Clinicopathologic Analysis and Prognostic Factors for Survival in Patients with Operable Ampullary Carcinoma: A Multi-Institutional Retrospective Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical and Histopathologic Features
2.3. Statistics
3. Results
3.1. Demographic Data
3.2. Recurrence and Life Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, R.; Friederichs, J.; Schuster, T.; Gertler, R.; Maak, M.; Becker, K.; Grebner, A.; Ulm, K.; Höfler, H.; Nekarda, H.; et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: A single-center analysis of 3026 patients over a 25-year time period. Ann. Surg. 2008, 248, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Enjoji, M. Carcinoma of the ampulla of vater. A clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer 1987, 59, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Albores-Saavedra, J.; Schwartz, A.M.; Batich, K.; Henson, D.E. Cancers of the ampulla of vater: Demographics, morphology, and survival based on 5625 cases from the SEER program. J. Surg. Oncol. 2009, 100, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.R.; Klimstra, D.S.; Moccia, R.D.; Conlon, K.C.; Brennan, M.F. Factors predictive of survival in ampullary carcinoma. Ann. Surg. 1998, 228, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, L. Staging of ampullary carcinoma by endoscopic ultrasonography. Endoscopy 1998, 30 (Suppl. S1), A128–A131. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, F.; Crippa, S.; Castelli, P.; Aleotti, F.; Pucci, A.; Partelli, S.; Zamboni, G.; Falconi, M. Management of ampullary neoplasms: A tailored approach between endoscopy and surgery. World J. Gastroenterol. 2015, 21, 7970–7987. [Google Scholar] [CrossRef]
- Bettschart, V.; Rahman, M.Q.; Engelken, F.J.F.; Madhavan, K.K.; Parks, R.W.; Garden, O.J. Presentation, treatment and outcome in patients with ampullary tumours. Br. J. Surg. 2004, 91, 1600–1607. [Google Scholar] [CrossRef]
- Morris-Stiff, G.; Alabraba, E.; Tan, Y.M.; Shapey, I.; Bhati, C.; Tanniere, P.; Mayer, D.; Buckels, J.; Bramhall, S.; Mirza, D.F. Assessment of survival advantage in ampullary carcinoma in relation to tumour biology and morphology. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2009, 35, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, C.; Limongelli, P.; Pai, M.; Ahmad, R.; Stamp, G.; Habib, N.; Williamson, R.; Jiao, L. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: An analysis of clinicopathological factors. J. Surg. Oncol. 