Nutritional and Lifestyle Features in a Mediterranean Cohort: An Epidemiological Instrument for Categorizing Metabotypes Based on a Computational Algorithm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Nutritional Indices
2.5. Statistical Data
3. Results
3.1. General Data
3.2. Dietary Intake Assessment
3.3. Adherence to Mediterranean Diet Pattern
3.4. Physical Activity Data
3.5. Quality of Life Results
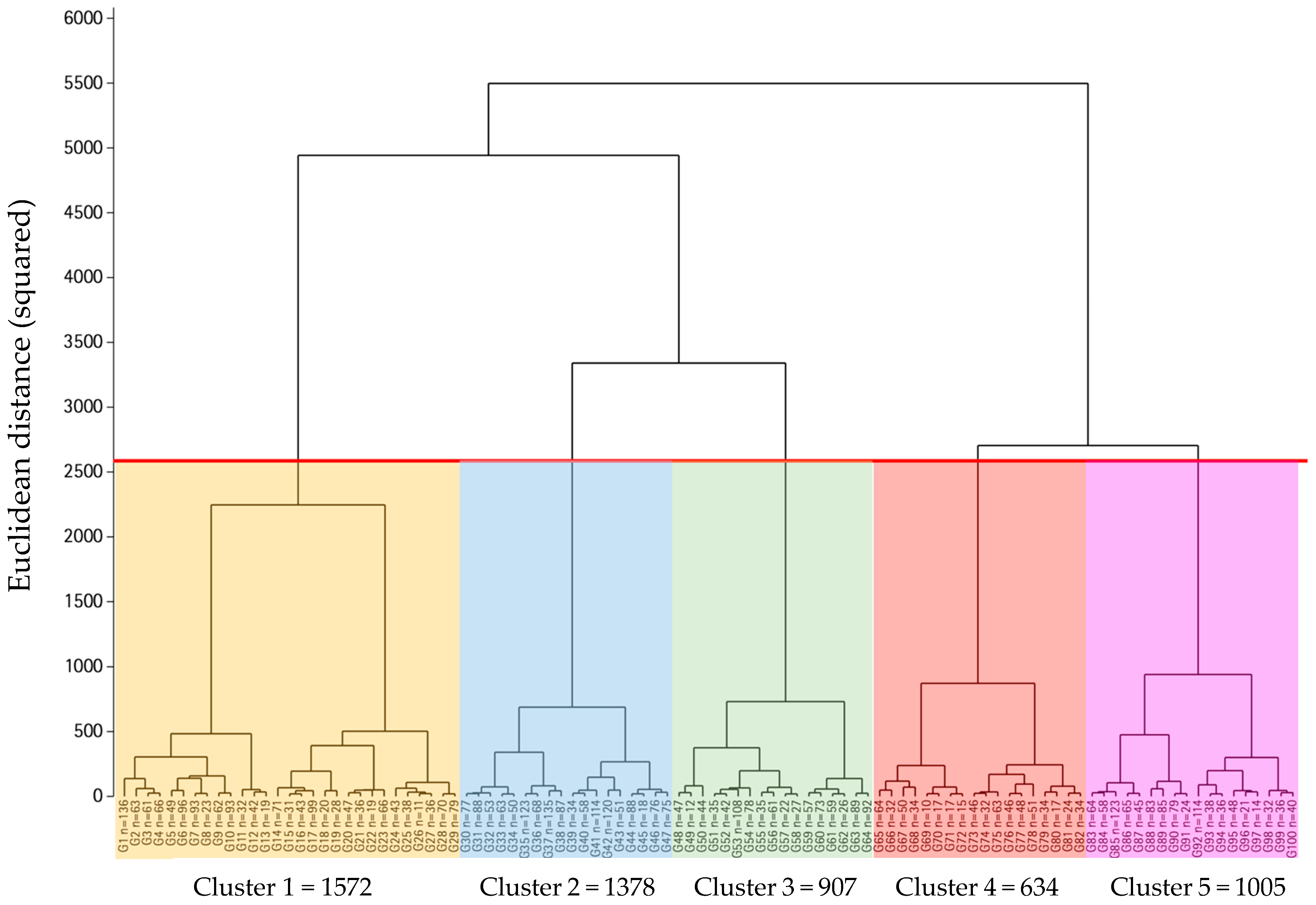
3.6. Nutritional Indices and Metabotype Computational Algorithm
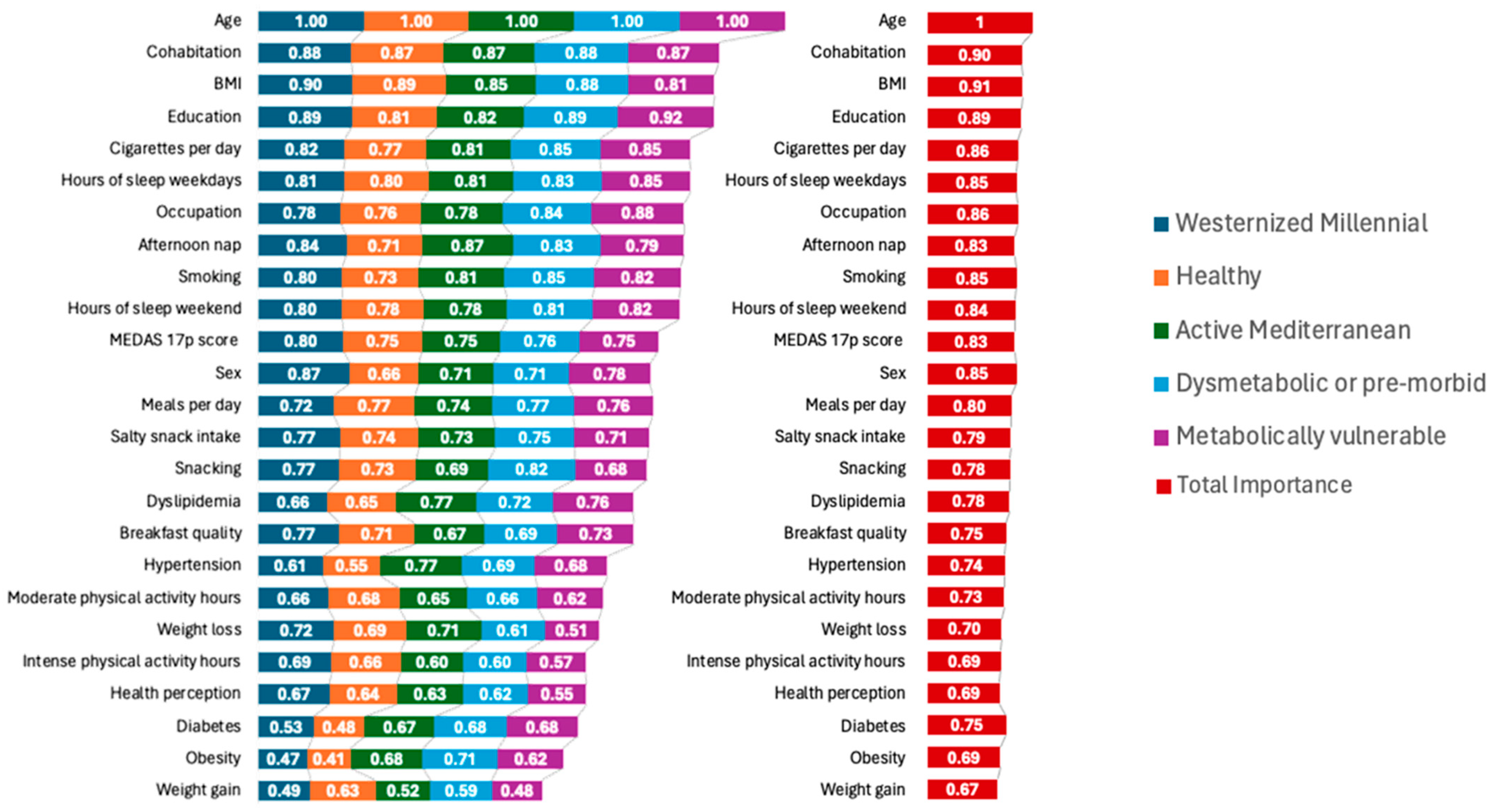
3.7. Metabotype Characterization
3.8. Metabotype Characterization
4. Discussion
Study Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M. Global trends of chronic non-communicable diseases risk factors. Eur. J. Public Health 2019, 29 (Suppl. S4), ckz185–ckz196. [Google Scholar] [CrossRef]
- Khawar Balwan, W.; Kour, S. Lifestyle Diseases: The Link between Modern Lifestyle and Threat to Public Health. Saudi J. Med. Pharm. Sci. 2021, 7, 179–184. [Google Scholar] [CrossRef]
- Rippe, J.M. Lifestyle Medicine: The Health Promoting Power of Daily Habits and Practices. Am. J. Lifestyle Med. 2018, 12, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Thompson, F.E.; Subar, A.F. Dietary assessment methodology. In Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 5–48. [Google Scholar] [CrossRef]
- Rollo, M.E.; Williams, R.L.; Burrows, T.; Kirkpatrick, S.I.; Bucher, T.; Collins, C.E. What are they really eating? A review on new approaches to dietary intake assessment and validation. Curr. Nutr. Rep. 2016, 5, 307–314. [Google Scholar] [CrossRef]
- Lepre, B.; Trigueiro, H.; Johnsen, J.T.; Khalid, A.A.; Ball, L.; Ray, S. Global architecture for the nutrition training of health professionals: A scoping review and blueprint for next steps. BMJ Nutr. Prev. Health 2022, 5, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Hall, A. Quality of Life and Value Assessment in Health Care. Health Care Anal. 2020, 28, 45–61. [Google Scholar] [CrossRef]
- Rozanski, A.; Sakul, S.; Narula, J.; Berman, D. Assessment of lifestyle “vital signs” in healthcare settings. Prog. Cardiovasc. Dis. 2023, 77, 107–118. [Google Scholar] [CrossRef]
- Sawicki, C.; Haslam, D.; Bhupathiraju, S. Utilising the precision nutrition toolkit in the path towards precision medicine. Proc. Nutr. Soc. 2023, 82, 359–369. [Google Scholar] [CrossRef]
- Kirk, D.; Kok, E.; Tufano, M.; Tekinerdogan, B.; Feskens, E.J.M.; Camps, G. Machine Learning in Nutrition Research. Adv. Nutr. 2022, 13, 2573–2589. [Google Scholar] [CrossRef] [PubMed]
- de Cuevillas, B.; Álvarez-álvarez, I.; Cuervo, M.; Fernández-Montero, A.; Navas-Carretero, S.; Martínez, J.A. Definition of nutritionally qualitative categorizing (Proto)nutritypes and a pilot quantitative nutrimeter for mirroring nutritional well-being based on a quality of life health related questionnaire. Nutr. Hosp. 2019, 36, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Hasbullah, F.Y.; Yusof, B.N.M.; Ghani, R.A.; Daud Z ’Azuan, M.; Appannah, G.; Abas, F.; Nurul Husna, S.; Hannah Izzati, M.K.; Murphy, H.R. Dietary Patterns, Metabolomic Profile, and Nutritype Signatures Associated with Type 2 Diabetes in Women with Postgestational Diabetes Mellitus: MyNutritype Study Protocol. Metabolites 2022, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Zhao, S.; Kline, D.; Brock, G.; Gooding, H.; Mattei, J.; Rodriguez-Artalejo, F.; Min, Y.I.; Rimm, E.B.; Tucker, K.L.; et al. Application of a lifestyle-based score to predict cardiovascular risk in African Americans: The Jackson heart study. J. Clin. Med. 2021, 10, 2252. [Google Scholar] [CrossRef] [PubMed]
- Moseley, L.E.; McConnell, L.; Garza, K.B.; Ford, C.R. Exploring the evolution of professional identity formation in health professions education. N. Dir. Teach. Learn. 2021, 2021, 11–27. [Google Scholar] [CrossRef]
- Gil Hernández, Á.; Wanden-Berghe, C.; Sanz-Valero, J. Scientific evidence in the process of communication on food and health: Sources of information. Rev. Española Comun. En Salud 2016, 7, 53–61. [Google Scholar] [CrossRef]
- Ribot-Rodriguez, R.; Higuera-Gomez, A.; San-Cristobal, R.; Martín-Hernández, R.; Micó, V.; Espinosa-Salinas, I.; Ramírez de Molina, A.; Martínez, J.A. Cardiometabolic Health Status, Ethnicity and Health-Related Quality of Life (HRQoL) Disparities in an Adult Population: NutrIMDEA Observational Web-Based Study. Int. J. Environ. Res. Public. Health 2022, 19, 2948. [Google Scholar] [CrossRef] [PubMed]
