Erdem, M.; Kıran, T.R.; Otlu, Ö.; İn, E. Diagnostic Value of Galectin-3 in Exacerbations of Chronic Obstructive Pulmonary Disease. Medicina 2024, 60, 529. https://doi.org/10.3390/medicina60040529
Berber NK, Atlı S, Geçkil AA, Erdem M, Kıran TR, Otlu Ö, İn E. Diagnostic Value of Galectin-3 in Exacerbations of Chronic Obstructive Pulmonary Disease. Medicina. 2024; 60(4):529. https://doi.org/10.3390/medicina60040529
Chicago/Turabian StyleBerber, Nurcan Kırıcı, Siahmet Atlı, Ayşegül Altıntop Geçkil, Mehmet Erdem, Tuğba Raika Kıran, Önder Otlu, and Erdal İn. 2024. "Diagnostic Value of Galectin-3 in Exacerbations of Chronic Obstructive Pulmonary Disease" Medicina 60, no. 4: 529. https://doi.org/10.3390/medicina60040529






