Transcoronary Gradients of Mechanosensitive MicroRNAs as Predictors of Collateral Development in Chronic Total Occlusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Catheterization and Transcoronary Gradient Assessment
2.3. Mechano-miR and Cytokine Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Study Cohort
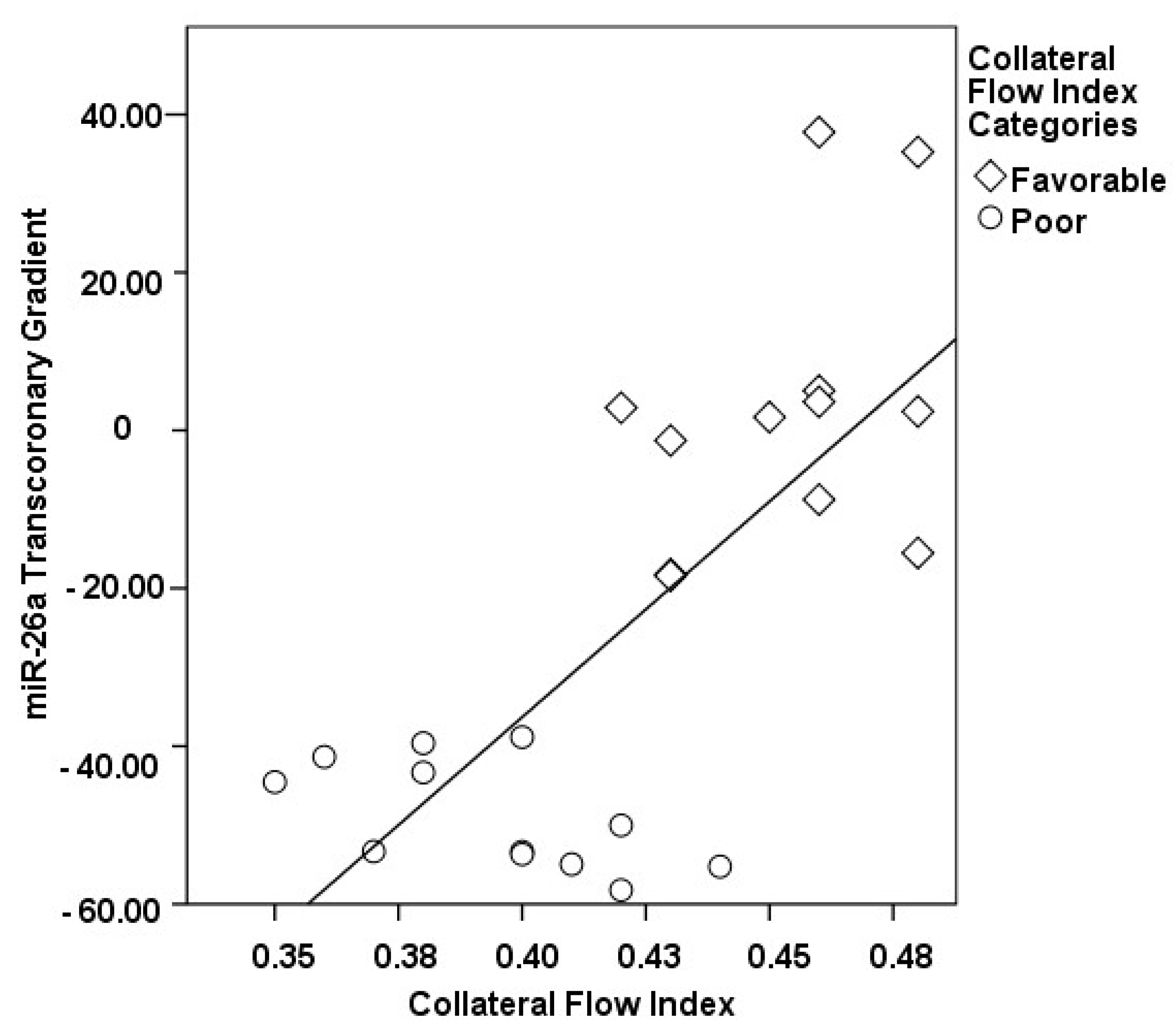
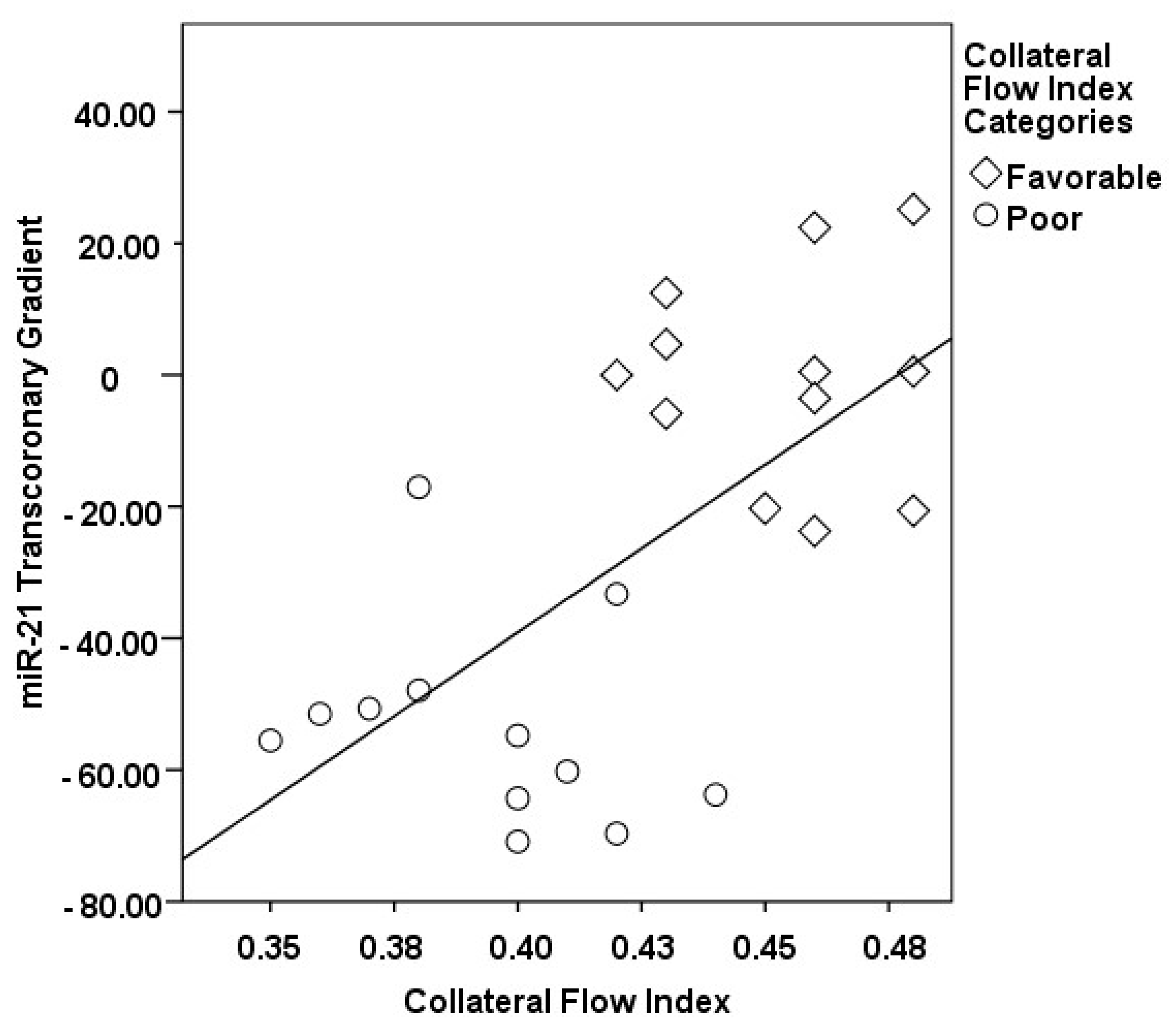
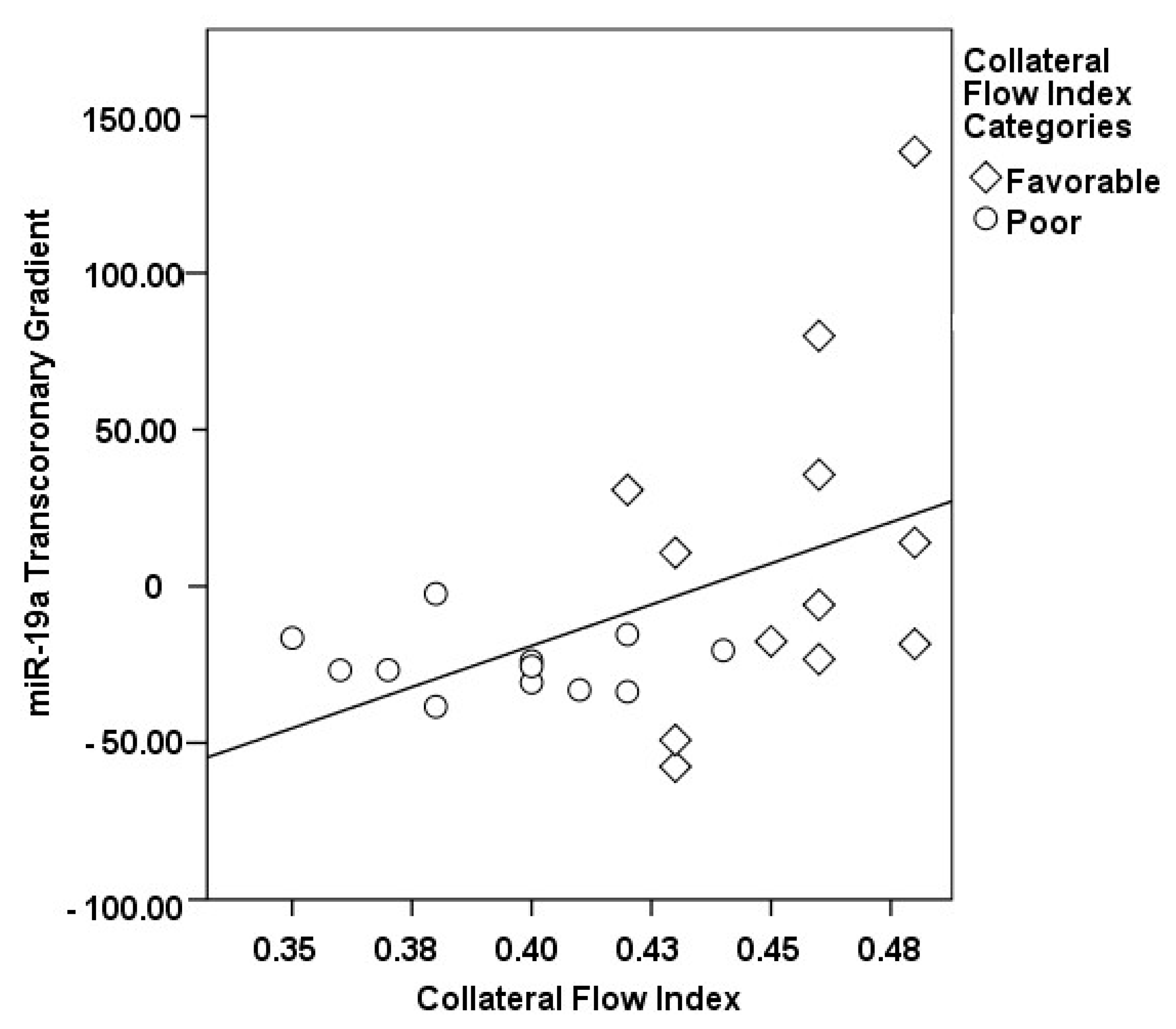
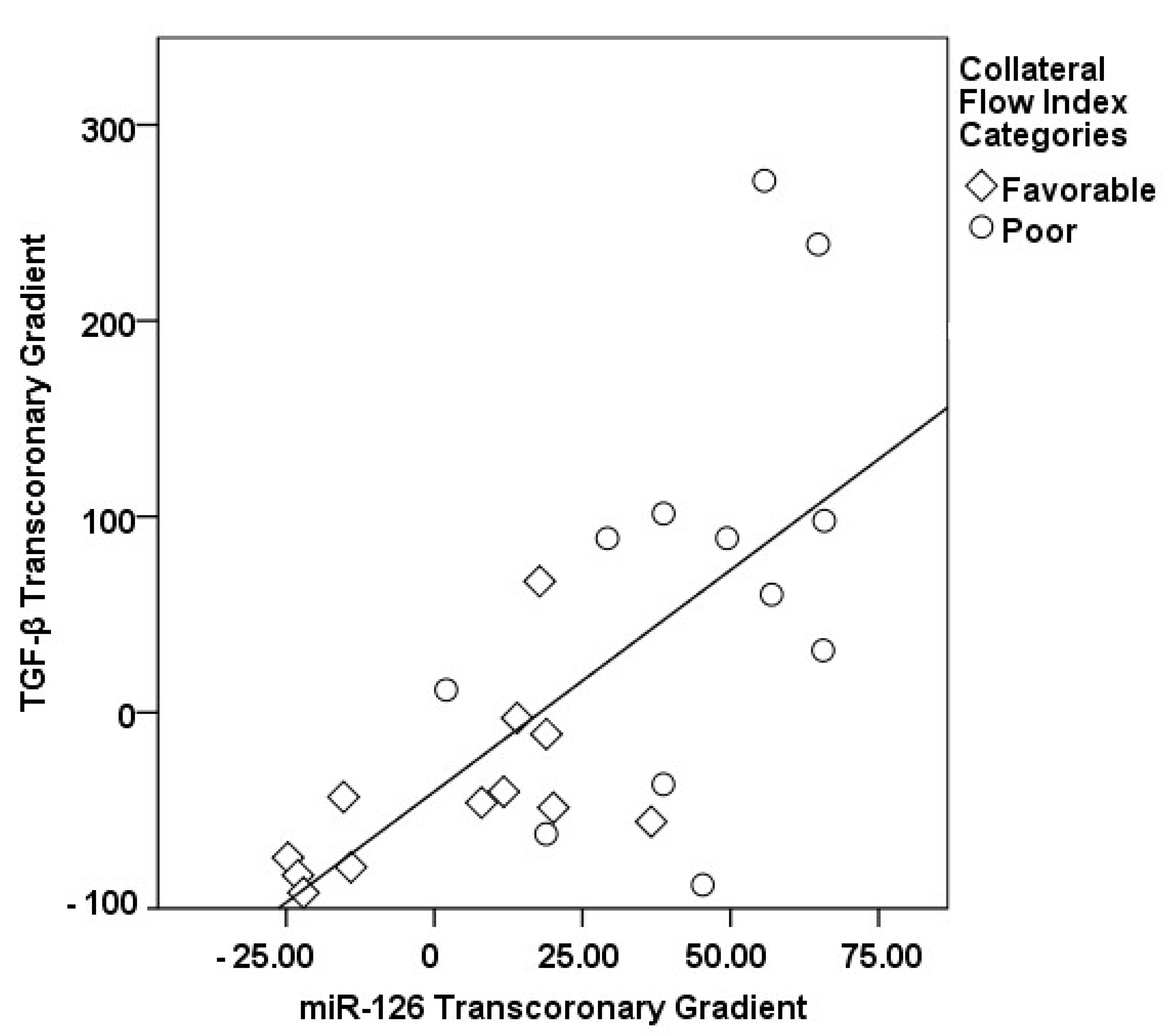
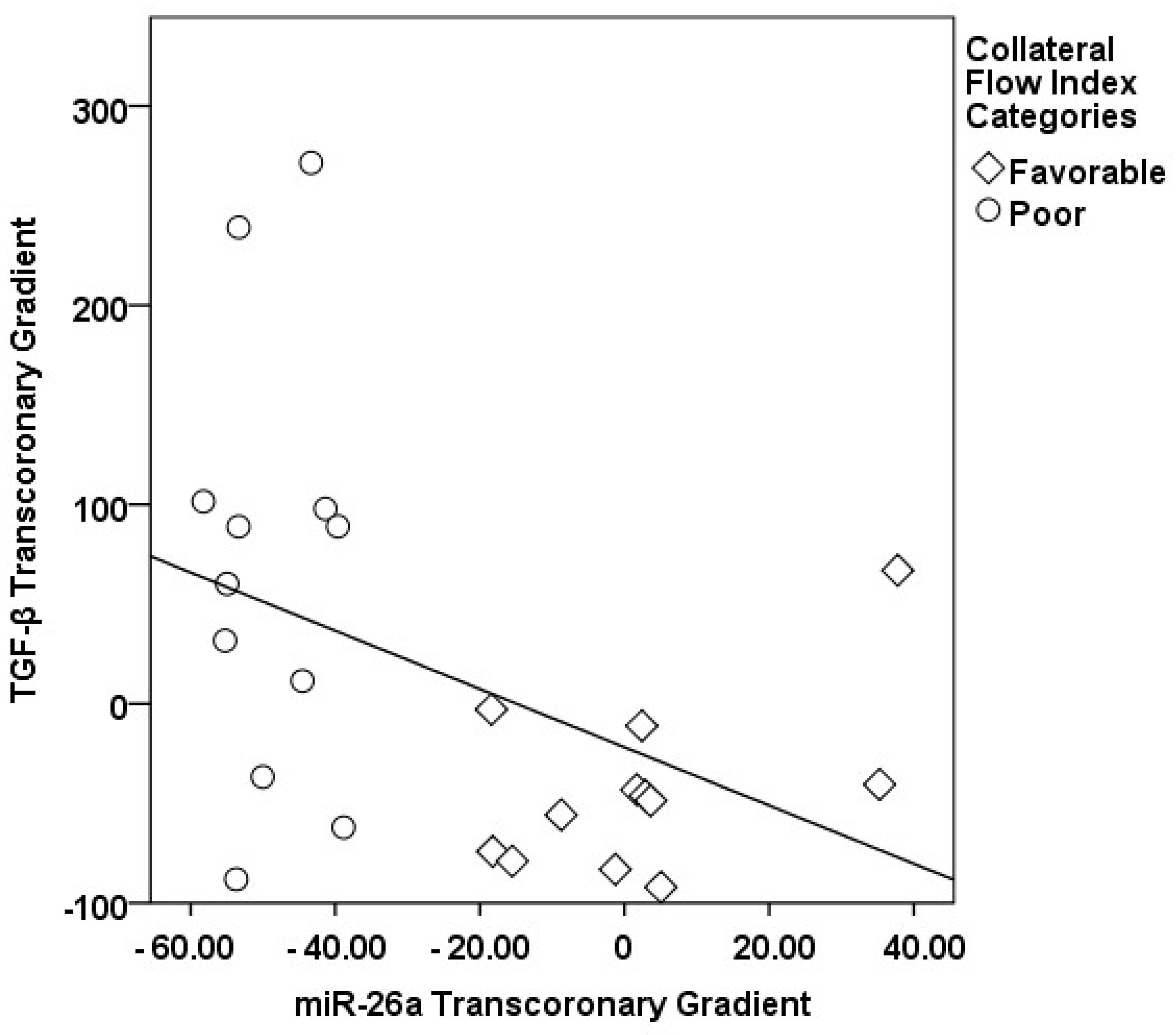
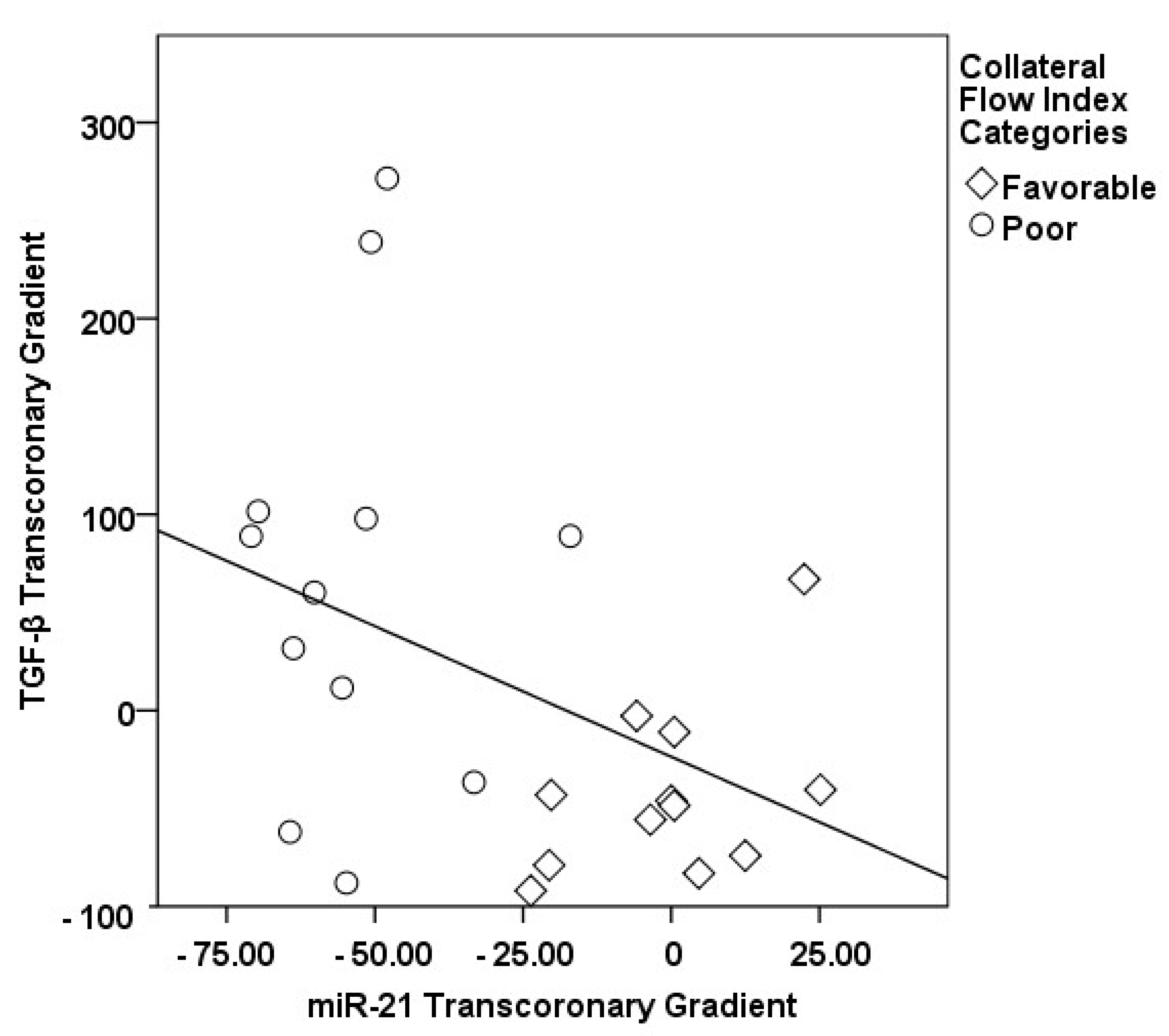
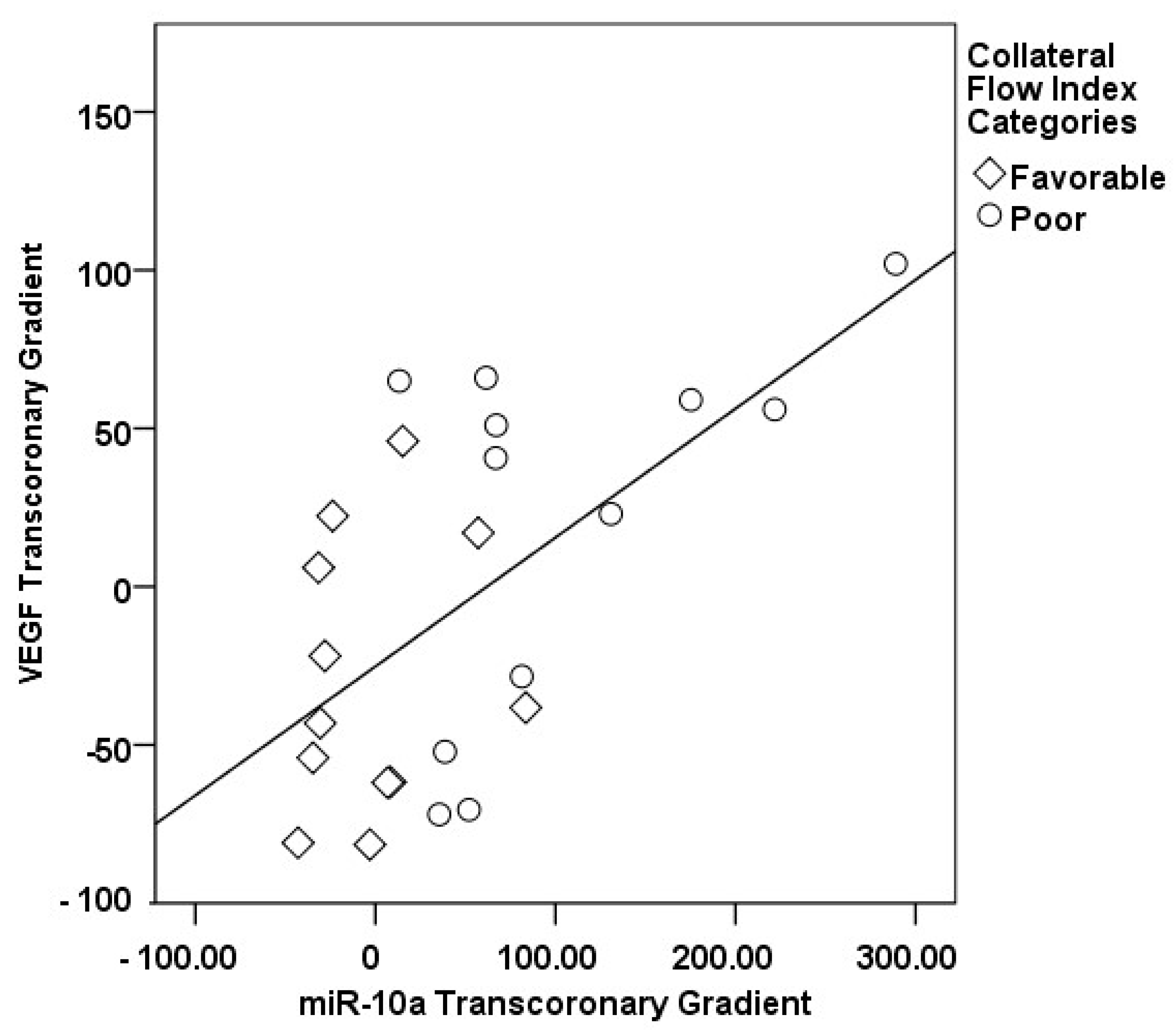
3.2. Transcoronary MicroRNA and Cytokine Profiles: Their Association with the Collateral Flow Index in Chronic Total Occlusion Patients
3.3. Multivariate Analysis of Factors Influencing the Collateral Flow Index in Chronic Total Occlusion Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koelbl, C.O.; Nedeljkovic, Z.S.; Jacobs, A.K. Coronary Chronic Total Occlusion (CTO): A Review. Rev. Cardiovasc. Med. 2018, 19, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Li, Z.; Wang, Q.; Lin, H.; Sun, H. Revascularization of chronic total occlusion coronary artery and cardiac regeneration. Front. Cardiovasc. Med. 2022, 9, 940808. [Google Scholar] [CrossRef] [PubMed]
- Zimarino, M.; D’Andreamatteo, M.; Waksman, R.; Epstein, S.E.; De Caterina, R. The dynamics of the coronary collateral circulation. Nat. Rev. Cardiol. 2014, 11, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hakimzadeh, N.; Elias, J.; Wijntjens, G.W.M.; Theunissen, R.; van Weert, A.; Smulders, M.W.; Akker, N.v.D.; Moerland, P.D.; Verberne, H.J.; Hoebers, L.P.; et al. Monocytic microRNA profile associated with coronary collateral artery function in chronic total occlusion patients. Sci. Rep. 2017, 7, 1532. [Google Scholar] [CrossRef] [PubMed]
- Hakimzadeh, N.; Nossent, A.Y.; van der Laan, A.M.; Schirmer, S.H.; de Ronde, M.W.; Pinto-Sietsma, S.J.; van Royen, N.; Quax, P.H.; Hoefer, I.E.; Piek, J.J. Circulating MicroRNAs Characterizing Patients with Insufficient Coronary Collateral Artery Function. PLoS ONE 2015, 10, e0137035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: Mechanosensitive athero-miRs. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Le, N.-T.; Sandhu, U.G.; Quintana-Quezada, R.A.; Hoang, N.M.; Fujiwara, K.; Abe, J.-I. Flow signaling and atherosclerosis. Cell Mol. Life Sci. 2017, 74, 1835–1858. [Google Scholar] [CrossRef] [PubMed]
