Advanced Interatrial Block across the Spectrum of Renal Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Electrocardiographic Measurements
2.3. Echocardiographic Measurements
2.4. Interatrial Block
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Relationship between Chronic Kidney Disease and Electrocardiographic Abnormalities
4.2. Interatrial Block and Left Atrial Enlargement
4.3. Main Findings
4.4. Clinical Perspectives
4.5. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayés de Luna, A.; Platonov, P.; Cosio, F.G.; Cygankiewicz, I.; Pastore, C.; Baranowski, R.; Bayés-Genis, A.; Guindo, J.; Viñolas, X.; Garcia-Niebla, J.; et al. Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. J. Electrocardiol. 2012, 45, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Legato, M.J.; Bull, M.B.; Ferrer, M.I. Atrial ultrastructure in patients with fixed intra-atrial block. Chest 1974, 65, 252–261. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Zhang, Z.M.; Loehr, L.R.; Chen, L.Y.; Alonso, A.; Soliman, E.Z. Electrocardiographic advanced interatrial block and atrial fibrillation risk in the general population. Am. J. Cardiol. 2016, 117, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.W.; Ghouse, J.; Kühl, J.T.; Platonov, P.G.; Graff, C.; Fuchs, A.; Rasmussen, P.V.; Pietersen, A.; Nordestgaard, B.G.; Torp-Pedersen, C.; et al. Risk prediction of atrial fibrillation based on electrocardiographic interatrial block. J. Am. Heart Assoc. 2018, 7, e008247. [Google Scholar] [CrossRef] [PubMed]
- Ariyarajah, V.; Puri, P.; Kranis, M.; Wilner, D.A.; Spodick, D.H. Prevalence of interatrial block in the Program of All-Inclusive Care for the Elderly (PACE). Am. J. Geriatr. Cardiol. 2006, 15, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Roessel, A.M.-V.; Álvarez-García, J.; de la Villa, B.G.; Cruz-Jentoft, A.J.; Vidán, M.T.; Díaz, J.L.; Redondo, F.J.F.; Guerrero, J.M.D.; Bayes-Genis, A.; et al. Interatrial block and atrial arrhythmias in centenarians: Prevalence, associations, and clinical implications. Heart Rhythm 2016, 13, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Power, D.A.; Lampert, J.; Camaj, A.; Bienstock, S.W.; Kocovic, N.; Bayes-Genis, A.; Miller, M.A.; Bayés-de-Luna, A.; Fuster, V. Cardiovascular Complications of Interatrial Conduction Block: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Marano, M. Letter to the editor—Prevalence of interatrial block during lifetime. Heart Rhythm 2016, 13, e90. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Papa, A.A.; Rago, A.; Ciardiello, C.; Marano, M.; Proietti, R.; Politano, L.; Nigro, G. Interatrial block to predict atrial fibrillation in myotonic dystrophy type 1. Neuromuscul. Disord. 2018, 28, 327–333. [Google Scholar] [CrossRef]
- Russo, V.; Albani, S.; Caturano, A.; Weisz, S.H.; Parisi, V.; Conte, M.; Zaccaro, L.; D’Andrea, A.; Al-Turky, A.; Marchel, M.; et al. The prognostic role of interatrial block among COVID-19 patients hospitalized in medicine wards. Eur. J. Clin. Investig. 2022, 52, e13781. [Google Scholar] [CrossRef]
- Massó-van Roessel, A.; Escobar-Robledo, L.A.; Dégano, I.R.; Grau, M.; Sala, J.; Ramos, R.; Marrugat, J.; Bayés de Luna, A.; Elosua, R. Analysis of the Association Between Electrocardiographic P-wave Characteristics and Atrial Fibrillation in the REGICOR Study. Rev. Española Cardiol. 2017, 70, 841–847. [Google Scholar] [CrossRef]
- Agarwal, Y.K.; Aronow, W.S.; Levy, J.A.; Spodick, D.H. Association of interatrial block with development of atrial fibrillation. Am. J. Cardiol. 2003, 91, 882. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, F.; Platonov, P.; Carlson, J.; Zareba, W.; Moss, A.J.; MADIT II Investigators. Abnormal P wave morphology is a predictor of atrial fibrillation in MADIT II patients. Ann. Noninvasive Electrocardiol. 2010, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Solak, Y.; Gul, E.E.; Kayrak, M.; Atalay, H.; Abdulhalikov, T.; Turk, S.; Covic, A.; Kanbay, M. Electrocardiographic P-wave characteristics in patients with end-stage renal disease: P-index and interatrial block. Int. Urol. Nephrol. 2013, 45, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Bayés-de-Luna, A.; Bacharova, L. New electrocardiographic aspects of the P wave: Its value in clinical cardiology. Ann. Noninvasive Electrocardiol. 2023, 28, e13053. [Google Scholar] [CrossRef]