- Goni Mateos, L.; Aray Miranda, M.; Martínez, A.H.; Cuervo Zapatel, M. Validation of a food groups frequency questionnaire based in an exchange system. Nutr. Hosp. 2016, 33, 1391–1399. [Google Scholar] [CrossRef]
- Conde-Pipó, J.; Bouzas, C.; Zurita-Ortega, F.; Olea-Serrano, F.; Tur, J.A.; Mariscal-Arcas, M. Adherence to a Mediterranean Diet Pattern, Physical Activity, and Physical Self-Concept in Spanish Older Adults. Nutrients 2022, 14, 2404. [Google Scholar] [CrossRef]
- Higuera-Gomez, A.; Ribot-Rodriguez, R.; San-Cristobal, R.; Martín-Hernández, R.; Mico, V.; Espinosa-Salinas, I.; Ramírez de Molina, A.; Martínez, J.A. HRQoL and nutritional well-being dissimilarities between two different online collection methods: Value for digital health implementation. Digit. Health 2022, 8, 20552076221138316. [Google Scholar] [CrossRef]
- Hasbani, N.R.; Ligthart, S.; Brown, M.R.; Heath, A.S.; Bebo, A.; Ashley, K.E.; Boerwinkle, E.; Morrison, A.C.; Folsom, A.R.; Aguilar, D.; et al. American Heart Association’s Life’s Simple 7: Lifestyle Recommendations, Polygenic Risk, and Lifetime Risk of Coronary Heart Disease. Circulation 2022, 145, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Moreno-Franco, B.; Ordovás, J.M.; León, M.; Casasnovas, J.A.; Peñalvo, J.L. Design and development of an instrument to measure overall lifestyle habits for epidemiological research: The Mediterranean Lifestyle (MEDLIFE) index. Public Health Nutr. 2015, 18, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Mattei, J.; Cook, N.R.; Hu, F.B.; Willett, W.C.; Chiuve, S.E.; Rimm, E.B.; Sesso, H.D. Association between a 20-year cardiovascular disease risk score based on modifiable lifestyles and total and cause-specific mortality among US men and women. J. Am. Heart Assoc. 2018, 7, e010052. [Google Scholar] [CrossRef]
- Tsai, M.C.; Yeh, T.L.; Hsu, H.Y.; Hsu, L.Y.; Lee, C.C.; Tseng, P.J.; Chien, K.L. Comparison of four healthy lifestyle scores for predicting cardiovascular events in a national cohort study. Sci. Rep. 2021, 11, 22146. [Google Scholar] [CrossRef]
- Schneeweiss, H.; Mathes, H. Factor analysis and principal components. J. Multivar. Anal. 1995, 55, 105–124. [Google Scholar] [CrossRef]
- D’agostino, R.B.; Russell, H.K. Scree Test. In Encyclopedia of Biostatistics; Wiley: Marshall, TX, USA, 2005. [Google Scholar] [CrossRef]
- Strauss, T.; Von Maltitz, M.J. Generalising ward’s method for use with manhattan distances. PLoS ONE 2017, 12, e0168288. [Google Scholar] [CrossRef]
- Apfel, N.; Liang, X. Agglomerative Hierarchical Clustering for Selecting Valid Instrumental Variables. arXiv 2021, arXiv:2101.05774. [Google Scholar]
- Smolinski, A.; Walczak, B.; Einax, J.W. Hierarchical clustering extended with visual complements of environmental data set. Chemom. Intell. Lab. Syst. 2002, 64, 45–54. [Google Scholar] [CrossRef]
- Halpin, B. Cluster Analysis Stopping Rules in Stata; Working Paper Series; University of Limerick, Department of Sociology: Limerick, Ireland, 2016. [Google Scholar]
- Nowak-Brzezińska, A. Enhancing the efficiency of a decision support system through the clustering of complex rule-based knowledge bases and modification of the inference algorithm. Complexity 2018, 2018, 2065491. [Google Scholar] [CrossRef]
- Cutler, A.; Cutler, D.R.; Stevens, J.R. Random Forests. In Ensemble Machine Learning; Springer: New York, NY, USA, 2012; pp. 157–175. [Google Scholar] [CrossRef]
- Weisell, R.C. Body mass index as an indicator of obesity. Asia Pac. J. Clin. Nutr. 2002, 11, S681–S684. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Kon, M. Two clustering methods based on the Ward’s method and dendrograms with interval-valued dissimilarities for interval-valued data. Int. J. Approx. Reason. 2021, 129, 103–121. [Google Scholar] [CrossRef]
- Kushner, R.F.; Sorensen, K.W. Lifestyle medicine: The future of chronic disease management. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Khunti, K.; Gillies, C.L.; Dhalwani, N.N.; Davies, M.J.; Yates, T.; Zaccardi, F. Healthy lifestyle and life expectancy in people with multimorbidity in the UK Biobank: A longitudinal cohort study. PLoS Med. 2020, 17, e1003332. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Jal, M.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Trends in the food and sports nutrition industry: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2405–2421. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; de Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J. Nutr. Nutr. 2016, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Roscoe, S.; Khatri, M.; Voshall, A.; Batra, S.; Kaur, S.; Deogun, J. Formal Concept Analysis Applications in Bioinformatics. ACM Comput. Surv. 2023, 55, 1–40. [Google Scholar] [CrossRef]
