Understanding CEAP Classification: Insights from an Italian Survey on Corona Phlebectatica and Recurrent Active Venous Ulcers by Vascular Specialists
Abstract
:1. Introduction
2. Material and Methods
- Clinical area: Clinical evaluation in relation to the presence of “corona phlebectatica” and its clinical/prognostic relevance, as well as the frequency and relevance of venous ulcer recurrences in patients with CVD.
- Risk factors: Considerations and behaviours of specialists in relation to risk factors for the evolution of CVD.
- Therapies: Considerations and therapeutic behaviours (physical, pharmacological, and surgical) in the management of a patient with CVD. In particular, they considered the presence of “corona phlebectatica” and the prevention of recurrence of venous ulcers.
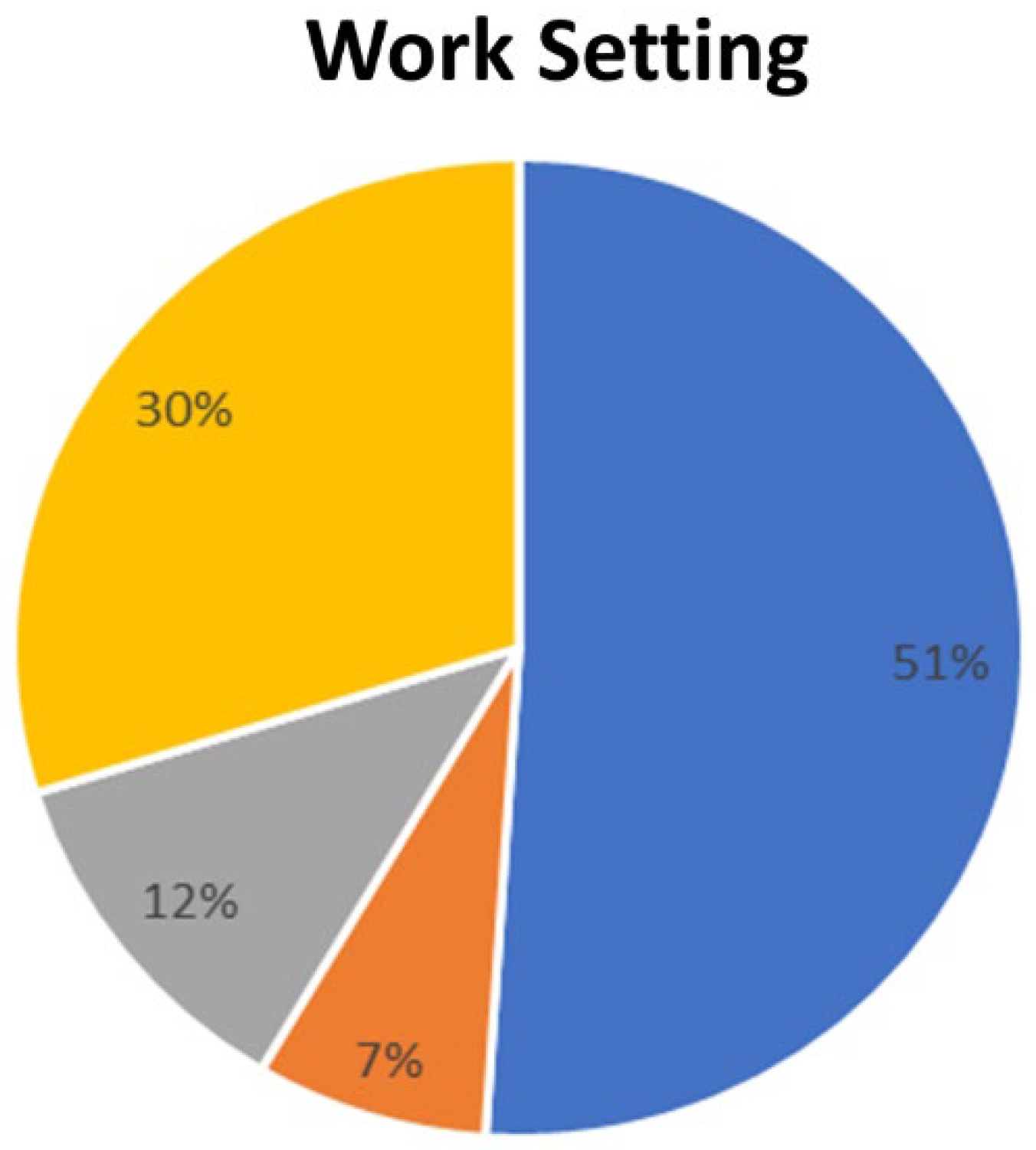
3. Results
3.1. Clinical Area
3.2. Risk Factors
3.3. Therapy
4. Discussion
Study Limitations
5. Conclusions
- (a)
- High frequency of venous ulcer recurrence at over 15%.