- Neth, P.; Nazari-Jahantigh, M.; Schober, A.; Weber, C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc. Res. 2013, 99, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Williams, D.; Sur, S.; Wang, J.Y.; Jo, H. Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vasc. Pharmacol. 2019, 114, 76–92. [Google Scholar] [CrossRef]
- Allahwala, U.K.; Khachigian, L.M.; Nour, D.; Ridiandres, A.; Billah, M.; Ward, M.; Weaver, J.; Bhindi, R. Recruitment and maturation of the coronary collateral circulation: Current understanding and perspectives in arteriogenesis. Microvasc. Res. 2020, 132, 104058. [Google Scholar] [CrossRef]
- Leistner, D.M.; Boeckel, J.-N.; Reis, S.M.; Thome, C.E.; De Rosa, R.; Keller, T.; Palapies, L.; Fichtlscherer, S.; Dimmeler, S.; Zeiher, A.M. Transcoronary gradients of vascular miRNAs and coronary atherosclerotic plaque characteristics. Eur. Heart J. 2016, 37, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Chung, W.-Y.; Herrmann, J.; Jordan, K.L.; Lerman, L.O.; Lerman, A. The association between circulating microRNA levels and coronary endothelial function. PLoS ONE 2014, 9, e109650. [Google Scholar] [CrossRef] [PubMed]
- Keulards, D.C.; Alsanjari, O.; Keeble, T.R.; Vlaar, P.-J.; Kelly, P.A.; Tang, K.H.; Khan, S.; Cockburn, J.; Pijls, N.H.; Hildick-Smith, D.; et al. Changes in coronary collateral function after successful chronic total occlusion percutaneous coronary intervention. EuroIntervention 2022, 18, e920–e928. [Google Scholar] [CrossRef] [PubMed]
- Somsen, Y.B.O.; de Winter, R.W.; Giunta, R.; Schumacher, S.P.; van Diemen, P.A.; Jukema, R.A.; Stuijfzand, W.J.; Danad, I.; Witte, B.I.L.; Verouden, N.J.; et al. Collateral grading systems in retrograde percutaneous coronary intervention of chronic total occlusions. Catheter. Cardiovasc. Interv. 2023, 102, 844–856. [Google Scholar] [CrossRef]
- Rossello, X.; Pujadas, S.; Serra, A.; Bajo, E.; Carreras, F.; Barros, A.; Cinca, J.; Pons-Lladó, G.; Vaquerizo, B. Assessment of Inducible Myocardial Ischemia, Quality of Life, and Functional Status After Successful Percutaneous Revascularization in Patients with Chronic Total Coronary Occlusion. Am. J. Cardiol. 2016, 117, 720–726. [Google Scholar] [CrossRef]