- Amin, A.M. The metabolic signatures of cardiometabolic diseases: Does the shared metabotype offer new therapeutic targets? Lifestyle Med. 2021, 2, e25. [Google Scholar] [CrossRef]
- Partearroyo, T.; Samaniego-Vaesken, M.d.L.; Ruiz, E.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Current food consumption amongst the spanish ANIBES Study population. Nutrients 2019, 11, 2663. [Google Scholar] [CrossRef] [PubMed]
- Pano, O.; Sayón-Orea, C.; Hershey, M.S.; Bes-Rastrollo, M.; Martínez-González, M.A.; Martínez, J.A. Development of a General Health Score Based on 12 Objective Metabolic and Lifestyle Items: The Lifestyle and Well-Being Index. Healthcare 2022, 10, 1088. [Google Scholar] [CrossRef]
- Muhammad, H.; Xie, C.; Sigel, C.S.; Doukas, M.; Alpert, L.; Simpson, A.L.; Fuchs, T.J. EPIC-Survival: End-to-end Part Inferred Clustering for Survival Analysis with Prognostic Stratification Boosting. Proc. Mach. Learn. Res. 2021, 143, 520–531. [Google Scholar]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; Mcginn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The Obese Without Cardiometabolic Risk Factor Clustering and the Normal Weight With Cardiometabolic Risk Factor Clustering. Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Veljković, M.; Pavlović, D.R.; Stojanović, N.M.; Džopalić, T.; Popović Dragonjić, L. Behavioral and Dietary Habits That Could Influence Both COVID-19 and Non-Communicable Civilization Disease Prevention-What Have We Learned Up to Now? Medicina 2022, 58, 1686. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Fuentes-Bol, C.; Ramos, D.; Carrasco, J.L.; Roman, B.; Bertomeu, I.F.; Cristià, E.; Geleva, D.; Serra-Majem, L. The Mediterranean diet in Spain: Adherence trends during the past two decades using the Mediterranean Adequacy Index. Public. Health Nutr. 2011, 14, 622–628. [Google Scholar] [CrossRef]
- Derrick, S.A.; Nguyen, S.T.; Marthens, J.R.; Dambacher, L.L.; Sikalidis, A.K.; Reaves, S.K. A Mediterranean-Style Diet Improves the Parameters for the Management and Prevention of Type 2 Diabetes Mellitus. Medicina 2023, 59, 1882. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Caprara, G. Mediterranean-type dietary pattern and physical activity: The winning combination to counteract the rising burden of non-communicable diseases (NCDS). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, H.F.; Lanham-New, S.; Whetton, A.D.; Geifman, N. Associations of Diet with Health Outcomes in the UK Biobank: A Systematic Review. Nutrients 2024, 16, 523. [Google Scholar] [CrossRef]
- Walker, M.E.; Song, R.J.; Xu, X.; Gerszten, R.E.; Ngo, D.; Clish, C.B.; Corlin, L.; Ma, J.; Xanthakis, V.; Jacques, P.F.; et al. Proteomic and metabolomic correlates of healthy dietary patterns: The framingham heart study. Nutrients 2020, 12, 1476. [Google Scholar] [CrossRef]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Dietary patterns and their association with food and nutrient intake in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam study. Br. J. Nutr. 2001, 85, 363–373. [Google Scholar] [CrossRef]
- Buckland, G.; Agudo, A.; Travier, N.; María Huerta, J.; Cirera, L.; Tormo, M.J.; Navarro, C.; Chirlaque, M.D.; Moreno-Iribas, C.; Ardanaz, E.; et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br. J. Nutr. 2011, 106, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Patino-Alonso, M.C.; Recio-Rodríguez, J.I.; Belio, J.F.M.; Colominas-Garrido, R.; Lema-Bartolomé, J.; Arranz, A.G.; Agudo-Conde, C.; Gomez-Marcos, M.A.; García-Ortiz, L.; EVIDENT Group. Factors associated with adherence to the mediterranean diet in the adult population. J. Acad. Nutr. Diet. 2014, 114, 583–589. [Google Scholar] [CrossRef]
- Redruello-Requejo, M.; Samaniego-Vaesken, M.d.L.; Puga, A.M.; Montero-Bravo, A.; Ruperto, M.; Rodríguez-Alonso, P.; Partearroyo, T.; Varela-Moreiras, G. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Intakes, Determinants and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2023, 15, 562. [Google Scholar] [CrossRef] [PubMed]
