Uterine Cesarean Scar Tissue—An Immunohistochemical Study
Abstract
1. Introduction
1.1. The Wound Healing Process
1.2. The Cluster of Differentiation 31 Antigen
1.3. Structural Proteins in the Repair and Healing Process
2. Material and Methods
- Group 1 consisted of 52 primipara participants.
- Group 2 consisted of 15 participants, 13–23 months.
- Group 3 consisted of 17 participants, 24–30 months.
- Group 4 consisted of 21 participants, 31–36 months.
- Group 5 consisted of 16 participants, 37–42 months.
- Group 6 consisted of 29 participants, 43–60 months.
- Group 7 consisted of 27 participants, more than 60 months.
2.1. Reagents Used for Immunohistochemical Determinations
2.2. Surgical Procedures
2.3. Morphological Study
2.4. Immunohistochemistry
2.5. Digital Image Analysis (Digital Computer-Assisted Analysis Technique)
2.6. Statistical Analysis
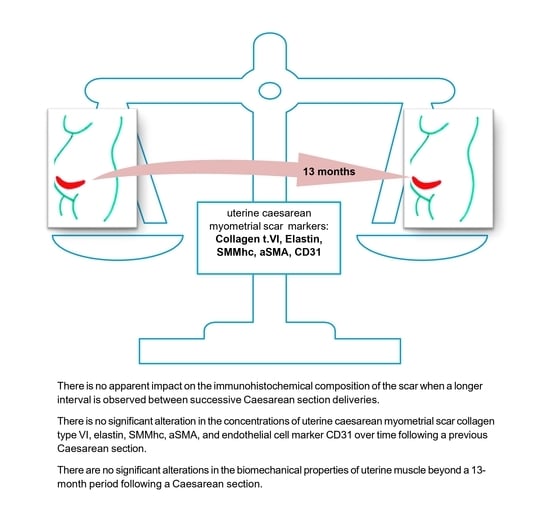
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aSMA | Alpha-smooth muscle actin |
| BM | basement membranes |
| CD31 | cluster of differentiation 31 |
| CS | cesarean section |
| ECM | extracellular matrix |
| IHC | immunohistochemistry |
| SMC | smooth muscle cells |
| SMMhc | smooth muscle myosin heavy chain |
References
- Hamilton, B.E.; Martin, J.A.; Osterman, M.J.; Curtin, S.C.; Matthews, T.J. Birth: Final Data for 2014. Natl. Vital. Stat. Rep. 2015, 64, 1–64. [Google Scholar] [PubMed]
- Melo-Cerda, I. Cesarean scar defect. Ginecol. Obstet. Mex. 2014, 82, 530–534. [Google Scholar] [PubMed]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef]
- Bauer, S.M.; Bauer, R.J.; Velazquez, O.C. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc. Endovascular Surg. 2005, 39, 293–306. [Google Scholar] [CrossRef]
- Takada, S.; Hojo, M.; Takebe, N.; Tanigaki, K.; Miyamoto, S. Role of Endothelial-to-Mesenchymal Transition in the Pathogenesis of Central Nervous System Hemangioblastomas. World Neurosurg. 2018, 117, e187–e193. [Google Scholar] [CrossRef]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Levillain, A.; Orhant, M.; Turquier, F.; Hoc, T. Contribution of collagen and elastin fibers to the mechanical behavior of an abdominal connective tissue. J. Mech. Behav. Biomed. Mater. 2016, 61, 308–317. [Google Scholar] [CrossRef]
- Nallasamy, S.; Yoshida, K.; Akins, M.; Myers, K.; Iozzo, R.; Mahendroo, M. Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagenand Elastic Fibers. Endocrinology 2017, 158, 950–962. [Google Scholar] [CrossRef]
- Varma, S.; Stéphenne, X.; Komuta, M.; Bouzin, C.; Ambroise, J.; Smets, F.; Reding, R.; Sokal, E.M. The histological quantification of alpha-smooth muscle actin predicts future graft fibrosis in pediatric liver transplant recipients. Pediatr. Transplant. 2017, 21, e12834. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchil Livingstone: London, UK, 2002. [Google Scholar]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Roeder, H.A.; Cramer, S.F.; Leppert, P.C. A look at uterine wound healing through a histopathological study of uterine scars. Reprod. Sci. 2012, 19, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Borel, J. Uterine collagens. General. Review. Rev. Fr. Gynecol. Obstet. 1991, 86, 715–722. [Google Scholar] [PubMed]