2009, 100, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Sohn, T.A.; Cameron, J.L.; Hruban, R.H.; Lillemoe, K.D.; Pitt, H.A. Periampullary adenocarcinoma: Analysis of 5-year survivors. Ann. Surg. 1998, 227, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Palta, M.; Patel, P.; Broadwater, G.; Willett, C.; Pepek, J.; Tyler, D.; Zafar, S.Y.; Uronis, H.; Hurwitz, H.; White, R.; et al. Carcinoma of the ampulla of Vater: Patterns of failure following resection and benefit of chemoradiotherapy. Ann. Surg. Oncol. 2012, 19, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Nappo, G.; Gentile, D.; Galvanin, J.; Capretti, G.; Ridolfi, C.; Petitti, T.; Spaggiari, P.; Carrara, S.; Gavazzi, F.; Repici, A.; et al. Trans-duodenal ampullectomy for ampullary neoplasms: Early and long-term outcomes in 36 consecutive patients. Surg. Endosc. 2020, 34, 4358–4368. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.C.; Vitale, G.C. Ampullary tumors: Endoscopic versus operative management. Surg. Innov. 2004, 11, 255–263. [Google Scholar] [CrossRef]
- Posner, S.; Colletti, L.; Knol, J.; Mulholland, M.; Eckhauser, F. Safety and long-term efficacy of transduodenal excision for tumors of the ampulla of Vater. Surgery 2000, 128, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Post, S.; Neuhaus, P.; Gellert, K.; Langrehr, J.; Ridwelski, K.; Schramm, H.; Fahlke, J.; Zuelke, C.; Burkart, C.; et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007, 297, 267–277. [Google Scholar] [CrossRef]
- Arkadopoulos, N.; Kyriazi, M.A.; Papanikolaou, I.S.; Vasiliou, P.; Theodoraki, K.; Lappas, C.; Oikonomopoulos, N.; Smyrniotis, V. Preoperative biliary drainage of severely jaundiced patients increases morbidity of pancreaticoduodenectomy: Results of a case-control study. World J. Surg. 2014, 38, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.K.; Miller, R.C.; Hsu, C.C.; Bhatia, S.; Pawlik, T.M.; Laheru, D.; Hruban, R.H.; Zhou, J.; Winter, J.M.; Haddock, M.G.; et al. Evaluation of adjuvant chemoradiation therapy for ampullary adenocarcinoma: The Johns Hopkins Hospital-Mayo Clinic collaborative study. Radiat. Oncol. Lond. Engl. 2011, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.B.; Maggard, M.A.; Manunga, J.; Tomlinson, J.S.; Reber, H.A.; Ko, C.Y.; Hines, O.J. Survival after resection of ampullary carcinoma: A national population-based study. Ann. Surg. Oncol. 2008, 15, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Kopelson, G.; Galdabini, J.; Warshaw, A.L.; Gunderson, L.L. Patterns of failure after curative surgery for extra-hepatic biliary tract carcinoma: Implications for adjuvant therapy. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.G.; Warshaw, A.L.; Convery, K.; Compton, C.C. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg. Gynecol. Obstet. 1993, 176, 33–38. [Google Scholar] [PubMed]