- Keustermans, G.C.; Hoeks, S.B.; Meerding, J.M.; Prakken, B.J.; de Jager, W. Cytokine assays: An assessment of the preparation and treatment of blood and tissue samples. Method 2013, 61, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kowdley, K.V. Method for microRNA isolation from clinical serum samples. Anal. Biochem. 2012, 431, 69–75. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Hemingway, H.; Lansky, A.J.; Knapp, G.; Pitt, B.; Seiler, C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur. Heart J. 2012, 33, 614–621. [Google Scholar] [CrossRef]
- Pohl, T.; Seiler, C.; Billinger, M.; Herren, E.; Wustmann, K.; Mehta, H.; Windecker, S.; Eberli, F.R.; Meier, B. Frequency distribution of collateral flow and factors influencing collateral channel development. Functional collateral channel measurement in 450 patients with coronary artery disease. J. Am. Coll. Cardiol. 2001, 38, 1872–1878. [Google Scholar] [CrossRef]
- Zorkun, C.; Akkaya, E.; Zorlu, A.; Tandoğan, I. Determinants of coronary collateral circulation in patients with coronary artery disease. Anadolu Kardiyol. Derg. 2013, 13, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.A.; Sandesara, P.B.; Jain, V.; Gold, M.E.; Vatsa, N.; Desai, S.R.; Hassan, M.E.; Yuan, C.; Ko, Y.A.; Alkhoder, A.; et al. High Sensitivity Troponin Level and Benefits of Chronic Total Occlusion Revascularization. J. Am. Heart Assoc. 2023, 12, e031431. [Google Scholar] [CrossRef]
- Artha, I.M.J.R.; Bakta, I.M.; Manuaba, I.B.P.; Wita, I.W.; Rohman, M.S.; Astawa, I.N.M.; Bhargah, A. The Effects of Percutaneous Coronary Intervention on Biomarkers and Quality of Life in Patients with Chronic Total Coronary Artery Obstruction. Cardiol. Res. 2023, 14, 69–78. [Google Scholar] [CrossRef]
- Dai, Y.; Chang, S.; Wang, S.; Shen, Y.; Li, C.; Huang, Z.; Lu, H.; Qian, J.; Ge, L.; Wang, Q.; et al. The preservation effect of coronary collateral circulation on left ventricular function in chronic total occlusion and its association with the expression of vascular endothelial growth factor A. Adv. Clin. Exp. Med. 2020, 29, 493–497. [Google Scholar] [CrossRef]