- Lucha-López, M.O.; Hidalgo-García, C.; Lucha-López, A.C.; Monti-Ballano, S.; Márquez-Gonzalvo, S.; Ferrández-Laliena, L.; Tricás-Vidal, H.J.; Tricás-Moreno, J.M. Determinants of Consumption of Vegetables among the Spanish Population: A Cross-Sectional Study. Foods 2023, 12, 4030. [Google Scholar] [CrossRef]
- Quarta, S.; Massaro, M.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; Jorge, R.; Andrade, V.; Philippou, E.; Zisimou, C.; Maksimova, V.; et al. Persistent moderate-to-weak mediterranean diet adherence and low scoring for plant-based foods across several southern european countries: Are we overlooking the mediterranean diet recommendations? Nutrients 2021, 13, 1432. [Google Scholar] [CrossRef]
- Ho, F.K.; Gray, S.R.; Welsh, P.; Petermann-Rocha, F.; Foster, H.; Waddell, H.; Anderson, J.; Lyall, D.; Sattar, N.; Gill, J.M.R.; et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: Prospective cohort study of UK Biobank participants. BMJ 2020, 368, m688. [Google Scholar] [CrossRef]
- Marcum, J.A. Nutrigenetics/Nutrigenomics, Personalized Nutrition, and Precision Healthcare. Curr. Nutr. Rep. 2020, 9, 338–345. [Google Scholar] [CrossRef]
- Wunsch, K.; Kienberger, K.; Niessner, C. Changes in Physical Activity Patterns Due to the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Int. J. Env. Res. Public Health 2022, 19, 2250. [Google Scholar] [CrossRef] [PubMed]
- Hershey, M.S.; Martínez-González, M.Á.; Álvarez-Álvarez, I.; Martínez Hernández, J.A.; Ruiz-Canela, M. The Mediterranean diet and physical activity: Better together than apart for the prevention of premature mortality. Br. J. Nutr. 2022, 128, 1413–1424. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Consejo General de Colegios Oficiales de Farmacéuticos. Plenufar 6. In Educación Nutricional en la Actividad Física; Consejo General de Colegios Oficiales de Farmacéuticos: Madrid, Spain, 2017. [Google Scholar]
- Pimenta, A.M.; de Deus Mendonça, R.; Lahortiga-Ramos, F.; Fernandez-Lazaro, C.I.; Martínez-González, M.A.; Sánchez-Villegas, A. Sedentary behaviors and risk of depression in the Seguimiento Universidad de Navarra cohort: The SUN Project. Cad. Saude Publica 2022, 38, e00076621. [Google Scholar] [CrossRef]
- Sbert, P.R.; González, A.A.Ĺ.O.; Fe, B.R.; Fe, N.R.; Bote, S.A.; Manent, J.I.R. Influence of physical activity, the Mediterranean diet and sociodemographic variables on the values of different cardiovascular risk scales. Med. J. Nutr. Metab. 2022, 15, 273–284. [Google Scholar] [CrossRef]
- Cabiedes-Miragaya, L.; Diaz-Mendez, C.; García-Espejo, I. Well-being and the lifestyle habits of the spanish population: The association between subjective well-being and eating habits. Int. J. Environ. Res. Public Health 2021, 18, 1553. [Google Scholar] [CrossRef] [PubMed]
- Pano, O.; Sayón-Orea, C.; Gea, A.; Bes-Rastrollo, M.; Martínez-González, M.Á.; Alfredo Martínez, J. Nutritional determinants of quality of life in a mediterranean cohort: The sun study. Int. J. Environ. Res. Public Health 2020, 17, 3897. [Google Scholar] [CrossRef] [PubMed]
- Díez-Espino, J.; Bui-Cosiales, P.; Babio, N.; Toledo, E.; Corella, D.; Ros, E.; Fitó, M.; Gómez-Gracia, E.; Estruch, R.; Fiol, M.; et al. Impacto de Life’s Simple 7 en la incidencia de eventos cardiovasculares mayores en adultos españoles con alto riesgo de la cohorte del estudio, PREDIMED. Rev. Esp. Cardiol. 2020, 73, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ribot-Rodríguez, R.; Higuera-Gómez, A.; San-Cristobal, R.; Micó, V.; Martínez, J.A. Comparison of Seven Healthy Lifestyle Scores Cardiometabolic Health: Age, Sex, and Lifestyle Interactions in the NutrIMDEA Web-Based Study. J. Epidemiol. Glob. Health 2023, 13, 653–663. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Ortolá, R.; Ruiz-Canela, M.; Garcia-Esquinas, E.; Martínez-Gómez, D.; Lopez-Garcia, E.; Martínez-González, M.Á.; Rodriguez-Artalejo, F. Association between the Mediterranean lifestyle, metabolic syndrome and mortality: A whole-country cohort in Spain. Cardiovasc. Diabetol. 2021, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cabrera, J.L.; García-Ríos, A.; Sotos-Prieto, M.; Quintana-Navarro, G.; Alcalá-Díaz, J.F.; Martín-Piedra, L.; Torres-Peña, J.D.; Luque, R.M.; Yubero-Serrano, E.M.; Delgado-Lista, J.; et al. Adherence to a Mediterranean lifestyle improves metabolic status in coronary heart disease patients: A prospective analysis from the CORDIOPREV study. J. Intern. Med. 2023, 293, 574–588. [Google Scholar] [CrossRef]