- Bhatia, S.; Miller, R.C.; Haddock, M.G.; Donohue, J.H.; Krishnan, S. Adjuvant therapy for ampullary carcinomas: The Mayo Clinic experience. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Chie, E.K.; Jang, J.-Y.; Kim, S.W.; Oh, D.-Y.; Im, S.-A.; Kim, T.-Y.; Bang, Y.-J.; Ha, S.W. Role of adjuvant chemoradiotherapy for ampulla of Vater cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 436–441. [Google Scholar] [CrossRef]
- Sikora, S.; Balachandran, P.; Dimri, K.; Rastogi, N.; Kumar, A.; Saxena, R.; Kapoor, V. Adjuvant chemo-radiotherapy in ampullary cancers. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2005, 31, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Valsangkar, N.P.; Ingkakul, T.; Correa-Gallego, C.; Mino-Kenudson, M.; Masia, R.; Lillemoe, K.D.; Castillo, C.F.-D.; Warshaw, A.L.; Liss, A.S.; Thayer, S.P. Survival in ampullary cancer: Potential role of different KRAS mutations. Surgery 2015, 157, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kawaida, H.; Kono, H.; Amemiya, H.; Hosomura, N.; Watanabe, M.; Saito, R.; Maruyama, S.; Shimizu, H.; Furuya, S.; Akaike, H.; et al. Stratification of Prognosis in Patients with Ampullary Carcinoma After Surgery by Preoperative Platelet-to-lymphocyte Ratio and Conventional Tumor Markers. Anticancer. Res. 2019, 39, 6923–6929. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, H.; Wang, S.; Xiang, J.; Liu, X. Prognostic impact of circulating tumor cells in patients with ampullary cancer. J. Cell Physiol. 2018, 233, 5014–5022. [Google Scholar] [CrossRef]
- John, T.G.; Greig, J.D.; Carter, D.C.; Garden, O.J. Carcinoma of the pancreatic head and periampullary region. Tumor staging with laparoscopy and laparoscopic ultrasonography. Ann. Surg. 1995, 221, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Enjoji, M.; Kitamura, K. Non-icteric ampullary carcinoma with a favorable prognosis. Am. J. Gastroenterol. 1990, 85, 994–999. [Google Scholar] [PubMed]