- Shoeibi, S.; Mozdziak, P.; Mohammadi, S. Important signals regulating coronary artery angiogenesis. Microvasc. Res. 2018, 117, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Qi, X.; Cui, Y.; Wu, Y.; Zhou, S.; Zhang, M. Serum VEGF: Diagnostic Value of Acute Coronary Syndrome from Stable Angina Pectoris and Prognostic Value of Coronary Artery Disease. Cardiol. Res. Pract. 2020, 2020, 6786302. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.A.; Mandinov, L.; Seiler, C.; Hess, O.M. Impact of exercise-induced coronary vasomotion on anti-ischemic therapy. Coron. Artery Dis. 2000, 11, 363–369. [Google Scholar] [CrossRef]
- Stähli, B.E.; Gebhard, C.; Gick, M.; Ferenc, M.; Mashayekhi, K.; Buettner, H.J.; Neumann, F.; Toma, A. Impact of anemia on long-term outcomes after percutaneous coronary intervention for chronic total occlusion. Catheter. Cardiovasc. Interv. 2018, 91, 226–233. [Google Scholar] [CrossRef]
- Cardinale, A.; Nastrucci, C.; Cesario, A.; Russo, P. Nicotine: Specific role in angiogenesis, proliferation and apoptosis. Crit. Rev. Toxicol. 2012, 42, 68–89. [Google Scholar] [CrossRef]
- Jo, H.-N.; Kang, H.; Lee, A.; Choi, J.; Chang, W.; Lee, M.-S.; Kim, J. Endothelial miR-26a regulates VEGF-Nogo-B receptor-mediated angiogenesis. BMB Rep. 2017, 50, 384–389. [Google Scholar] [CrossRef]
- Hutcheson, R.; Chaplin, J.; Hutcheson, B.; Borthwick, F.; Proctor, S.; Gebb, S.; Jadhav, R.; Smith, E.; Russell, J.C.; Rocic, P. miR-21 normalizes vascular smooth muscle proliferation and improves coronary collateral growth in metabolic syndrome. FASEB J. 2014, 28, 4088–4099. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, G.; Wang, X.; Zhang, B.; Zhao, T.; Wu, L. Correlation analysis of serum miR-21 and miR-210 with hs-CRP, TNF-α, IL-6, and ICAM-1 in patients with sepsis after burns. Burns 2022, 48, 633–638. [Google Scholar] [CrossRef] [PubMed]
- van Solingen, C.; Seghers, L.; Bijkerk, R.; Duijs, J.M.; Roeten, M.K.; van Oeveren-Rietdijk, A.M.; Baelde, H.J.; Monge, M.; Vos, J.B.; de Boer, H.C.; et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J. Cell. Mol. Med. 2009, 13, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Zhou, K.; Kao, G.; Xiao, J. MicroRNA-126 and VEGF enhance the function of endothelial progenitor cells in acute myocardial infarction. Exp. Ther. Med. 2022, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Ahmadi, R.; Fallah, S.; Fadaei, R.; Kazemian, E. Lower Expression of miR-10a in Coronary Artery Disease