- Mata-Fernández, A.; Hershey, M.S.; Pastrana-Delgado, J.C.; Sotos-Prieto, M.; Ruiz-Canela, M.; Kales, S.N.; Martínez-González, M.A.; Fernandez-Montero, A. A Mediterranean lifestyle reduces the risk of cardiovascular disease in the “Seguimiento Universidad de Navarra” (SUN) cohort. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1728–1737. [Google Scholar] [CrossRef]
- Gooding, H.C.; Ning, H.; Gillman, M.W.; Shay, C.; Allen, N.; Goff, D.C.; Lloyd-Jones, D., Jr.; Chiuve, S. Application of a lifestyle-based tool to estimate premature cardiovascular disease events in young adults the coronary artery risk development in young adults (CARDIA) study. JAMA Intern. Med. 2017, 177, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Delgado-Velandia, M.; Fernández-Felix, B.M.; Zamora, J.; Ortolá, R.; García-Esquinas, E.; Rimm, E.B.; Rodríguez-Artalejo, F. Performance and validation of the Healthy Heart Score model for predicting 12-year cardiovascular mortality in a nationwide Mediterranean population. Rev. Española Cardiol. (Engl. Ed.) 2023, 76, 444–452. [Google Scholar] [CrossRef]
- Roshan, A.; Choo, J.; Lim, C. Readability, Understandability, and Actionability of Online Cardiovascular Risk Assessment Tools and Patient Educational Material: A Systematic Review. Glomerular Dis. 2022, 3, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Wallisch, C.; Heinze, G.; Rinner, C.; Mundigler, G.; Winkelmayer, W.C.; Dunkler, D. Re-estimation improved the performance of two Framingham cardiovascular risk equations and the Pooled Cohort equations: A nationwide registry analysis. Sci. Rep. 2020, 10, 8140. [Google Scholar] [CrossRef]
- Kraus, S.; Schiavone, F.; Pluzhnikova, A.; Invernizzi, A.C. Digital transformation in healthcare: Analyzing the current state-of-research. J. Bus. Res. 2021, 123, 557–567. [Google Scholar] [CrossRef]
- Higuera-Gómez, A.; Ribot-Rodríguez, R.; Micó, V.; Cuevas-Sierra, A.; San Cristóbal, R.; Martínez, J.A. Lifestyle and Health-Related Quality of Life Relationships Concerning Metabolic Disease Phenotypes on the Nutrimdea Online Cohort. Int. J. Environ. Res. Public Health 2023, 20, 767. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, E.; de la O, V.; Fernández, C.; Calle-Pascual, A.L.; Larrad, A.; Espadas, J.L.; Martinez, J.A. Categorized Dietotype Emergence by Exploratory Factorial Analyses with Axial Nutrition–Health Precision Potential. Proceedings 2023, 91, 135. [Google Scholar] [CrossRef]
- Micó, V.; San-Cristobal, R.; Martín, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Fitó, M.; Alonso-Gómez, Á.M.; Wärnberg, J.; Vioque, J.; et al. Morbid liver manifestations are intrinsically bound to metabolic syndrome and nutrient intake based on a machine-learning cluster analysis. Front. Endocrinol. 2022, 13, 936956. [Google Scholar] [CrossRef]
- Merino, J. Precision nutrition in diabetes: When population-based dietary advice gets personal. Diabetologia 2022, 65, 1839–1848. [Google Scholar] [CrossRef]
- Zhu, R.; Fogelholm, M.; Larsen, T.M.; Poppitt, S.D.; Silvestre, M.P.; Vestentoft, P.S.; Jalo, E.; Navas-Carretero, S.; Huttunen-Lenz, M.; Taylor, M.A.; et al. A High-Protein, Low Glycemic Index Diet Suppresses Hunger but Not Weight Regain after Weight Loss: Results from a Large, 3-Years Randomized Trial (PREVIEW). Front. Nutr. 2021, 8, 685648. [Google Scholar] [CrossRef]
- Jayatilake, S.M.D.A.C.; Ganegoda, G.U. Involvement of Machine Learning Tools in Healthcare Decision Making. J. Healthc. Eng. 2021, 2021, 6679512. [Google Scholar] [CrossRef] [PubMed]
- Bamia, C.; Orfanos, P.; Ferrari, P.; Overvad, K.; Hundborg, H.H.; Tjønneland, A.; Olsen, A.; Kesse, E.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; et al. Dietary patterns among older Europeans: The EPIC-Elderly study. Br. J. Nutr. 2005, 94, 100–113. [Google Scholar] [CrossRef]
- Barden, A.E.; Huang, R.C.; Beilin, L.J.; Rauschert, S.; Tsai, I.J.; Oddy, W.H.; Mori, T.A. Identifying young adults at high risk of cardiometabolic disease using cluster analysis and the Framingham 30-yr risk score. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Cardilli, L.; Reed, J.L.; Saunders, T.J.; Kite, C.; Douillette, K.; Fournier, K.; Buckley, J.P. A comparison of self-reported and device measured sedentary behaviour in adults: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.P.; Pereira, J.L.; Barbosa, J.P.D.A.S.; Mello AV de Onita, B.M.; Fisberg, R.M.; Florindo, A.A. Validity of self-reported body mass and height: Relation with sex, age, physical activity, and cardiometabolic risk factors. Braz. J. Epidemiol. 2021, 24, e210043. [Google Scholar] [CrossRef]