- Makipour, H.; Cooperman, A.; Danzi, J.T.; Farmer, R.G. Carcinoma of the ampulla of Vater: Review of 38 cases with emphasis on treatment and prognostic factors. Ann. Surg. 1976, 183, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.T.; Grenert, J.P.; Rubenstein, L.; Stewart, L.; Way, L.W. Tumors of the ampulla of vater: Histopathologic classification and predictors of survival. J. Am. Coll. Surg. 2008, 207, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Klempnauer, J.; Ridder, G.J.; Maschek, H.; Pichlmayr, R. Carcinoma of the ampulla of vater: Determinants of long-term survival in 94 resected patients. HPB Surg. World J. Hepatic Pancreat. Biliary Surg. 1998, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Shibata, K.; Iwaki, K.; Kai, S.; Ohta, M.; Kitano, S. Ampullary cancer and preoperative jaundice: Possible indication of the minimal surgery. Hepatogastroenterology 2009, 56, 1194–1198. [Google Scholar] [PubMed]
- Kamisawa, T.; Tu, Y.; Egawa, N.; Nakajima, H.; Horiguchi, S.-I.; Tsuruta, K.; Okamoto, A. Clinicopathologic features of ampullary carcinoma without jaundice. J. Clin. Gastroenterol. 2006, 40, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Pamukcuoglu, M.; Oksuzoglu, B.; Abali, H.; Akoglu, M.; Atalay, F.; Budakoglu, B.; Uncu, D.; Ozdemir, N.Y.; Guler, T.; Zengin, N. Prognostic factors and clinicopathological characteristics of carcinoma of ampulla Vateri. Int. Surg. 2008, 93, 214–219. [Google Scholar] [PubMed]
- Kimura, W.; Futakawa, N.; Zhao, B. Neoplastic diseases of the papilla of Vater. J. Hepatobiliary Pancreat. Surg. 2004, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Kundhal, P.S.; McGilvray, I.D.; Cattral, M.S.; Taylor, B.; Langer, B.; Grant, D.R.; Zogopoulos, G.; Shah, S.A.; Greig, P.D.; et al. Predictors of failure after pancreaticoduodenectomy for ampullary carcinoma. J. Am. Coll. Surg. 2006, 202, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Outcome of Pancreaticoduodenectomy and Impact of Adjuvant Therapy for Ampullary Carcinomas—PubMed [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/10863064-outcome-of-pancreaticoduodenectomy-and-impact-of-adjuvant-therapy-for-ampullary-carcinomas/?from_term=Outcome+of+pancreaticoduodenectomy+and+impact+of+adjuvant+therapy+for+ampullary+carcinomas (accessed on 11 March 2020).
- Winter, J.M.; Cameron, J.L.; Olino, K.; Herman, J.M.; De Jong, M.C.; Hruban, R.H.; Wolfgang, C.L.; Eckhauser, F.; Edil, B.H.; Choti, M.A.; et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: Implications for surgical strategy and long-term prognosis. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment Tract. 