- (b)
- Short times for the development of venous ulcer recurrence (1–5 years).
- (c)
- The presence of “corona phlebectatica” is an unfavourable prognostic marker towards the progression of CVD.
- (d)
- Importance of the analysis of patient-related risk factors and strict re-evaluation of the patient.
- (e)
- The extreme importance of a combined approach of compression therapy + pharmacological therapy (GAGs, sulodexide, MPFF), contextually (before, waiting, after) of any hemodynamic correction.
- (f)
- Networks should be established between hospitals and primary care physician and nursing services’ community or wound care centres to ensure continuity of the care of patients.
Key Messages Section
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eklof, B.; Perrin, M.; Delis, K.T.; Rutherford, R.B.; Gloviczki, P. Updated terminology of chronic venous disorders: The VEIN-TERM transatlantic interdisciplinary consensus document. J. Vasc. Surg. 2009, 49, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Jünger, M.; Steins, A.; Hahn, M.; Häfner, H.M. Microcirculatory dysfunction in chronic venous insufficiency (CVI). Microcirculation 2000, 7, S3–S12. [Google Scholar] [CrossRef]
- Beebe, H.G.; Bergan, J.J.; Bergqvist, D.; Eklof, B.; Eriksson, I.; Goldman, M.P.; Greenfield, L.J.; Hobson, R.W., 2nd; Juhan, C.; Kistner, R.L.; et al. Classification and grading of chronic venous disease in the lower limbs. A consensus statement. Eur. J. Vasc. Endovasc. Surg. 1996, 12, 487–491; discussion 491–492. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Bonkemeyer Millan, S.; Gan, R.; Townsend, P.E. Venous Ulcers: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 298–305. [Google Scholar] [PubMed]
- Uhl, J.F.; Cornu-Thenard, A.; Satger, B.; Carpentier, P.H. Clinical analysis of the corona phlebectatica. J. Vasc. Surg. 2012, 55, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, R.E.; Suchkov, I.A.; Puchkova, G.A.; Zhelezinskiĭ, V.P.; Shanaev, I.N. Anatomical aspects of formation of corona phlebectatica. Angiol. Sosud. Khir 2017, 23, 66–70. [Google Scholar] [PubMed]
- Robertson, L.; Lee, A.J.; Gallagher, K.; Carmichael, S.J.; Evans, C.J.; McKinstry, B.H.; Fraser, S.C.; Allan, P.L.; Weller, D.; Ruckley, C.V.; et al. Risk factors for chronic ulceration in patients with varicose veins: A case control study. J. Vasc. Surg. 2009, 49, 1490–1498. [Google Scholar] [CrossRef]
- Salim, S.; Machin, M.; Patterson, B.O.; Onida, S.; Davies, A.H. Global Epidemiology of Chronic Venous Disease: A Systematic Review with Pooled Prevalence Analysis. Ann. Surg. 2021, 274, 971–976. [Google Scholar] [CrossRef]
- De Maeseneer, M.G.; Kakkos, S.K.; Aherne, T.; Baekgaard, N.; Black, S.; Blomgren, L.; Giannoukas, A.; Gohel, M.; de Graaf, R.; Hamel-Desnos, C.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 184–267. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Shi, J.; Li, L.; Ma, Y.; Zhao, H.; Qin, P.; Ma, P. Prevention strategies for the recurrence of venous leg ulcers: A scoping review. Int. Wound J. 2024, 21, e14759. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.; Ramelet, A.A.; Schmeller, W.; Brunner, U.V. Management of leg ulcers. Curr. Probl. Dermatol. 1999, 27, 4–7. [Google Scholar] [CrossRef]
- Evans, C.J.; Fowkes, F.G.; Ruckley, C.V.; Lee, A.J. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J. Epidemiol. Community Health 1999, 53, 149–153. [Google Scholar] [CrossRef]
- Lee, A.J.; Robertson, L.A.; Boghossian, S.M.; Allan, P.L.; Ruckley, C.V.; Fowkes, F.G.; Evans, C.J. Progression of varicose veins and chronic venous insufficiency in the general population in the Edinburgh Vein Study. J. Vasc. Surg. Venous Lymphat. Disord. 2015, 3, 18–26. [Google Scholar] [CrossRef]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef]