and its Association with Pro/Anti-Inflammatory Cytokines. Clin. Lab. 2018, 64, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Landskroner-Eiger, S.; Qiu, C.; Perrotta, P.; Siragusa, M.; Lee, M.Y.; Ulrich, V.; Luciano, A.K.; Zhuang, Z.W.; Corti, F.; Simons, M.; et al. Endothelial miR-17~92 cluster negatively regulates arteriogenesis via miRNA-19 repression of WNT signaling. Proc. Natl. Acad. Sci. USA 2015, 112, 12812–12817. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Okamoto, A.; Yokota, M.; Shimizu, K.; Asai, T.; Dewa, T.; Oku, N. Development of a miR-92a delivery system for anti-angiogenesis-based cancer therapy. J. Gene Med. 2013, 15, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gorski, D.H. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood 2008, 111, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.W.; Qiu, H.; Jo, H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H1762–H1769. [Google Scholar] [CrossRef]
- Ji, X.; Hua, H.; Shen, Y.; Bu, S.; Yi, S. Let-7d modulates the proliferation, migration, tubulogenesis of endothelial cells. Mol. Cell. Biochem. 2019, 462, 75–83. [Google Scholar] [CrossRef]
- Qatrun Nada, D.; Masniza, M.L.; Abdullah, N.; Marlini, M.; Elias, M.H.; Pathmanathan, S.G.; Hayati, A.R.; Fadlul Azim, F.; Hamid, A.A.; Nur Fariha, M.M. Distinct microRNA expression pattern in breast cancer cells following anti-neoplastic treatment: A systematic review and functional analysis of microRNA target genes. Malays. J. Pathol. 2022, 44, 367–385. [Google Scholar] [PubMed]
- Liu, S.; Ni, S.; Wang, C.; Yang, K.; Yang, Y.; Li, L.; Liu, J.; Wang, Y.; Qin, Y.; Zhang, M. Association of serum cytokines with coronary chronic total occlusion and their role in predicting procedural outcomes. Hell. J. Cardiol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Götze, A.M.; Schubert, C.; Jung, G.; Dörr, O.; Liebetrau, C.; Hamm, C.W.; Schmitz-Rixen, T.; Troidl, C.; Troidl, K. IL10 Alters Peri-Collateral Macrophage Polarization and Hind-Limb Reperfusion in Mice after Femoral Artery Ligation. Int. J. Mol. Sci. 2020, 21, 2821. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Jandt, E.; Krack, A.; Schwarz, G.; Mutschke, O.; Kuethe, F.; Ferrari, M.; Figulla, H.R. Growth factors in the collateral circulation of chronic total coronary occlusions: Relation to duration of occlusion and collateral function. Circulation 2004, 110, 1940–1945. [Google Scholar] [CrossRef]