- Marano, M.; D’Amato, A.; Tomasino, G.; Izzo, F.; Capasso, M.; Auletta, E. Prevalence and determinants of interatrial block in hemodialysis patients. Int. Urol. Nephrol. 2015, 47, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Elosua, R.; Ibarrola, M.; de Andrés, M.; Díez-Villanueva, P.; Bayés-Genis, A.; Baranchuk, A.; Bayés-de-Luna, A.; BAYES Registry Investigators. Advanced interatrial block and P-wave duration are associated with atrial fibrillation and stroke in older adults with heart disease: The BAYES registry. EP Europace 2020, 22, 1001–1008. [Google Scholar]
- Lampert, J.; Power, D.; Havaldar, S.; Govindarajulu, U.; Kawamura, I.; Maan, A.; Miller, M.A.; Menon, K.; Koruth, J.; Whang, W.; et al. Interatrial Block Association with Adverse Cardiovascular Outcomes in Patients Without a History of Atrial Fibrillation. Clin. Electrophysiol. 2023, 9, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Istolahti, T.; Eranti, A.; Huhtala, H.; Lyytikäinen, L.P.; Kähönen, M.; Lehtimäki, T.; Eskola, M.; Anttila, I.; Jula, A.; Bayés de Luna, A.; et al. The prevalence and prognostic significance of interatrial block in the general population. Ann. Med. 2020, 52, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Martínez-Larrú, M.E.; Ibarrola, M.; Santos, A.; Díez-Villanueva, P.; Bayés-Genis, A.; Baranchuk, A.; Bayés-De-Luna, A.; Elosua, R. Interatrial block and cognitive impairment in the BAYES prospective registry. Int. J. Cardiol. 2020, 321, 95–98. [Google Scholar] [CrossRef]
- Gutierrez, A.; Norby, F.L.; Maheshwari, A.; Rooney, M.R.; Gottesman, R.F.; Mosley, T.H.; Lutsey, P.L.; Oldenburg, N.; Soliman, E.Z.; Alonso, A.; et al. Association of abnormal P-wave indices with dementia and cognitive decline over 25 years: ARIC-NCS (the atherosclerosis risk in communities neurocognitive study). J. Am. Heart Assoc. 2019, 8, e014553. [Google Scholar] [CrossRef]
- Herrera, C.; Bruña, V.; Abizanda, P.; Díez-Villanueva, P.; Formiga, F.; Torres, R.; Carreras, J.; Ayala, R.; Martin-Sánchez, F.J.; Bayés-Genis, A.; et al. Relation of Interatrial Block to Cognitive Impairment in Patients ≥ 70 Years of Age (From the CAMBIAD Case-control Study). Am. J. Cardiol. 2020, 136, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, M.; Covino, S.; Petillo, R.; Russo, V. Interatrial block as a first clinical presentation of atrial cardiomyopathy related to a novel LMNA variant: A case report. Eur. Heart J. Case Rep. 2023, 7, ytad532. [Google Scholar] [CrossRef] [PubMed]
- Proietti, R.; Russo, V.; Sagone, A.; Viecca, M.; Spodick, D.H. Interatrial block: An under-recognized electrocardiographic diagnosis with important clinical-therapeutic implications. G. Ital. Cardiol. 2014, 15, 561–568. (In Italian) [Google Scholar]
- Maheshwari, A.; Norby, F.L.; Soliman, E.Z.; Alraies, M.C.; Adabag, S.; O’Neal, W.T.; Alonso, A.; Chen, L.Y. Relation of prolonged P-wave duration to risk of sudden cardiac death in the general population (from the atherosclerosis risk in communities study). Am. J. Cardiol. 2017, 119, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Gorodeski, E.Z.; Johnson, V.M.; Sullivan, L.M.; Hamburg, N.M.; Benjamin, E.J.; Ellinor, P.T. P wave duration is associated with cardiovascular and all-cause mortality outcomes: The national health and nutrition examination survey. Heart Rhythm 2011, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Marano, M.; D’Amato, A. Advanced interatrial block in hemodialysis patients. Hemodial. Int. 2016, 20, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Bayés de Luna, A.; de Ribot, R.F.; Trilla, E.; Julia, J.; Garcia, J.; Sadurni, J.; Riba, J.; Sagues, F. Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J. Electrocardiol. 1985, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Rosca, M.; Lancellotti, P.; Popescu, B.A.; Pierard, L.A. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart 2011, 97, 1982–1989. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Dagostino, R.B.; Belanger, A.J.; Wolf, P.A.; Levy, D. Left Atrial Size and the Risk of Stroke and Death—The Framingham Heart-Study. Circulation 1995, 92, 835–841. [Google Scholar] [CrossRef]
- Aurigemma, G.P.; Gottdiener, J.S.; Arnold, A.M.; Chinali, M.; Hill, J.C.; Kitzman, D. Left Atrial Volume and Geometry in Healthy Aging the Cardiovascular Health Study. Circ. Cardiovasc. Imaging 2009, 2, 282–289. [Google Scholar] [CrossRef]
- Kou, S.; Caballero, L.; Dulgheru, R.; Voilliot, D.; De Sousa, C.; Kacharava, G.; Athanassopoulos, G.D.; Barone, D.; Baroni, M.; Cardim, N.; et al. Echocardiographic reference ranges for normal cardiac chamber size: Results from the NORRE study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 680–690. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611644. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Moura, L.; Popescu, B.A.; Agricola, E.; Monin, J.L.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; et al. European Association of Echocardiography recommendation for the assessment of valvular regurgitation. Part 1: Aortic and pulmonary regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 223244. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303371. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; Mitrofanova, L.B.; Chirreikin, L.V.; Olsson, S.B. Morphology of inter-atrial conduction routes in patients with atrial fibrillation. Europace 2002, 4, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Johner, N.; Namdar, M.; Shah, D.C. Intra- and interatrial conduction abnormalities: Hemodynamic and arrhythmic significance. J. Interv. Card. Electrophysiol. 2018, 52, 293–302. [Google Scholar] [CrossRef]
- Decreased, G. Definition and classification of CKD. Kidney Int. 2013, 3, 19–62. [Google Scholar]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L.; Lipman, M.L.; Mann, J.F.E. Chronic kidney disease: Eff ects on the cardiovascular system. Circulation 2007, 116, 85–97. [Google Scholar] [CrossRef]
- Kestenbaum, B.; Rudser, K.D.; Shlipak, M.G.; Fried, L.F.; Newman, A.B.; Katz, R.; Sarnak, M.J.; Seliger, S.; Stehman-Breen, C.; Prineas, R.; et al. Kidney function, electrocardiographic findings, and cardiovascular events among older adults. Clin. J. Am. Soc. Nephrol. 2007, 2, 501–508. [Google Scholar] [CrossRef]
- Deo, R.; Shou, H.; Soliman, E.Z.; Yang, W.; Arkin, J.M.; Zhang, X.; Townsend, R.R.; Go, A.S.; Shlipak, M.G.; Feldman, H.I. Electrocardiographic Measures and Prediction of Cardiovascular and Noncardiovascular Death in CKD. J. Am. Soc. Nephrol. 2016, 27, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yum, Y.; Cha, J.J.; Joo, H.J.; Park, J.H.; Hong, S.J.; Yu, C.W.; Lim, D.S. Prevalence and Clinical Impact of Electrocardiographic Abnormalities in Patients with Chronic Kidney Disease. J. Clin. Med. 2022, 11, 5414. [Google Scholar] [CrossRef] [PubMed]
- Turakhia, M.P.; Blankestijn, P.J.; Carrero, J.J.; Clase, C.M.; Deo, R.; Herzog, C.A.; Kasner, S.E.; Passman, R.S.; Pecoits-Filho, R.; Reinecke, H.; et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur. Heart J. 2018, 39, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. 2006 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, Cardiovascular Special Studies; National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Division of Kidney, Urologic, and Hematologic Diseases: Bethesda, MD, USA, 2006.