- Luther, D.J.; Thodeti, C.K.; Shamhart, P.E.; Adapala, R.K.; Hodnichak, C.; Weihrauch, D.; Bonaldo, P.; Chilian, W.M.; Meszaros, J.G. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ. Res. 2012, 110, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, C.S.; Buhimschi, I.A.; Yu, C.; Wang, H.; Sharer, D.J.; Diamond, M.P.; Petkova, A.P.; Garfield, R.E.; Saade, G.R.; Weiner, C.P. The effect of dystocia and previous cesarean uterine scar on the tensile properties of the lower uterine segment. Am. J. Obstet. Gynecol. 2006, 194, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Pollio, F.; Staibano, S.; Mascolo, M.; Salvatore, G.; Persico, F.; De Falco, M.; Di Lieto, A. Uterine dehiscence in term pregnant patients with one previous cesarean delivery: Growth factor immunoexpression and collagen content in the scarred lower uterine segment. Am. J. Obstet. Gynecol. 2006, 194, 527–534. [Google Scholar] [CrossRef]
- House, M.; Kaplan, D.L.; Socrate, S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin. Perinatol. 2009, 33, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Jayes, F.L.; Segars, J.H. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet. Gynecol. Int. 2014, 2014, 783289. [Google Scholar] [CrossRef]
- Woessner, J.F.; Brewer, T.H. Formation and breakdown of collagen and elastin in the human uterus during pregnancy and post-partum involution. Biochem. J. 1963, 89, 75–82. [Google Scholar] [CrossRef]
- Dawning, K.T.; Billah, M.; Raparia, E.; Shah, A.; Silverstein, M.C.; Ahmad, A.; Boutis, G.S. The role of mode of delivery on elastic fiber architecture and vaginal vault elasticity: A rodent model study. J. Mech. Behav. Biomed. Mater. 2014, 29, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Dhital, B.; Gul-E-Noor, F.; Downing, K.T.; Hirsch, S.; Boutis, G.S. Pregnancy-Induced Dynamical and Structural Changes of Reproductive Tract Collagen. Biophys. J. 2016, 111, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Morrione, T.G.; Seifter, S. Alteration in the collagen content of the human uterus during pregnancy and post partum involution. J. Exp. Med. 1962, 115, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.J.; Franzblau, C. Increase in urinary desmosine and pyridinoline during postpartum involution of the uterus in humans. Proc. Soc. Exp. Biol. Med. 1995, 210, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Word, R.A.; Pathi, S.; Schaffer, J.I. Pathophysiology of pelvic organ prolapse. Obstet. Gynecol. Clin. North. Am. 2009, 36, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Roman, C.D.; Choy, H.; Nanney, L.; Riordan, C.; Parman, K.; Johnson, D.; Beauchamp, R.D. Vascular endothelial growth factor-mediated angiogenesis inhibition and postoperative wound healing in rats. J. Surg. Res. 2002, 105, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, D. Neovascularisation in wound healing. J. Wound Care 2008, 17, 298–300, 302. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ferreira, A.M.; Oberyszyn, T.M. Regulation of scar formation by vascular endothelial growth factor. Lab. Investig. 2008, 88, 579–590. [Google Scholar] [CrossRef]