- Ravelli, M.N.; Schoeller, D.A. Traditional Self-Reported Dietary Instruments Are Prone to Inaccuracies and New Approaches Are Needed. Front. Nutr. 2020, 7, 538983. [Google Scholar] [CrossRef]
| Variables | Frequency | Men | Women | p 1 |
|---|---|---|---|---|
| Age (years) | 47.63 ± 17.81 | 47.24 ± 17.06 | 0.422 | |
| Weight (kg) | 80.87 ± 13.23 | 65.05 ± 12.03 | <0.001 | |
| BMI (kg/m2) | 26.32 ± 4.11 | 24.48 ± 4.53 | <0.001 | |
| MEDAS (0–17 p) | 8.58 ± 3.04 | 9.43 ± 2.87 | <0.001 | |
| IPAQ (kcal/week) | 3442 ± 4168 | 1746 ± 2095 | <0.001 | |
| SF-12 (PD) (0–100 p) | 33.3 ± 6.1 | 33.6 ± 6.1 | 0.073 | |
| SF-12 (MD) (0–100 p) | 30.7 ± 6.7 | 30.6 ± 6.7 | 0.578 | |
| Number of meals/day (%) | <2 | 6.60 | 4.25 | 0.001 |
| 3–5 | 92.16 | 94.65 | ||
| >6 | 1.24 | 1.10 | ||
| Number of glasses of water/day (%) | <4 | 38.37 | 39.73 | 0.066 |
| 4–7 | 28.27 | 29.79 | ||
| >7 | 33.17 | 30.48 | ||
| Hours of sleep per night/day (M-F) (%) | <5 h | 4.37 | 3.95 | 0.003 |
| 5–9 h | 91.07 | 91.02 | ||
| >9 h | 4.57 | 5.03 | ||
| Hours of sleep per night/day (S-S) (%) | <5 h | 3.23 | 3.27 | 0.031 |
| 5–9 h | 77.29 | 74.60 | ||
| >9 h | 19.48 | 22.13 |
| Food Group | Never/ Almost Never | Times Per Month | Times Per Week | Times Per Day | p 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–3 | 1–2 | 3–4 | 5–6 | 1–2 | 3–4 | >5 | |||||||||||
| M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | ||
| Whole daily | 37.5 | 46.3 | 6.8 | 7.1 | 11.5 | 12.2 | 10.0 | 8.9 | 3.5 | 2.4 | 27.7 | 20.6 | 2.6 | 2.2 | 0.4 | 0.3 | <0.001 |
| Semi-skimmed Dairy | 31.7 | 23.0 | 3.9 | 3.8 | 6.2 | 5.4 | 7.7 | 8.6 | 3.8 | 4.6 | 41.3 | 47.3 | 4.5 | 6.3 | 0.9 | 1.0 | <0.001 |
| Eggs | 1.1 | 1.6 | 3.1 | 3.6 | 30.9 | 35.2 | 44.4 | 45.3 | 11.6 | 8.4 | 7.6 | 5.3 | 1.1 | 0.6 | 0.2 | 0.1 | 0.103 |
| Lean meats | 2.7 | 5.7 | 5.1 | 6.3 | 36.4 | 39.4 | 39.3 | 37.2 | 7.4 | 6.0 | 8.4 | 5.0 | 0.5 | 0.3 | 0.2 | 0.1 | <0.001 |
| Fatty meats | 15.7 | 26.3 | 17.2 | 22.4 | 47.7 | 39.7 | 15.0 | 8.7 | 1.8 | 1.1 | 2.4 | 1.6 | 0.2 | 0.1 | 0.0 | 0.0 | <0.001 |
| Whitefish | 9.2 | 7.7 | 16.0 | 12.5 | 59.3 | 59.4 | 12.9 | 17.7 | 1.2 | 1.6 | 1.3 | 1.0 | 0.1 | 0.2 | 0.1 | 0.0 | 0.051 |
| Bluefish | 12.7 | 10.5 | 21.0 | 18.5 | 55.9 | 59.8 | 8.7 | 9.4 | 1.1 | 0.7 | 0.6 | 1.0 | 0.0 | 0.1 | 0.6 | 0.0 | 0.010 |
| Vegetables | 4.0 | 1.6 | 3.0 | 1.4 | 17.2 | 10.8 | 24.9 | 22.0 | 10.0 | 11.8 | 36.6 | 45.4 | 3.7 | 5.8 | 0.6 | 1.3 | <0.001 |
| Fruits | 5.7 | 3.5 | 2.0 | 1.7 | 6.6 | 5.2 | 11.2 | 10.3 | 6.2 | 6.5 | 44.7 | 48.7 | 19.7 | 21.8 | 4.0 | 2.4 | <0.001 |
| Nuts | 24.3 | 22.2 | 13.8 | 13.0 | 22.9 | 22.8 | 13.4 | 14.0 | 3.7 | 3.8 | 19.7 | 22.8 | 1.4 | 1.1 | 0.7 | 0.5 | 0.064 |
| Legumes | 3.9 | 4.0 | 8.8 | 8.8 | 61.5 | 63.6 | 22.1 | 20.1 | 1.6 | 1.4 | 2.0 | 1.8 | 0.1 | 0.3 | 0.0 | 0.1 | 0.785 |
| Olive oil | 2.2 | 1.9 | 1.0 | 0.7 | 3.1 | 2.7 | 7.0 | 5.9 | 6.8 | 5.1 | 62.4 | 64.3 | 14.2 | 16.6 | 3.2 | 2.9 | 0.47 |
| Other fats | 50.6 | 52.6 | 13.7 | 13.7 | 19.2 | 17.4 | 7.0 | 6.4 | 1.3 | 1.5 | 7.5 | 8.1 | 0.4 | 0.3 | 0.3 | 0.0 | 0.133 |
| Refined grains | 37.3 | 40.4 | 7.2 | 7.9 | 15.4 | 17.1 | 10.4 | 9.2 | 3.1 | 2.5 | 22.1 | 20.0 | 4.0 | 2.7 | 0.3 | 0.3 | 0.026 |
| Whole grains | 56.9 | 43.3 | 6.9 | 8.1 | 11.7 | 13.9 | 6.1 | 8.0 | 2.2 | 2.6 | 15.1 | 22.6 | 1.0 | 1.2 | 0.1 | 0.3 | <0.001 |
| Processed bakery goods | 38.5 | 43.8 | 14.4 | 16.5 | 22.3 | 20.6 | 10.5 | 8.1 | 2.3 | 1.7 | 11.5 | 8.9 | 0.4 | 0.4 | 0.1 | 0.2 | <0.001 |
| Sugars | 35.4 | 41.5 | 8.5 | 8.6 | 11.8 | 13.5 | 8.4 | 6.9 | 2.8 | 2.4 | 30.4 | 25.1 | 2.1 | 1.7 | 0.6 | 0.4 | <0.001 |
| Alcohol | 25.2 | 44.6 | 13.4 | 14.1 | 26.7 | 27.7 | 11.2 | 6.1 | 4.2 | 1.5 | 16.5 | 5.6 | 2.5 | 0.4 | 0.3 | 0.1 | <0.001 |
| Water | 0.9 | 1.0 | 0.3 | 0.3 | 0.5 | 0.5 | 3.1 | 2.9 | 2.4 | 2.5 | 17.3 | 14.9 | 32.8 | 32.4 | 42.7 | 45.7 | 0.855 |
| Question | Positive Scale (+1 p) | Percentages (%) | |||
|---|---|---|---|---|---|
| Total | Men | Women | p 1 | ||
| 1. Use of extra virgin olive oil | Yes | 89.5 | 88.9 | 90.1 | 0.180 |
| 2. Servings of vegetables/day | ≥2 servings | 51.2 | 45.9 | 54.3 | <0.001 |
| 3. Pieces of fruit/day | ≥3 pieces | 36.8 | 35.6 | 37.4 | 0.0166 |
| 4. Servings of red meat, processed meats, or sausages/week | 0–1 serving | 50.0 | 41.5 | 55.4 | <0.001 |