2010, 14, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.C.; Bhandari, M.; Astill, D.S.; Wilson, T.G.; Kow, L.; Brooke-Smith, M.; Toouli, J.; Padbury, R.T.A. Predicting patient survival after pancreaticoduodenectomy for malignancy: Histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB 2010, 12, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pomianowska, E.; Westgaard, A.; Mathisen, Ø.; Clausen, O.P.F.; Gladhaug, I.P. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann. Surg. Oncol. 2013, 20, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Zhao, Y.; Ye, M.; Yang, Y.; Zhao, J.; Huang, Y.; Wan, Y. Carcinoma of the ampulla of Vater: Factors influencing long-term survival of 127 patients with resection. World J. Surg. 2007, 31, 137–143; discussion 144–146. [Google Scholar] [CrossRef] [PubMed]
- Sudo, T.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Hashimoto, Y.; Ohge, H.; Shimamoto, F.; Sueda, T. Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig. Dis. Sci. 2008, 53, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- El Nakeeb, A.; El Sorogy, M.; Ezzat, H.; Said, R.; El Dosoky, M.; El Gawad, M.A.; Elsabagh, A.M.; El Hanafy, E. Predictors of long-term survival after pancreaticoduodenectomy for peri-ampullary adenocarcinoma: A retrospective study of 5-year survivors. Hepatobiliary Pancreat. Dis. Int. HBPD Int. 2018, 17, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Sikora, S.S.; Kapoor, S.; Krishnani, N.; Kumar, A.; Saxena, R.; Kapoor, V.K. Long-term survival and recurrence patterns in ampullary cancer. Pancreas 2006, 32, 390–395. [Google Scholar] [CrossRef]
- Roder, J.D.; Schneider, P.M.; Stein, H.J.; Siewert, J.R. Number of lymph node metastases is significantly associated with survival in patients with radically resected carcinoma of the ampulla of Vater. Br. J. Surg. 1995, 82, 1693–1696. [Google Scholar] [CrossRef]
- Schmidt, C.M.; Powell, E.S.; Yiannoutsos, C.T.; Howard, T.J.; Wiebke, E.A.; Wiesenauer, C.A.; Baumgardner, J.A.; Cummings, O.W.; Jacobson, L.E.; Broadie, T.A.; et al. Pancreaticoduodenectomy: A 20-year experience in 516 patients. Arch. Surg. 1960. 2004, 139, 718–725; discussion 725–727. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Shyr, Y.-M.; Chou, S.-C.; Wang, S.-E. The role of lymph nodes in predicting the prognosis of ampullary carcinoma after curative resection. World J. Surg. Oncol. 2015, 13, 224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, W.S.; Choi, D.W.; Choi, S.H.; Heo, J.S.; You, D.D.; Lee, H.G. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J. Surg. Oncol. 