- Wietecha, M.S.; DiPietro, L.A. Therapeutic Approaches to the Regulation of Wound Angiogenesis. Adv. Wound Care 2013, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Bangal, V.B.; Giri, P.A.; Shinde, K.K. Vaginal birth after cesarean section. N. Am. J. Med. Sci. 2013, 5, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Guise, J.M.; McDonagh, M.S.; Osterweil, P.; Nygren, P.; Chan, B.K.S.; Helfand, M. Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. BMJ 2004, 329, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Ehsan, A.; Arif, S.; Murtaza, J.; Hanif, A. Evaluating trial of scar in patients with a history of caesarean section. N. Am. J. Med. Sci. 2011, 3, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Leindecker, S.; Spong, C.Y.; Hauth, J.C.; Bloom, S.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. The MFMU Cesarean Registry: Factors affecting the success of trial of labor after previous cesarean delivery. Am. J. Obstet. Gynecol. 2005, 193 Pt 2, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Hauth, J.C.; Bloom, S.L.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. Risk of uterine rupture with a trial of labor in women with multiple and single prior cesarean delivery. Obstet. Gynecol. 2006, 108, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Macones, G.A.; Cahill, A.G.; Stamilio, D.M.; Odibo, A.; Peipert, J.; Stevens, E.J. Can uterine rupture in patients attempting vaginal birth after cesarean delivery be predicted? Am. J. Obstet. Gynecol. 2006, 195, 1148–1152. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Rosas-Bermudez, A.; Kafury-Goeta, A.C. Birth spacing and risk of adverse perinatal outcomes: A metaanalysis. JAMA 2006, 295, 1809–1823. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Rosas-Bermudez, A.; Kafury-Goeta, A.C. Effects of birth spacing on maternal health: A systematic review. Am. J. Obstet. Gynecol. 2007, 196, 297–308. [Google Scholar] [CrossRef]
- Esposito, M.A.; Menihan, C.A.; Malee, M.P. Association of interpregnancy interval with uterine scar failure in labor: A case–control study. Am. J. Obstet. Gynecol. 2000, 183, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.M.; Zelop, C.M. Vaginal birth after cesarean: Assessing maternal and perinatal risks- -contemporary management. Clin. Obstet. Gynecol. 2006, 49, 147–153. [Google Scholar] [CrossRef]
- Birth after Previous Caesarean Birth. Green-Top Guideline Number 45; Royal College of Obstetricians and Gynaecologists: London, UK, 2015. [Google Scholar]
- Bujold, E.; Gauthier, R.J. Risk of uterine rupture associated with an interdelivery interval between 18 and 24 months. Obstet. Gynecol. 2010, 115, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Bujold, E.; Mehta, S.H.; Bujold, C.; Gauthier, R.J. Interdelivery interval and uterine rupture. Am. J. Obstet. Gynecol. 2002, 187, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Cahill, A.G.; Macones, G.A. Vaginal birth after cesarean delivery: Evidence-based practice. Clin. Obstet. Gynecol. 2007, 50, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Shipp, T.D.; Zelop, C.M.; Repke, J.T.; Cohen, A.; Lieberman, E. Interdelivery interval and risk of symptomatic uterine rupture. Obstet. Gynecol. 2001, 97, 175–177. [Google Scholar]
- Stamilio, D.M.; DeFranco, E.; Pare, E.; Odibo, A.O.; Peipert, J.F.; Allsworth, J.E.; Stevens, E.; Macones, G.A. Short interpregnancy interval risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet. Gynecol. 2007, 110, 1075–1082. [Google Scholar] [CrossRef]







| Interpregnancy Interval (Months) | Maternal Age (Years) * | Maternal Weight (kg) * | Gestational Age (Weeks) * | Birth Weight (kg) * | |