- Loboda, A.; Sobczak, M.; Jozkowicz, A.; Dulak, J. TGF-β1/Smads and miR-21 in Renal Fibrosis and Inflammation. Mediat. Inflamm. 2016, 2016, 8319283. [Google Scholar] [CrossRef]

| Parameters | Healthy Subjects Group I (n = 30) | CAD without CTO Group II (n = 33) | CTO Patients Group III (n = 63) | p-Value |
|---|---|---|---|---|
| Age, years | 54.8 ± 12.0 | 52.0 ± 11.0 | 55.3 ± 10.8 | 0.374 |
| Male, % | 80 | 69.7 | 81 | 0.427 |
| BMI, kg/m2 | 28.6 ± 4.0 | 28.7 ± 4.9 | 27.9 ± 3.8 | 0.593 |
| Hypertension, % | 60 | 65 | 69.4 | 0.672 |
| Dyslipidemia, % | 46.7 | 63.6 | 66.7 | 0.170 |
| Diabetes, % | 20 | 21.2 | 17.5 | 0.895 |
| Current smoking, % | 53.3 | 48.5 | 39.7 | 0.294 |
| Acetylsalicylic acid, % | 10 | 100 | 89.9 | <0.001 |
| Clopidogrel, % | 3.3 | 21.2 | 23.8 | 0.050 |
| Beta blocking agents, % | 33.3 | 57.6 | 68.3 | 0.006 |
| Calcium channel blockers, % | 13.3 | 15.2 | 14.3 | 0.979 |
| RAAS inhibitors, % | 70 | 66.7 | 69.8 | 0.942 |
| Statins, % | 46.7 | 45.5 | 55.6 | 0.562 |
| LV EF, % | 59.2 ± 6.3 | 61.5 ± 5.7 | 59.6 ± 5.9 | 0.228 |
| LV diastolic dysfunction, % | 46.7 | 48.5 | 73 | 0.014 |
| Culprit lesion | ||||
| LAD, % | - | 33.3 | 42.4 | 0.204 |
| Cx, % | - | 35.5 | 31.7 | |
| RCA, % | - | 31.2 | 25.9 | |
| Symptom duration | ||||
| 3–12 months, % | 0 | 18.2 | 47.1 | <0.001 |
| >12 months, % | 0 | 18.2 | 45.1 | |
| Ischemic burden | ||||
| <5% | 100 | 48.1 | 21.4 | <0.001 |
| 5–10% | 0 | 29.6 | 42.9 | |
| >10% | 0 | 22.2 | 35.7 | |
| Hemoglobin, g/dL | 13.3 ± 1.8 | 13.0 ± 2.2 | 13.5 ± 1.8 | 0.429 |
| Creatinine, g/dL | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.344 |
| Leukocyte count, ×109/L | 10.0 ± 2.2 | 11.5 ± 2.8 | 10.4 ± 3.5 | 0.156 |
| NLR | 4.4 ± 3.8 | 4.4 ± 3.0 | 4.2 ± 5.6 | 0.983 |
| Glucose, mg/dL | 112.5 ± 39.2 | 121.9 ± 36.5 | 128.8 ± 47.9 | 0.285 |
| Total cholesterol, mg/dL | 183.5 ± 47.2 | 198.2 ± 40.2 | 194.0 ± 42.1 | 0.377 |
| LDL cholesterol, mg/dL | 116.3 ± 28.3 | 113.0 ± 37.6 | 109.5 ± 35.8 | 0.702 |
| HDL cholesterol, mg/dL | 36.6 ± 9.2 | 42.9 ± 13.0 | 42.1 ± 12.8 | 0.078 |
| Non-HDL cholesterol, mg/dL | 146.9 ± 47.2 | 155.3 ± 40.8 | 152.0 ± 41.1 | 0.733 |
| Remnant cholesterol, mg/dL | 29.7 ± 39.0 | 26.2 ± 34.8 | 27.1 ± 24.8 | 0.900 |