| 5. Servings of butter, margarine, or cream/week | <1 serving | 83.3 | 82.4 | 84.0 | 0.134 |
| 6. Consumption of sugary drinks/week | <1 drink | 73.8 | 68.1 | 77.5 | <0.001 |
| 7. Servings of legumes/week | ≥3 servings | 30.1 | 30.4 | 29.7 | 0.626 |
| 8. Servings of fish or seafood/week | ≥3 servings | 39.4 | 36.3 | 41.3 | <0.001 |
| 9. Pastry consumption/week | ≤2 servings | 69.0 | 65.2 | 71.3 | <0.001 |
| 10. Nut consumption/week | ≥3 servings | 37.6 | 35.8 | 38.6 | 0.041 |
| 11. Greater consumption of white meat compared to red meat | Yes | 76.8 | 69.2 | 81.7 | <0.001 |
| 12. Dishes seasoned with sofrito/week | ≥2 times | 63.7 | 65.7 | 62.4 | 0.016 |
| 13. Consumption of sugary drinks | No | 62.2 | 56.7 | 65.7 | <0.001 |
| 14. Servings of white bread/day | 0–1 time | 59.9 | 51.2 | 65.3 | <0.001 |
| 15. Servings of cereals and whole grain foods/week | ≥5 servings | 22.3 | 20.3 | 23.2 | 0.012 |
| 16. Servings of refined bread, rice, and/or pasta/week | ≤2 servings | 54.0 | 48.0 | 57.7 | <0.001 |
| 17. Wine consumption | Yes | 88.5 | 83.2 | 92.0 | <0.001 |
| Type of Physical Activity | 0 d (%) | 1 d (%) | 2 d (%) | 3 d (%) | 4 d (%) | 5 d (%) | >5 d (%) | |
|---|---|---|---|---|---|---|---|---|
| Intense | Frequency | 44.1 | 10.5 | 12.9 | 12.8 | 7.5 | 6.1 | 6.2 |
| Moderate | 29.0 | 13.3 | 16.3 | 13.7 | 7.6 | 7.9 | 12.2 | |
| Walking (>10′) | 8.0 | 4.9 | 8.3 | 10.2 | 8.2 | 12.5 | 48.0 | |
| 1–30′ | 30–60′ | 1–2 h | 2–3 h | >3 h | ||||
| Intense | Timing | 52.8 | 25.1 | 16.6 | 3.1 | 2.3 | n.a | n.a |
| Moderate | 50.1 | 29.3 | 15.0 | 2.9 | 2.8 | n.a | n.a | |
| Walking (>10′) | 37.6 | 38.0 | 18.0 | 3.6 | 2.8 | n.a | n.a | |
| 1–2 h | 2–4 h | 5–7 h | 8–10 h | >10 h | ||||
| Sitting time | Timing | 13.8 | 32.1 | 33.5 | 15.7 | 4.9 | n.a | n.a |
| Question | Poor | Fair | Good | V. Good | Excellent | |
|---|---|---|---|---|---|---|
| 1. How would you rate your health compared to people of your age? | 2.4 | 17.2 | 49.9 | 23.5 | 6.9 | |
| No, it does not limit me at all | Yes, it limits me a little | Yes, it limits me | ||||
| 2. Does your current health limit you from moderate efforts, such as moving a table, vacuuming, bowling, or walking for more than an hour? | 80.6 | 14.0 | 5.3 | |||
| 3. Does your current health limit you from climbing several flights of stairs? | 75.3 | 17.2 | 7.4 | |||
| No | Yes | |||||
| 4. During the past 4 weeks, have you been limited to doing less than you would have liked due to your physical health? | 81.7 | 18.3 | ||||
| 5. During the past 4 weeks, have you had to stop doing some tasks at work or in your daily activities because of your physical health? | 85.3 | 14.7 | ||||
| 6. During the past 4 weeks, have you done less than you would have liked to do because of any emotional problems (such as feeling sad, depressed, or anxious)? | 80.6 | 19.4 | ||||
| 7. During the past 4 weeks, have you not done your work or daily activities as carefully as usual because of any emotional problems (such as feeling sad, depressed, or anxious)? | 82.7 | 17.3 | ||||
| Not at all | A little | Moderate | Quite a bit | A lot | ||
| 8. To what extent has pain made it difficult for you to do your usual work (including work outside the home and household chores)? | 62.1 | 22.2 | 8.5 | 5.1 | 2.2 | |
| Never | Only sometimes | Sometimes | Often | Almost always | Always | |
| 9. How often have you felt calm and peaceful? | 2.4 | 8.6 | 28.4 | 45.8 | 0.0 | 14.7 |
| 10. How often have you had a lot of energy? | 3.4 | 12.3 | 35.2 | 37.8 | 0.0 | 11.2 |
| 11. How often have you felt discouraged and sad? | 19.1 | 40.2 | 30.4 | 9.1 | 0.0 | 1.2 |
| 12. How often have physical health or emotional problems made it difficult for you to participate in social activities (such as visiting friends or family)? | 43.3 | 7.8 | 45.7 | 0.0 | 2.6 | 0.6 |
| Westernized Millennial | Healthy | Active Mediterranean | Dysmetabolic/ Pre-Morbid | Metabolically Vulnerable/Pro-Morbid | |
|---|---|---|---|---|---|
| Frequency (%) | 28 | 25 | 17 | 18 | 12 |
| Age (years) | 39.8 ± 15.9 | 43.1 ± 14.6 | 47.2 ± 15.9 | 56.8 ± 15.7 | 59.1 ± 16.4 |
| Female (%) | 55.15 | 90.2 | 18.74 | 85.07 | 35.96 |
| Employment | |||||
| Unemployment | 6.04 | 2.98 | 1.98 | 5.07 | 8.36 |
| Full-time student | 17.43 | 9 | 6.84 | 2.29 | 1.74 |
| Permanent illness/disability | 0.89 | 0.22 | 0.55 | 2.09 | 3.94 |
| Retired | 5.66 | 6.46 | 14.77 | 32.64 | 30.13 |
| Homemaker | 2.48 | 4.35 | 1.54 | 10.75 | 6.47 |