2012, 105, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.C.; Coban, I.; Adsay, N.V.; Sarmiento, J.M.; Chu, C.K.; Staley, C.A.; Galloway, J.R.; Kooby, D.A. Important prognostic factors in adenocarcinoma of the ampulla of Vater. Am. Surg. 2009, 75, 754–760; discussion 761. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Koh, K.; Kawabe, T.; Son, E.; Yoshikawa, H.; Yasutomi, M. Importance of microperineural invasion as a prognostic factor in ampullary carcinoma. Br. J. Surg. 1997, 84, 1399–1401. [Google Scholar] [PubMed]
- Yamaguchi, K.; Nishihara, K. Long- and short-term survivors after pancreatoduodenectomy for ampullary carcinoma. J. Surg. Oncol. 1992, 50, 195–200. [Google Scholar] [CrossRef] [PubMed]
- el-Ghazzawy, A.G.; Wade, T.P.; Virgo, K.S.; Johnson, F.E. Recent experience with cancer of the ampulla of Vater in a national hospital group. Am. Surg. 1995, 61, 607–611. [Google Scholar] [PubMed]
- Klein, F.; Jacob, D.; Bahra, M.; Pelzer, U.; Puhl, G.; Krannich, A.; Andreou, A.; Gül, S.; Guckelberger, O. Prognostic factors for long-term survival in patients with ampullary carcinoma: The results of a 15-year observation period after pancreaticoduodenectomy. HPB Surg. World J. Hepatic Pancreat. Biliary Surg. 2014, 2014, 970234. [Google Scholar] [CrossRef]
- Beger, H.G.; Treitschke, F.; Gansauge, F.; Harada, N.; Hiki, N.; Mattfeldt, T. Tumor of the ampulla of Vater: Experience with local or radical resection in 171 consecutively treated patients. Arch. Surg. 1999, 134, 526–532. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The ESPAC-3 periampullary cancer randomized trial. JAMA 2012, 308, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hsu, C.C.; Winter, J.M.; Pawlik, T.M.; Laheru, D.; Hughes, M.A.; Donehower, R.; Wolfgang, C.; Akbar, U.; Schulick, R.; et al. Adjuvant chemoradiation versus surgery alone for adenocarcinoma of the ampulla of Vater. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 92, 244–248. [Google Scholar] [CrossRef]
- Acharya, A.; Markar, S.R.; Sodergren, M.H.; Malietzis, G.; Darzi, A.; Athanasiou, T.; Khan, A.Z. Meta-analysis of adjuvant therapy following curative surgery for periampullary adenocarcinoma. Br. J. Surg. 2017, 104, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Doepker, M.P.; Thompson, Z.J.; Centeno, B.A.; Kim, R.D.; Wong, J.; Hodul, P.J. Clinicopathologic and survival analysis of resected ampullary adenocarcinoma. J. Surg. Oncol. 2016, 114, 170–175. [Google Scholar] [CrossRef]