| TGF-β, ng/mL | 769.8 ± 506.7 | 1016.0 ± 606.9 | 967.8 ± 480.7 | 0.201 |
| IL-1β, ng/mL | 4.5 ± 4.3 | 6.7 ± 4.7 | 7.6 ± 5.0 1 | 0.042 |
| MMP-2, ng/mL | 464.7 ± 233.2 | 349.6 ± 194.6 | 311.9 ± 200.2 1 | 0.016 |
| IL-10, ng/mL | 12.2 ± 7.1 | 10.4 ± 4.3 | 12.5 ± 5.8 | 0.370 |
| VEGF, ng/mL | 683.3 ± 501.6 | 386.5 ± 214.7 1 | 363.2 ± 211.8 1 | 0.001 |
| hs-Troponin, ng/mL | 0.4 ± 0.9 | 5.5 ± 14.3 | 6.3 ± 10.1 1 | 0.032 |
| NT-pro BNP, pg/mL | 166.8 ± 86.6 | 262.1 ± 154.7 | 217.0 ± 178.4 | 0.092 |
| hs-CRP, ng/mL | 3.6 ± 4.0 | 3.1 ± 2.5 | 3.4 ± 2.1 | 0.785 |
| Parameters | Favorable Collateral (n = 17) | Poor Collateral (n = 19) | p-Value |
|---|---|---|---|
| Age, years | 55.5 ± 8.2 | 55.1 ± 12.6 | 0.907 |
| Male, % | 82.4 | 73.7 | 0.532 |
| Hypertension, % | 70.6 | 73.7 | 0.836 |
| Diabetes, % | 29.4 | 10.5 | 0.153 |
| Dyslipidemia, % | 64.7 | 63.2 | 0.923 |
| Current smoking, % | 41.2 | 26.3 | 0.445 |
| RAAS inhibitors, % | 64.7 | 73.7 | 0.559 |
| Statins, % | 52.9 | 63.2 | 0.535 |
| Beta blocking agents, % | 70.6 | 68.4 | 0.888 |
| Calcium channel blockers, % | 5.9 | 26.3 | 0.101 |
| Acetylsalicylic acid, % | 94.1 | 94.7 | 0.935 |
| Clopidogrel, % | 23.5 | 15.8 | 0.558 |
| Culprit Lesion | |||
| LAD, % | 42.4 | 43.2 | 0.359 |
| Cx, % | 11.8 | 15.8 | |
| RCA, % | 45.9 | 41.1 | |
| Symptom duration | |||
| 3–12 months, % | 52.9 | 58.8 | 0.345 |
| >12 months, % | 35.3 | 41.2 | |
| Ischemic burden | |||
| <5% | 21.4 | 20.0 | 0.979 |
| 5–10% | 35.7 | 33.3 | |
| >10% | 42.9 | 46.7 | |
| CFI | 0.45 ± 0.02 | 0.38 ± 0.03 | <0.001 |
| LV EF, % | 59.18 ± 6.28 | 59.37 ± 6.12 | 0.927 |
| LV diastolic dysfunction, % | 70.6 | 68.4 | 0.888 |
| Hemoglobin, g/dL | 13.57 ± 2.43 | 13.27 ± 1.71 | 0.672 |
| Leukocyte count, ×109/L | 10.67 ± 4.11 | 9.68 ± 3.16 | 0.423 |
| Platelet count, ×109/L | 235.53 ± 53.66 | 231.21 ± 53.54 | 0.811 |
| Neutrophil, ×109/L | 5.04 ± 2.39 | 4.92 ± 1.40 | 0.847 |
| Lymphocyte count, ×109/L | 1.56 ± 0.56 | 1.80 ± 0.90 | 0.385 |
| NLR | 5.69 ± 9.89 | 3.49 ± 1.31 | 0.356 |
| Glucose, mg/dL | 105.18 ± 29.12 | 137.11 ± 51.59 | 0.032 |
| Creatinine, mg/dL | 0.99 ± 0.44 | 0.90 ± 0.23 | 0.447 |
| Total Cholesterol, mg/dL | 189.29 ± 38.08 | 191.00 ± 36.31 | 0.891 |
| HDL Cholesterol, mg/dL | 46.35 ± 15.80 | 39.68 ± 10.14 | 0.137 |