| Paid employment | 67.49 | 77 | 74.31 | 47.16 | 49.37 |
| Smoking | |||||
| Non-smoker | 68.13 | 79.32 | 69.46 | 65.67 | 48.26 |
| Ex-smoker | 11.51 | 13.86 | 20.29 | 21.49 | 27.76 |
| Current smoker | 20.36 | 6.82 | 10.25 | 12.84 | 23.97 |
| Nap daily | 31.93 | 21.77 | 45.87 | 47.76 | 61.04 |
| Sleeping weekdays (7–8 h/d) | 62.02 | 66.26 | 60.31 | 49.95 | 47.79 |
| Sleeping weekends (7–8 h/d) | 53.88 | 62.92 | 63.73 | 52.44 | 49.21 |
| Disease | |||||
| Obesity | 1.34 | 0.44 | 9.7 | 15.12 | 39.27 |
| T2D | 2.04 | 1.16 | 7.28 | 19.2 | 28.08 |
| HBP | 6.74 | 5.15 | 20.18 | 39.9 | 50 |
| Dyslipidemia | 9.73 | 10.09 | 20.51 | 42.79 | 49.21 |
| BMI (kg/m2) | 23.6 ± 3.7 | 22.5 ± 6.4 | 26.4 ± 3.7 | 26.9 ± 3.7 | 30.7 ± 4.5 |
| Snacking (per day) | 53.18 | 30.55 | 29.55 | 40.4 | 68.61 |
| Meals (4 per day) | 30.28 | 35.41 | 36.49 | 31.94 | 26.81 |
| Use of salt habitually | 3.56 | 2.03 | 3.09 | 2.29 | 5.21 |
| MEDAS-17 p | 10.9 ± 2.1 | 6.3 ± 2.4 | 10.5 ± 2.1 | 6.8 ± 2.2 | 10.9 ± 2.1 |
| Characteristic food (%) | |||||
| Whole milk (1–2/week) | 3.63 | 1.67 | 2.21 | 1.59 | 5.52 |
| Semi-skimmed milk (1–2/week) | 4.07 | 7.91 | 6.84 | 9.35 | 5.36 |
| Eggs (3–4/week) | 15.78 | 14.66 | 24.04 | 12.94 | 19.72 |
| Lean meats (3–4/week) | 14.69 | 10.23 | 17.86 | 10.85 | 14.67 |
| Fatty meats (1–2/week) | 23.03 | 5.01 | 10.14 | 7.46 | 32.18 |
| Whitefish (1–2/week) | 8.72 | 22.35 | 25.91 | 26.37 | 11.67 |
| Fatty fish (1–2/week) | 5.98 | 14.44 | 17.42 | 11.04 | 7.26 |
| Vegetables (1–2/day) | 1.91 | 11.10 | 7.72 | 7.36 | 0.95 |
| Fruits (3–4/day) | 1.08 | 3.77 | 6.84 | 3.18 | 1.26 |
| Nuts (+5/week) | 13.17 | 32.95 | 34.18 | 24.98 | 10.09 |
| Legumes (3–4/week) | 2.10 | 4.79 | 5.51 | 3.18 | 3.31 |
| Olive oil (+5/day) | 2.42 | 2.76 | 4.63 | 3.38 | 2.68 |
| Other fats (3–4/week) | 16.16 | 5.44 | 4.96 | 4.58 | 18.45 |
| Refined grains (1–2/week) | 48.60 | 24.89 | 29.44 | 32.14 | 50.16 |
| Whole grains(1–2/week) | 15.90 | 47.31 | 41.90 | 35.22 | 9.46 |
| Pastries (1–2/week) | 37.47 | 7.91 | 7.94 | 11.34 | 45.74 |
| Sugars (1–2/week) | 61.32 | 22.71 | 24.81 | 26.87 | 62.30 |
| Alcohol (1–2/week) | 22.33 | 11.18 | 31.64 | 14.93 | 39.91 |
| Water (+5/day) | 36.07 | 56.10 | 57.66 | 41.79 | 25.55 |
| Health status perception (good or excellent) | 27.35 | 41.22 | 42.67 | 10.45 | 4.1 |
| Physical and psychological state | |||||
| Limited by physical health? | 10.43 | 3.99 | 5.51 | 43.98 | 46.69 |
| Cut back on work or daily activities? | 7 | 2.83 | 3.42 | 37.41 | 40.06 |
| Limited by emotional issues? | 17.75 | 8.56 | 4.63 | 38.61 | 37.7 |
| Problems with work/daily activities? | 16.22 | 7.98 | 3.53 | 33.93 | 33.6 |
| Moderate PA (d/week) | |||||
| 1 | 13.49 | 15.02 | 14.44 | 10.05 | 12.93 |
| 2 | 16.98 | 19.23 | 16.98 | 14.53 | 13.41 |
| 3 | 11.45 | 14.37 | 16.1 | 10.25 | 8.04 |
| 4 | 7.12 | 7.26 | 8.82 | 3.78 | 3.47 |
| 5 | 6.23 | 9.29 | 8.82 | 5.17 | 3.47 |
| +5 | 7.44 | 10.3 | 16.54 | 7.96 | 4.73 |
| No PA | 37.28 | 24.53 | 18.3 | 48.26 | 53.94 |
| Time moderate PA | |||||
| 0–30 min/day | 53.05 | 39.62 | 31.09 | 63.48 | 71.45 |
| 30–60 min/day | 26.97 | 37.08 | 36.38 | 23.78 | 16.4 |
| 1–2 h/day | 14.31 | 18.21 | 22.93 | 10.15 | 6.15 |
| 2–3 h/day | 3.12 | 2.39 | 4.85 | 1.19 | 3.15 |
| +3 h/d | 2.54 | 2.69 | 4.74 | 1.39 | 2.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Perea, A.; Fernández-Cruz, E.; de la O-Pascual, V.; Gonzalez-Zorzano, E.; Moreno-Aliaga, M.J.; Tur, J.A.; Martinez, J.A. Nutritional and Lifestyle Features in a Mediterranean Cohort: An Epidemiological Instrument for Categorizing Metabotypes Based on a Computational Algorithm. Medicina 2024, 60, 610. https://doi.org/10.3390/medicina60040610
García-Perea A, Fernández-Cruz E, de la O-Pascual V, Gonzalez-Zorzano E, Moreno-Aliaga MJ, Tur JA, Martinez JA. Nutritional and Lifestyle Features in a Mediterranean Cohort: An Epidemiological Instrument for Categorizing Metabotypes Based on a Computational Algorithm. Medicina. 2024; 60(4):610. https://doi.org/10.3390/medicina60040610
Chicago/Turabian StyleGarcía-Perea, Aquilino, Edwin Fernández-Cruz, Victor de la O-Pascual, Eduardo Gonzalez-Zorzano, María J. Moreno-Aliaga, Josep A. Tur, and J. Alfredo Martinez. 2024. "Nutritional and Lifestyle Features in a Mediterranean Cohort: An Epidemiological Instrument for Categorizing Metabotypes Based on a Computational Algorithm" Medicina 60, no. 4: 610. https://doi.org/10.3390/medicina60040610