- Scotton, M.F.; Miot, H.A.; Abbade, L.P. Factors that influence healing of chronic venous leg ulcers: A retrospective cohort. An. Bras. Dermatol. 2014, 89, 414–422. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, H.B.; Marston, W.A.; Farber, M.A.; Mendes, R.R.; Owens, L.V.; Young, M.L.; Daniel, P.F.; Keagy, B.A. Recurrence of chronic venous ulcers on the basis of clinical, etiologic, anatomic, and pathophysiologic criteria and air plethysmography. J. Vasc. Surg. 2002, 35, 723–728. [Google Scholar] [CrossRef]
- Finlayson, K.; Wu, M.L.; Edwards, H.E. Identifying risk factors and protective factors for venous leg ulcer recurrence using a theoretical approach: A longitudinal study. Int. J. Nurs. Stud. 2015, 52, 1042–1051. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Zhu, B.; Morrow, J.R.; Aldrich, M.B.; Sahihi, A.; Harlin, S.A.; Fife, C.E.; O’Donnell, T.F., Jr.; Sevick-Muraca, E.M. Degradation of lymphatic anatomy and function in early venous insufficiency. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 720–730.e722. [Google Scholar] [CrossRef]
- Gloviczki, P.; Lawrence, P.F.; Wasan, S.M.; Meissner, M.H.; Almeida, J.; Brown, K.R.; Bush, R.L.; Di Iorio, M.; Fish, J.; Fukaya, E.; et al. The 2023 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities. Part II: Endorsed by the Society of Interventional Radiology and the Society for Vascular Medicine. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101670. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.H.; Cornu-Thénard, A.; Uhl, J.F.; Partsch, H.; Antignani, P.L. Appraisal of the information content of the C classes of CEAP clinical classification of chronic venous disorders: A multicenter evaluation of 872 patients. J. Vasc. Surg. 2003, 37, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Uhl, J.F.; Cornu-Thénard, A.; Carpentier, P.H.; Widmer, M.T.; Partsch, H.; Antignani, P.L. Clinical and hemodynamic significance of corona phlebectatica in chronic venous disorders. J. Vasc. Surg. 2005, 42, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Bindlish, S.; Ng, J.; Ghusn, W.; Fitch, A.; Bays, H.E. Obesity, thrombosis, venous disease, lymphatic disease, and lipedema: An obesity medicine association (OMA) clinical practice statement (CPS) 2023. Obes. Pillars 2023, 8, 100092. [Google Scholar] [CrossRef]
- de Moraes Silva, M.A.; Nelson, A.; Bell-Syer, S.E.; Jesus-Silva, S.G.; Miranda, F., Jr. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst. Rev. 2024, 3, Cd002303. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lessiani, G.; Gazzabin, L.; Cocco, G.; Corvino, A.; D’Ardes, D.; Boccatonda, A. Understanding CEAP Classification: Insights from an Italian Survey on Corona Phlebectatica and Recurrent Active Venous Ulcers by Vascular Specialists. Medicina 2024, 60, 618. https://doi.org/10.3390/medicina60040618
Lessiani G, Gazzabin L, Cocco G, Corvino A, D’Ardes D, Boccatonda A. Understanding CEAP Classification: Insights from an Italian Survey on Corona Phlebectatica and Recurrent Active Venous Ulcers by Vascular Specialists. Medicina. 2024; 60(4):618. https://doi.org/10.3390/medicina60040618
Chicago/Turabian StyleLessiani, Gianfranco, Luca Gazzabin, Giulio Cocco, Antonio Corvino, Damiano D’Ardes, and Andrea Boccatonda. 2024. "Understanding CEAP Classification: Insights from an Italian Survey on Corona Phlebectatica and Recurrent Active Venous Ulcers by Vascular Specialists" Medicina 60, no. 4: 618. https://doi.org/10.3390/medicina60040618
APA StyleLessiani, G., Gazzabin, L., Cocco, G., Corvino, A., D’Ardes, D., & Boccatonda, A. (2024). Understanding CEAP Classification: Insights from an Italian Survey on Corona Phlebectatica and Recurrent Active Venous Ulcers by Vascular Specialists. Medicina, 60(4), 618. https://doi.org/10.3390/medicina60040618