| LDL Cholesterol, mg/dL | 111.88 ± 26.86 | 101.47 ± 35.09 | 0.329 |
| Non-HDL Cholesterol, mg/dL | 142.94 ± 35.64 | 151.32 ± 38.26 | 0.503 |
| Remnant Cholesterol, mg/dL | 21.65 ± 21.43 | 36.32 ± 30.44 | 0.108 |
| High-sensitive Troponin, ng/mL | 6.63 ± 12.04 | 5.99 ± 8.40 | 0.853 |
| NT-Pro BNP, pg/mL | 195.75 ± 119.01 | 269.73 ± 237.12 | 0.268 |
| hs-CRP, ng/L | 3.59 ± 2.24 | 2.70 ± 2.25 | 0.257 |
| ∆ TGF-β, % | −35.30 ± 49.21 | 107.33 ± 148.21 | 0.003 |
| ∆ IL-1β, % | −87.09 ± 127.50 | −44.73 ± 142.03 | 0.417 |
| ∆ IL-10, % | 12.90 ± 35.68 | 13.11 ± 41.23 | 0.988 |
| ∆ VEGF, % | −27.78 ± 41.39 | 25.05 ± 52.03 | 0.006 |
| ∆ MMP-2, % | 12.78 ± 68.89 | −26.81 ± 82.38 | 0.173 |
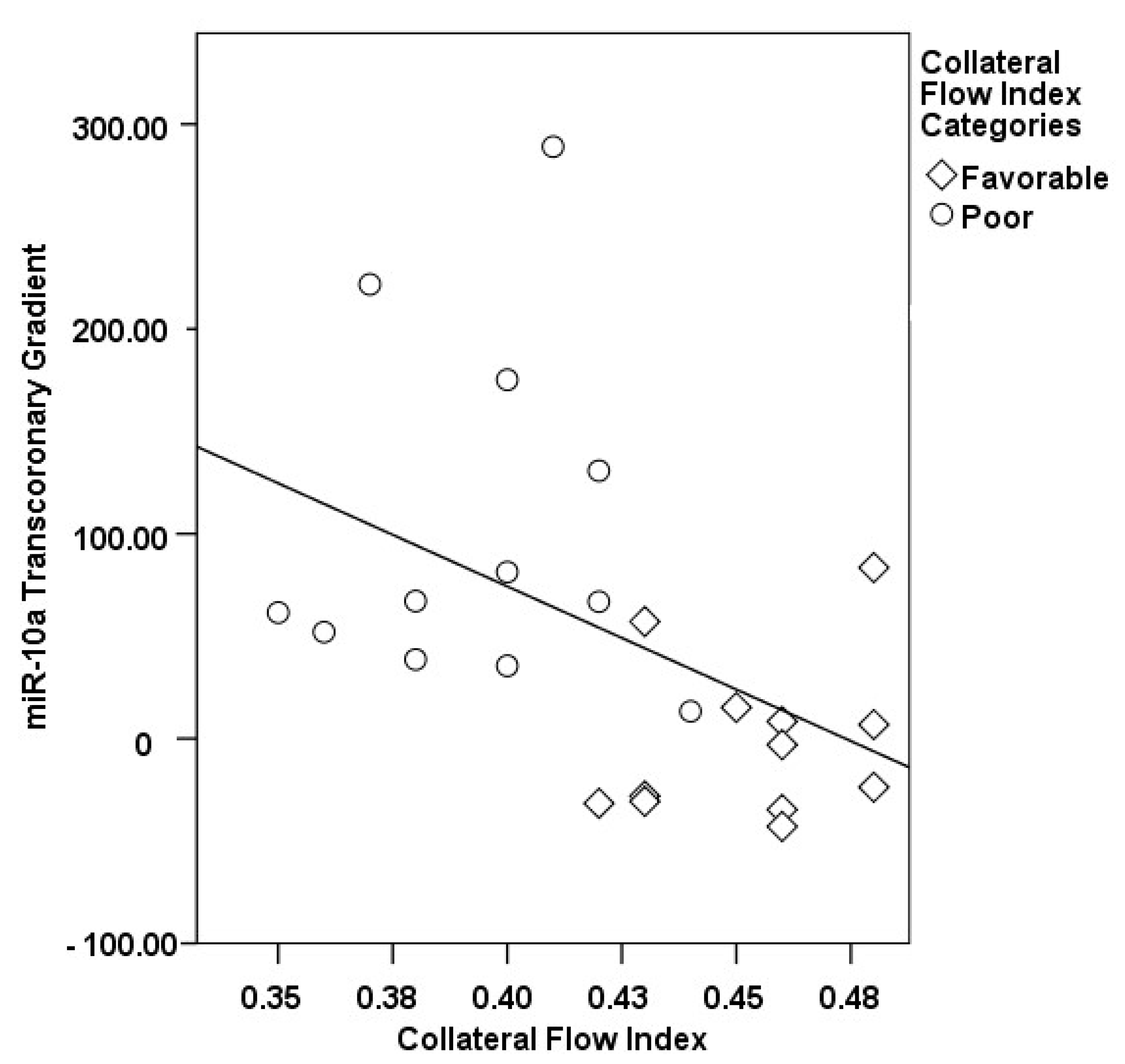
| ∆ miR-10a, % | −1.98 ± 39.17 | 102.76 ± 84.65 | 0.001 |
| ∆ miR-19a, % | 11.49 ± 55.21 | −24.46 ± 9.79 | 0.037 |
| ∆ miR-21, % | −53.30 ± 15.43 | −0.69 ± 15.82 | <0.001 |
| ∆ miR-23b, % | −21.36 ± 23.64 | 3.49 ± 28.38 | 0.029 |
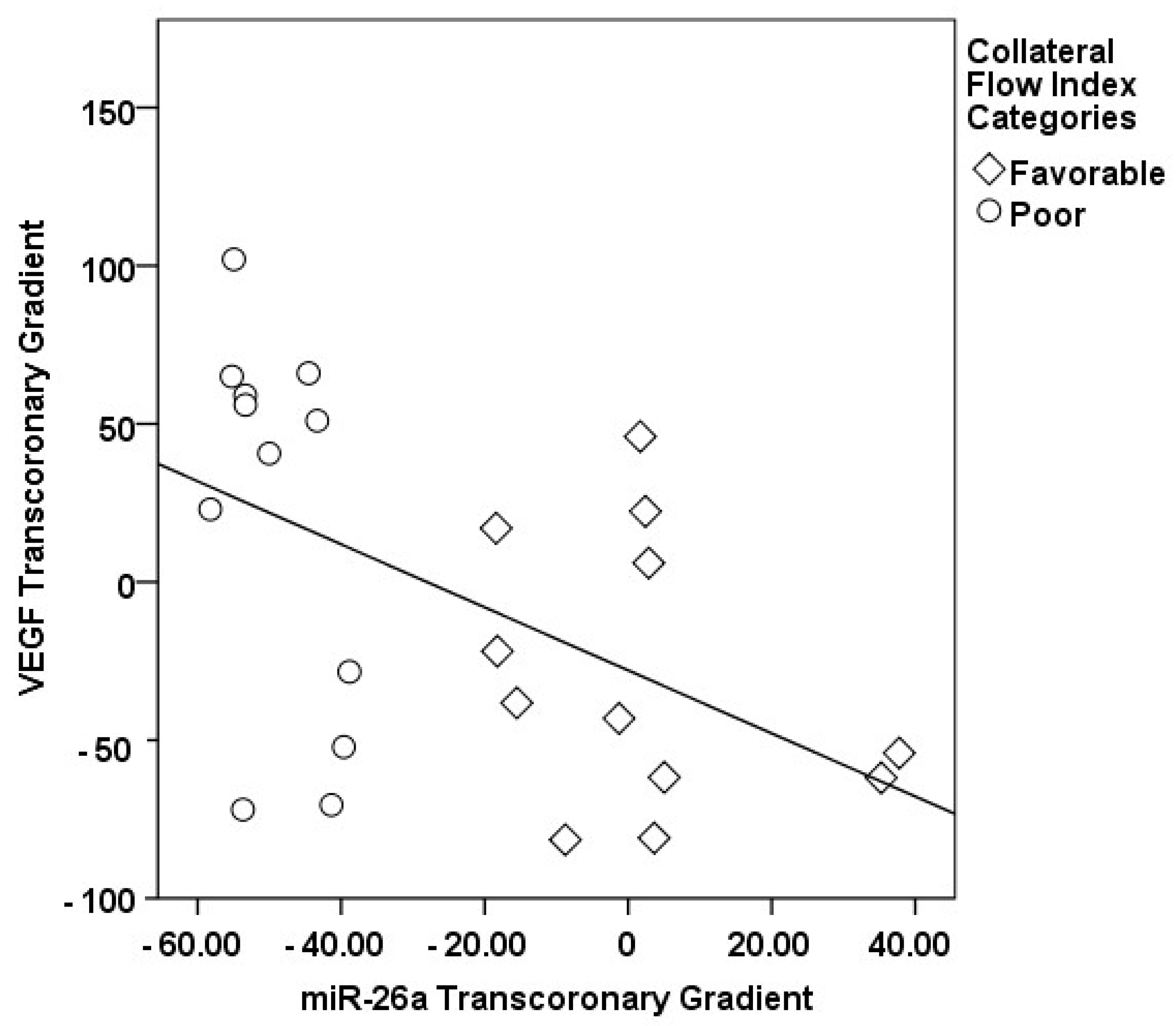
| ∆ miR-26a, % | −48.88 ± 6.89 | 2.20 ± 18.23 | <0.001 |
| ∆ miR-92a, % | 54.72 ± 67.35 | 5.42 ± 33.58 | 0.033 |
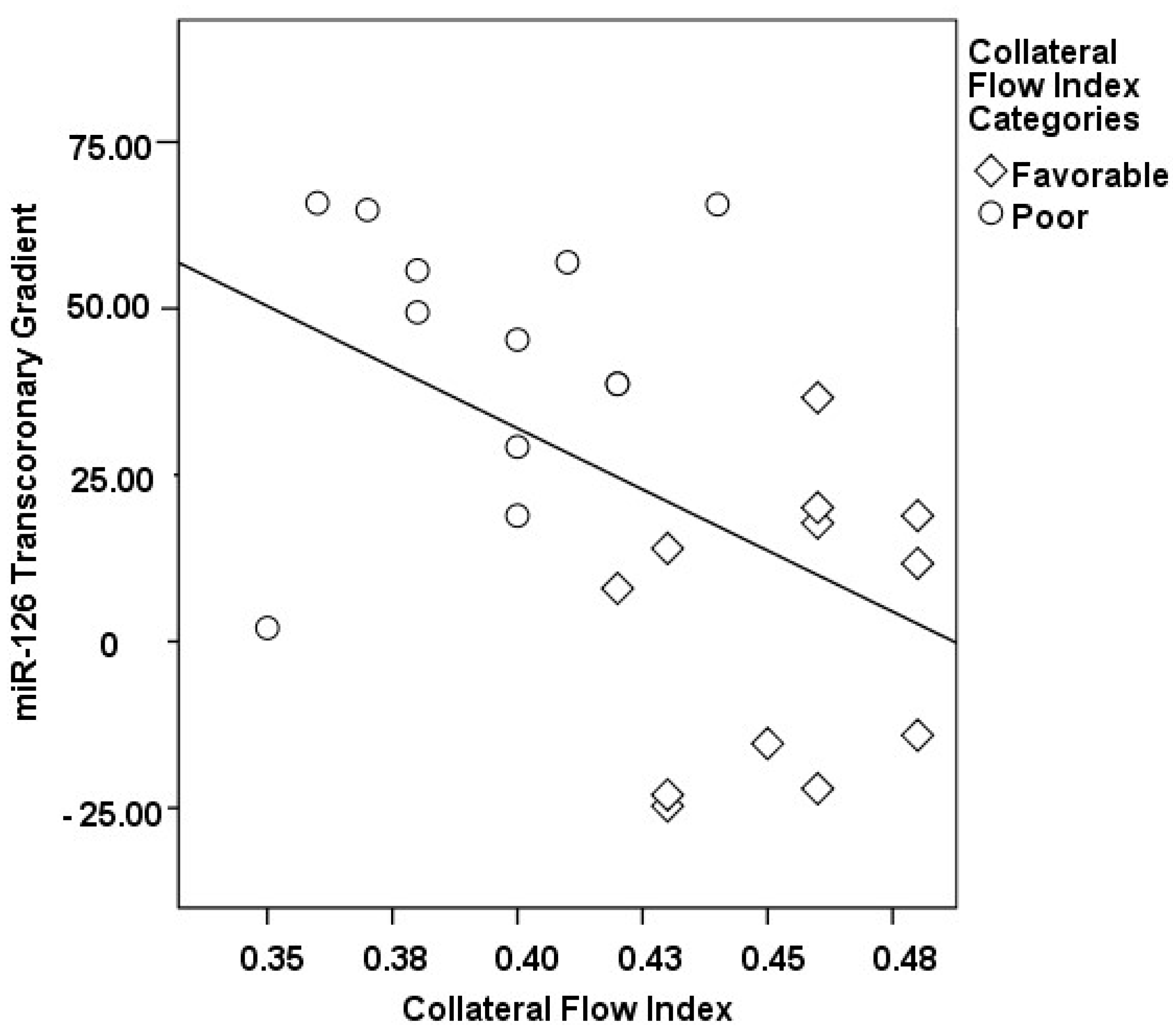
| ∆ miR-126, % | 44.26 ± 19.96 | 2.32 ± 20.91 | <0.001 |
| ∆ miR-130a, % | 63.79 ± 37.58 | 2.98 ± 24.70 | <0.001 |
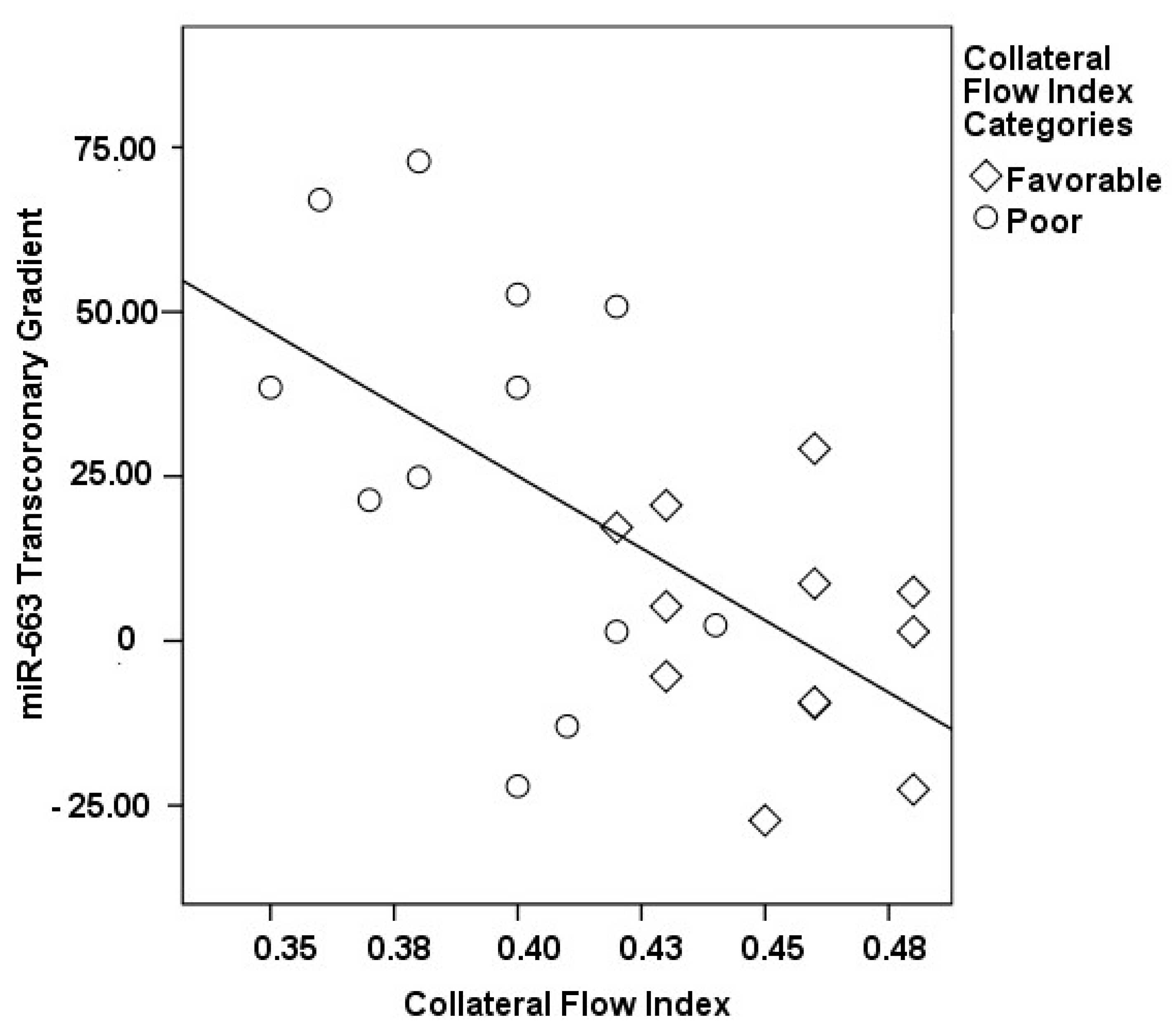
| ∆ miR-663, % | 27.94 ± 30.87 | 1.32 ± 16.99 | 0.016 |
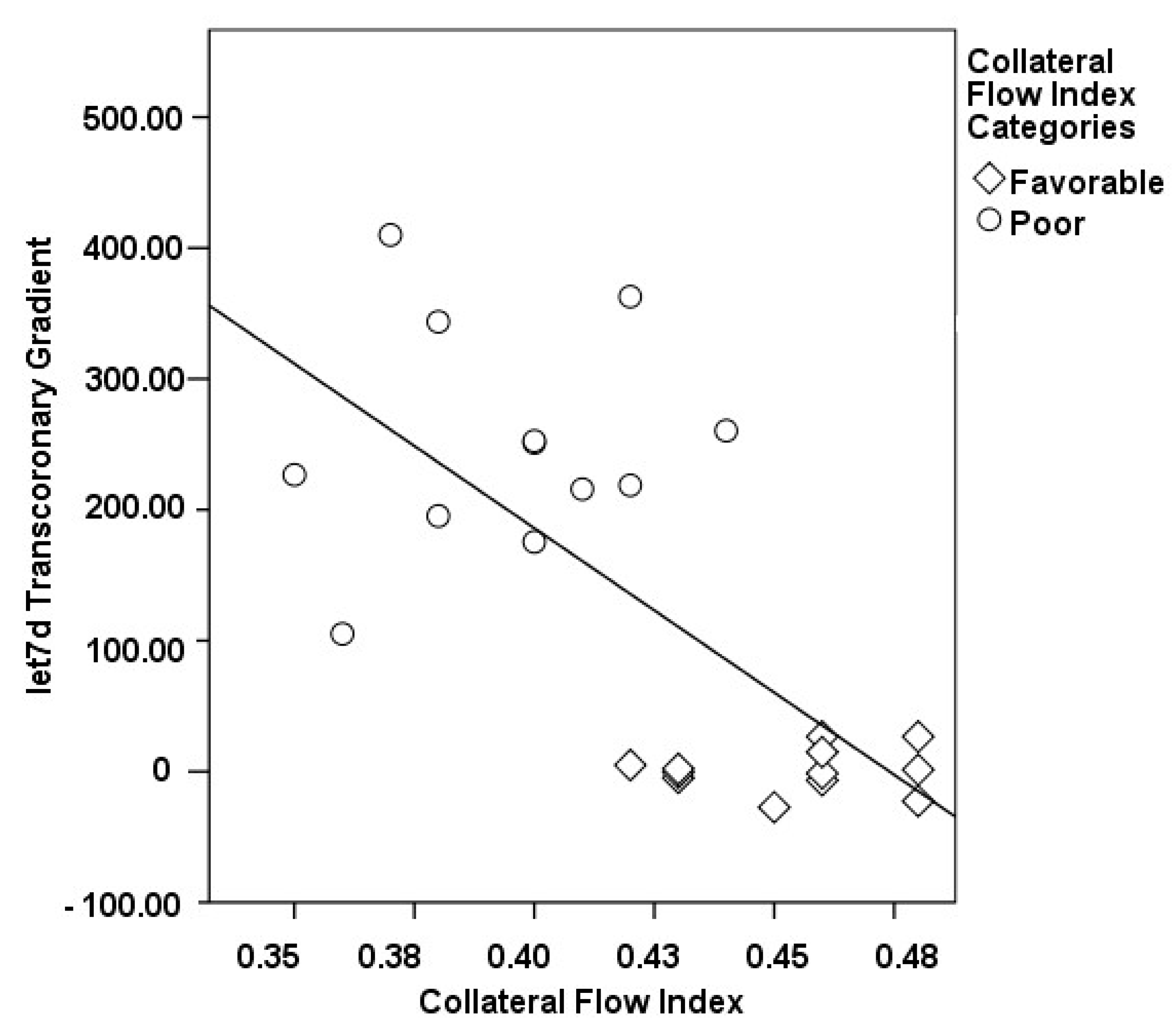
| ∆ let7d, % | 251.46 ± 84.97 | 1.10 ± 16.46 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vural, M.G.; Temel, H.Y.; Turunc, E.; Akdemir, R.; Tatli, E.; Agac, M.T. Transcoronary Gradients of Mechanosensitive MicroRNAs as Predictors of Collateral Development in Chronic Total Occlusion. Medicina 2024, 60, 590. https://doi.org/10.3390/medicina60040590
Vural MG, Temel HY, Turunc E, Akdemir R, Tatli E, Agac MT. Transcoronary Gradients of Mechanosensitive MicroRNAs as Predictors of Collateral Development in Chronic Total Occlusion. Medicina. 2024; 60(4):590. https://doi.org/10.3390/medicina60040590
Chicago/Turabian StyleVural, Mustafa Gökhan, Hulya Yilmaz Temel, Ezgi Turunc, Ramazan Akdemir, Ersan Tatli, and Mustafa Tarik Agac. 2024. "Transcoronary Gradients of Mechanosensitive MicroRNAs as Predictors of Collateral Development in Chronic Total Occlusion" Medicina 60, no. 4: 590. https://doi.org/10.3390/medicina60040590