- Li, H.-B.; Zhao, F.-Q.; Zhou, J. Prognostic Nomogram for Disease-Specific Survival in Patients with Non-metastatic Ampullary Carcinoma After Surgery. Ann. Surg. Oncol. 2019, 26, 1079–1085. [Google Scholar] [CrossRef]

| Characteristics | No. of Patients, n = 197 (%) |
|---|---|
| Age, years | |
| Median | 60 |
| Range | 29–90 |
| 50 | 39 (19.8) |
| ≥50 | 158(80.2) |
| Sex | |
| Mele | 120 (60.9) |
| Female | 77 (39.1) |
| Performance status | |
| 0–1 | 154 (78.1) |
| 2–3 | 43 (21.9) |
| TNM stage | |
| I | 67 (34) |
| II | 103 (52.2) |
| III | 27 (13.8) |
| T stage | |
| 1–2 | 99 (50.2) |
| 3–4 | 98 (49.8) |
| N stage | |
| Positive | 90 (45.6) |
| Negative | 107 (54.4) |
| Histological grade | |
| Well differentiated | 58 (29.4) |
| Moderately differentiated | 75 (38) |
| Poorly differentiated | 17 (8.6) |
| Pathological subtype | |
| Adenocarcinoma | 170 (86.2) |
| Mucinous | 18 (9.1) |
| Other | 9 (4.6) |
| Lymphovascular invasion | |
| Yes | 69 (35) |
| No | 101 (51.2) |
| Perineural invasion | |
| Yes | 50 (25.3) |
| No | 120 (60.9) |
| Surgical margin | |
| Positive | 13 (6.5) |
| Negative | 184 (93.5) |
| Adjuvant treatment | |
| CT | 74 (37.5) |
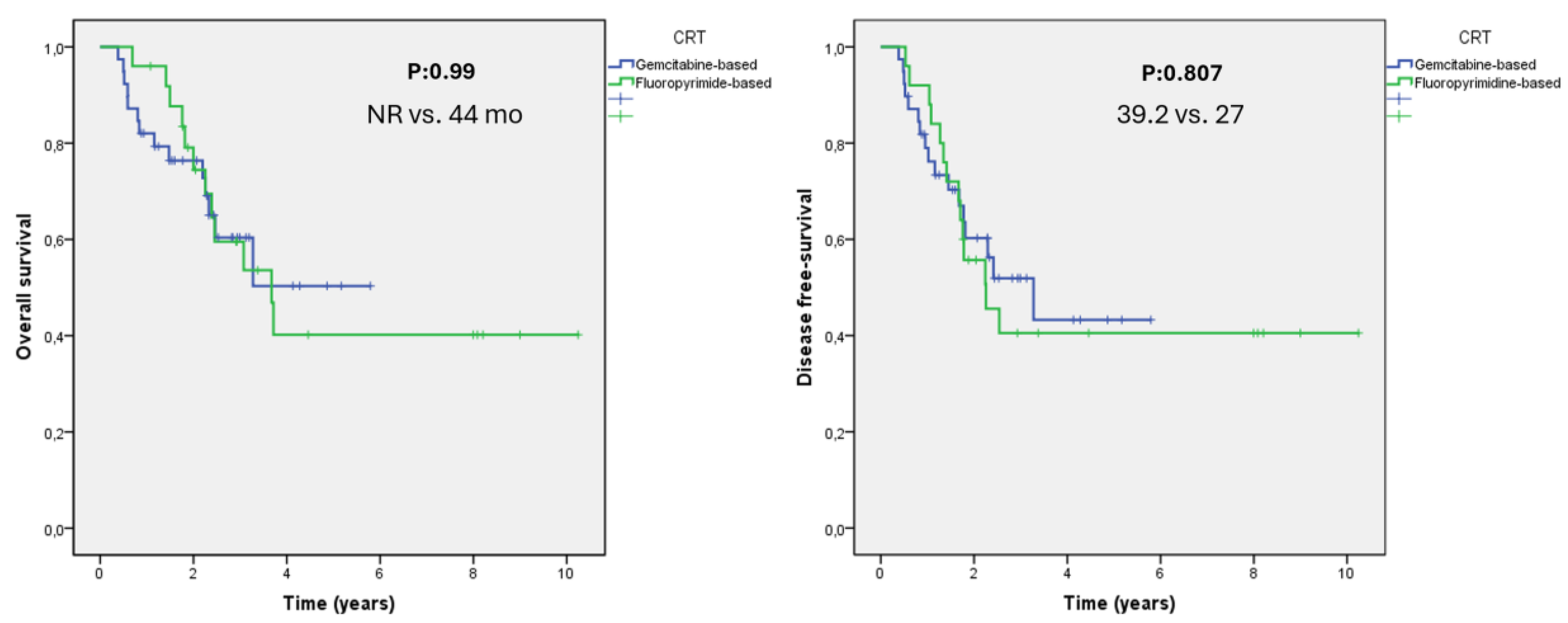
| Gemcitabine | 43 |
| Fluoropyrimidine | 31 |
| CRT | 64 (32.4) |
| Gemcitabine | 39 |
| Fluoropyrimidine | 25 |
| Observation | 59 (29.9) |
| Jaundice at diagnosis | |
| Yes | 119 (60.5) |
| No | 78 (39.5) |
| Patients | n (%) | 3-Year Survival, % | 5-Year Survival, % | mOS (mo) | p | mDFS (mo) | p |
|---|---|---|---|---|---|---|---|
| All patient groups | 197 | 65 | 51 | 63.4 | 40.9 | ||
| Age, years | |||||||
| <50 | 39 (19.8) | 91 | 66 | 78.8 | 0.045 | 54.3 | 0.270 |
| ≥50 | 158 (80.2) | 58 | 48 | 53.1 | 30.5 | ||
| Performance status | |||||||
| 0–1 | 154 (78.2) | 66 | 55 | 108.5 | 0.048 | 41.6 | 0.1 |
| 2–3 | 43 (21.8) | 59 | 37 | 46 | 28.4 | ||
| Nodal involvement | |||||||
| Positive | 90 (45.6) | 53 | 32 | 38.8 | 0.001 | 28.4 | 0.027 |
| Negative | 107 (54.4) | 75 | 62 | 141.9 | 67.8 | ||
| Histological grade | |||||||
| Well differentiated | 58 (29.4) | 71 | 61 | NR | 0.042 | 54.3 | 0.082 |
| Moderately differentiated | 75 (38) | 58 | 51 | 63.1 | 38.3 | ||
| Poorly differentiated | 17 (8.6) | 51 | 26 | 36.9 | 26.9 | ||
| Lymphovascular invasion | |||||||
| Yes | 69 (35) | 51 | 37 | 36.9 | <0.001 | 27 | <0.001 |
| No | 101 (51.2) | 75 | 66 | NR | 108.5 | ||
| Perineural invasion | |||||||
| Yes | 50 (25.3) | 55 | 39 | 39.2 | 0.007 | 38.8 | 0.089 |
| No | 120 (60.9) | 70 | 61 | 108.8 | 67.8 | ||
| T stage | |||||||