|---|---|---|---|---|---|
| Group I | - | 28.05 ± 3.71 | 75.04 ± 8.09 | 38.6 ± 1.2 | 3.256 ± 0.556 |
| Group II | 20.07 ± 3.03 | 32.5 ± 4.46 | 82.45 ± 18.61 | 38.67 ± 0.98 | 3.354 ± 0.279 |
| Group III | 26.35 ± 2.45 | 31.27 ± 4.13 | 78.63 ± 12.62 | 38.42 ± 1.52 | 3.318 ± 0.674 |
| Group IV | 34.75 ± 1.29 | 34.81 ± 5.0 | 81.21 ± 14.57 | 37.95 ± 2.85 | 3.281 ± 0.683 |
| Group V | 44.25 ± 3.39 | 33.71 ± 7.12 | 81.39 ± 15.92 | 38.06 ± 2.14 | 3.215 ± 0.568 |
| Group VI | 54.69 ± 5.11 | 33.36 ± 4.56 | 80.61 ± 12.36 | 38.14 ± 1.15 | 3.334 ± 0.328 |
| Group VII | 92.61 ± 33.10 | 32.82 ± 4.83 | 81.96 ± 11.63 | 37.93 ± 2.1 | 3.231 ± 0.656 |
| Parameter | Actin Area (aSMA) | Myosin Area (SMMhc) | Elastin Area | Collagen Area (Collagen t.VI) | CD 31 Area (PECAM-1) |
|---|---|---|---|---|---|
| Group I | 65,933.65 ± 33,522.77 | 5870.08 ± 5939.30 | 5002.57 ± 3823.45 | 23,379.68 ± 14,945.39 | 3553.13 ± 2932.26 |
| Group II | 40,730.74 ± 27,791.32 | 7198.77 ± 6749.24 | 5522.69 ± 6390.30 | 14,122.49 ± 11,119.03 | 2490.21 ± 1523.59 |
| Group III | 37,578.51 ± 33,305.28 | 7554.65 ± 8615.21 | 4972.82 ± 4437.13 | 24,045.47 ± 21,182.52 | 2013.35 ± 1587.92 |
| Group IV | 28,598.35 ± 23,383.7 | 8097.26 ± 8845.94 | 3805.66 ± 4234.11 | 13,136.13 ± 11,771.61 | 2070.71 ± 1417.69 |
| Group V | 40,118.16 ± 21,442.87 | 10,338.99 ± 9192.84 | 7909.23 ± 9559.51 | 17,991.79 ± 13,068.37 | 2760.05 ± 2155.23 |
| Group VI | 37,833.75 ± 26,360.83 | 7569.92 ± 6656.56 | 9693.78 ± 17235.27 | 26,225.81 ± 20,777.57 | 2084.36 ± 1282.20 |
| Group VII | 43,394.51 ± 30,965.22 | 4837.22 ± 6096.49 | 5604.20 ± 5449.66 | 19,540.48 ± 15,068.75 | 2380.13 ± 1564.63 |
| Parameter | Actin % (aSMA) | Myosin % (SMMhc) | Elastin % | Collagen % (Collagen t.VI) | CD 31% (PECAM-1) |
|---|---|---|---|---|---|
| Group I | 41.33 ± 14.68 | 3.07 ± 4.3 | 2.11 ± 3.19 | 14.65 ± 10.91 | 2.06 ± 1.21 |
| Group II | 29.92 ± 19.23 | 4.75 ± 4.93 | 3.59 ± 4.64 | 9.94 ± 7.83 | 1.54 ± 0.83 |
| Group III | 21.72 ± 17.8 | 4.18 ± 5.16 | 3.19 ± 3.31 | 12.63 ± 8.14 | 1.10 ± 0.67 |
| Group IV | 19.28 ± 16.38 | 5.32 ± 5.64 | 2.27 ± 3.17 | 9.05 ± 8.43 | 1.34 ± 1.0 |
| Group V | 24.88 ± 15.83 | 6.33 ± 6.16 | 2.74 ± 3.64 | 12.12 ± 9.62 | 1.45 ± 0.79 |
| Group VI | 23.77 ± 17.43 | 4.32 ± 3.06 | 4.39 ± 4.78 | 13.76 ± 9.30 | 1.24 ± 0.8 |
| Group VII | 30.16 ± 22.2 | 2.69 ± 3.9 | 3.43 ± 4.07 | 12.49 ± 10.78 | 1.43 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziętek, M.; Świątkowska-Feund, M.; Ciećwież, S.; Machałowski, T.; Szczuko, M. Uterine Cesarean Scar Tissue—An Immunohistochemical Study. Medicina 2024, 60, 651. https://doi.org/10.3390/medicina60040651
Ziętek M, Świątkowska-Feund M, Ciećwież S, Machałowski T, Szczuko M. Uterine Cesarean Scar Tissue—An Immunohistochemical Study. Medicina. 2024; 60(4):651. https://doi.org/10.3390/medicina60040651
Chicago/Turabian StyleZiętek, Maciej, Małgorzata Świątkowska-Feund, Sylwester Ciećwież, Tomasz Machałowski, and Małgorzata Szczuko. 2024. "Uterine Cesarean Scar Tissue—An Immunohistochemical Study" Medicina 60, no. 4: 651. https://doi.org/10.3390/medicina60040651
APA StyleZiętek, M., Świątkowska-Feund, M., Ciećwież, S., Machałowski, T., & Szczuko, M. (2024). Uterine Cesarean Scar Tissue—An Immunohistochemical Study. Medicina, 60(4), 651. https://doi.org/10.3390/medicina60040651