| 1–2 | 99 (50.2) | 73 | 59 | 108.5 | 0.018 | 54.3 | 0.116 |
| 3–4 | 98 (49.8) | 56 | 42 | 39.3 | 30.6 | ||
| TNM stage | |||||||
| I | 67 (34) | 75 | 64 | 108.5 | 0.06 | 67.8 | 0.192 |
| II | 103 (52.2) | 61 | 47 | 53.1 | 34.4 | ||
| III | 27 (13.8) | 55 | 36 | 38.8 | 26.3 | ||
| Surgical margin | |||||||
| Negative | 184 (93.5) | 67 | 54 | 20.1 | 0.01 | 41.6 | 0.058 |
| Positive | 13 (6.6) | 38 | 38 | 78.8 | 15.5 | ||
| Adjuvant treatment | |||||||
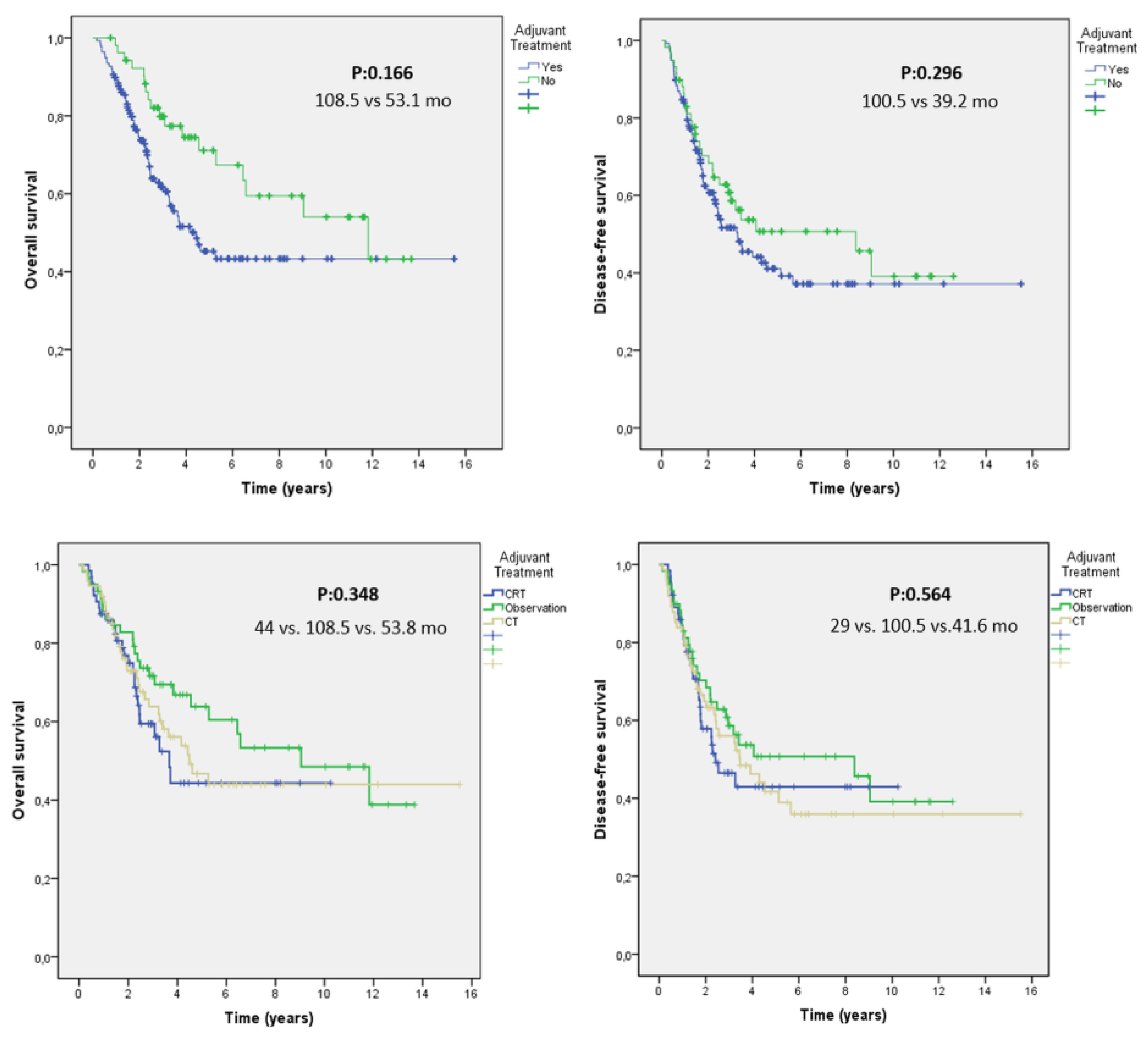
| None | 59 (29.9) | 71 | 71 | 108.5 | 0.348 | 100.5 | 0.564 |
| CRT | 64 (32.5) | 58 | 44 | 44 | 29 | ||
| CT | 74 (37.6) | 64 | 46 | 53.8 | 41.6 |
| Factors | Relative Risk | 95% CI | p-Value |
|---|---|---|---|
| Nodal Status | |||
| Positive | 1.98 | 1.08–3.65 | 0.027 |
| Negative | |||
| Surgical Margin | |||
| Positive | 2.61 | 1.09–6.24 | 0.03 |
| Negative | |||
| Tumor Stage | |||
| T1–T2 | 1.76 | 0.96–3.2 | 0.064 |
| T3–T4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, N.S.; Cavdar, E.; Ozdemir, N.Y.; Yuksel, S.; Iriagac, Y.; Erdem, G.U.; Odabas, H.; Hacibekiroglu, I.; Karaagac, M.; Ucar, M.; et al. Clinicopathologic Analysis and Prognostic Factors for Survival in Patients with Operable Ampullary Carcinoma: A Multi-Institutional Retrospective Experience. Medicina 2024, 60, 818. https://doi.org/10.3390/medicina60050818
Demirci NS, Cavdar E, Ozdemir NY, Yuksel S, Iriagac Y, Erdem GU, Odabas H, Hacibekiroglu I, Karaagac M, Ucar M, et al. Clinicopathologic Analysis and Prognostic Factors for Survival in Patients with Operable Ampullary Carcinoma: A Multi-Institutional Retrospective Experience. Medicina. 2024; 60(5):818. https://doi.org/10.3390/medicina60050818
Chicago/Turabian StyleDemirci, Nebi Serkan, Eyyup Cavdar, Nuriye Yildirim Ozdemir, Sinemis Yuksel, Yakup Iriagac, Gokmen Umut Erdem, Hatice Odabas, Ilhan Hacibekiroglu, Mustafa Karaagac, Mahmut Ucar, and et al. 2024. "Clinicopathologic Analysis and Prognostic Factors for Survival in Patients with Operable Ampullary Carcinoma: A Multi-Institutional Retrospective Experience" Medicina 60, no. 5: 818. https://doi.org/10.3390/medicina60050818
APA StyleDemirci, N. S., Cavdar, E., Ozdemir, N. Y., Yuksel, S., Iriagac, Y., Erdem, G. U., Odabas, H., Hacibekiroglu, I., Karaagac, M., Ucar, M., Ozturk, B., & Bozkaya, Y. (2024). Clinicopathologic Analysis and Prognostic Factors for Survival in Patients with Operable Ampullary Carcinoma: A Multi-Institutional Retrospective Experience. Medicina, 60(5), 818. https://doi.org/10.3390/medicina60050818