- Herzog, C.A.; Li, S.; Weinhandl, E.D.; Strief, J.W.; Collins, A.J.; Gilbertson, D.T. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005, 68, 818–825. [Google Scholar] [CrossRef]
- Covic, A.; Diaconita, M.; Gusbeth-Tatomir, P.; Covic, M.; Botezan, A.; Ungureanu, G.; Goldsmith, D.J. Hemodialysis increase QTc interval but not QTc dispersion in ESRD patients without manifest cardiac disease. Nephrol. Dial. Transplant. 2002, 17, 2170–2177. [Google Scholar] [CrossRef]
- Malhis, M.; Al-Bitar, S.; Farhood, S.; Zaiat, K.A. Changes in QT intervals in patients with end-stage renal disease before and after hemodialysis. Saudi J. Kidney Dis. Transplant. 2010, 21, 460–465. [Google Scholar] [PubMed]
- Yetkin, E.; Ileri, M.; Tandogan, I.; Boran, M.; Yanik, A.; Hisar, I.; Kutlu, M.; Çehreli, S.; Korkmaz, Ş.; Göksel, S. Increased QT interval dispersion after hemodialysis: Role of peridialytic electrolyte gradients. Angiology 2000, 51, 499–504. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Shimizu, W.; Yan, G.X.; Sicouri, S. Cellular basis for QT dispersion. J. Electrocardiol. 1998, 30, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lőorincz, I.; Mátyus, J.; Zilahi, Z.; Kun, C.; Karányi, Z.; Kakuk, G. QT Dispersion in Patients with End-Stage Renal Failure and during Hemodialysis. J. Am. Soc. Nephrol. 1999, 10, 1297–1302. [Google Scholar] [CrossRef]
- Patzer, R.E.; Flueckiger, P.; Pastan, S.; Goyal, A.; McClellan, W.W. Associations of ECG interval prolongations with mortality among ESRD patients evaluated for renal transplantation. Ann. Transplant. 2014, 19, 257–268. [Google Scholar] [CrossRef]
- Dobre, M.; Brateanu, A.; Rashidi, A.; Rahman, M. Electrocardiogram abnormalities and cardiovascular mortality in elderly patients with CKD. Clin. J. Am. Soc. Nephrol. 2012, 7, 949–956. [Google Scholar] [CrossRef]
- Vázquez Ruiz de Castroviejo, E.; Sánchez Perales, C.; López López, J.; García Cortés, M.J.; Aragón Extremera, V.; Guzmán Herrera, M.; Fajardo Pineda, A.; Lozano Cabezas, C. Análisis de la prevalencia y los factores predisponentes de los bloqueos de rama en los pacientes que inician diálisis [Prevalence of and predisposing factors for bundle branch block in patients starting dialysis]. Rev. Esp. Cardiol. 2008, 61, 719–725. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Bruña, V.; Comella, A.; de la Rosa, A.; Díaz-González, L.; Ruiz-Ortiz, M.; Lacalzada-Almeida, J.; Lucía, A.; Boraita, A.; Bayés-de-Luna, A.; et al. Left Atrial Enlargement in Competitive Athletes and Atrial Electrophysiology. Rev. Esp. Cardiol. 2022, 75, 421–428. [Google Scholar] [CrossRef]
- Josephson, M.E.; Kastor, J.A.; Morganroth, J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and haemodynamic correlates. Am. J. Cardiol. 1977, 39, 967. [Google Scholar] [CrossRef]
- de Luna, A.B.; Massó-van Roessel, A.; Robledo, L.A.E. The Diagnosis and Clinical Implications of Interatrial Block. Eur. Cardiol. 2015, 10, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bayés de Luna, A.; Baranchuk, A.; Alberto Escobar Robledo, L.; Massó van Roessel, A.; Martínez-Sellés, M. Diagnosis of interatrial block. J. Geriatr. Cardiol. 2017, 14, 161–165. [Google Scholar]
- Bejarano-Arosemena, R.; Martínez-Sellés, M. Interatrial Block, Bayés Syndrome, Left Atrial Enlargement, and Atrial Failure. J. Clin. Med. 2023, 12, 7331. [Google Scholar] [CrossRef]
- Guerra, J.M.; Vilahur, G.; Bayés De Luna, A.; Cabrera, J.A.; Martínez-Sellés, M.; Mendieta, G.; Baranchuk, A.; Sánchez-Quintana, D. Interatrial Block Can Occur in the Absence of Left Atrial Enlargement: New Experimental Model. Pacing Clin. Electrophysiol. 2020, 43, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Kalçık, M.; Yetim, M.; Doğan, T.; Eser, B.; Doğan, T.; Bekar, L.; Çelik, O.; Karavelioğlu, Y. Echocardiographic predictors of interatrial block in patients with severe chronic kidney disease. Int. Urol. Nephrol. 2020, 52, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Severi, S.; Pogliani, D.; Fantini, G.; Fabbrini, P.; Vigano, M.R.; Galbiati, E.; Bonforte, G.; Vincenti, A.; Stella, A.; Genovesi, S. Alterations of atrialelectrophysiologyinduced by electrolytevariations: Combinedcomputational and P-waveanalysis. Europace 2010, 12, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Marano, M.; D’Amato, A.; de Luna, A.B.; Baranchuk, A. Hemodialysis affects interatrial conduction. Ann. Noninvasive Electrocardiol. 2015, 20, 299–300. [Google Scholar] [CrossRef]
- Enriquez, A.; Marano, M.; D’Amato, A.; de Luna, A.B.; Baranchuk, A. Second-degree interatrial block in hemodialysis patients. Case Rep. Cardiol. 2015, 2015, 468493. [Google Scholar] [CrossRef]
- Bayés de Luna, A.; Baranchuk, A.; Niño Pulido, C.; Martínez-Sellés, M.; Bayés-Genís, A.; Elosua, R.; Elizari, M.V. Second-degree interatrial block: Brief review and concept. Ann. Noninvasive Electrocardiol. 2018, 23, e12583. [Google Scholar] [CrossRef]


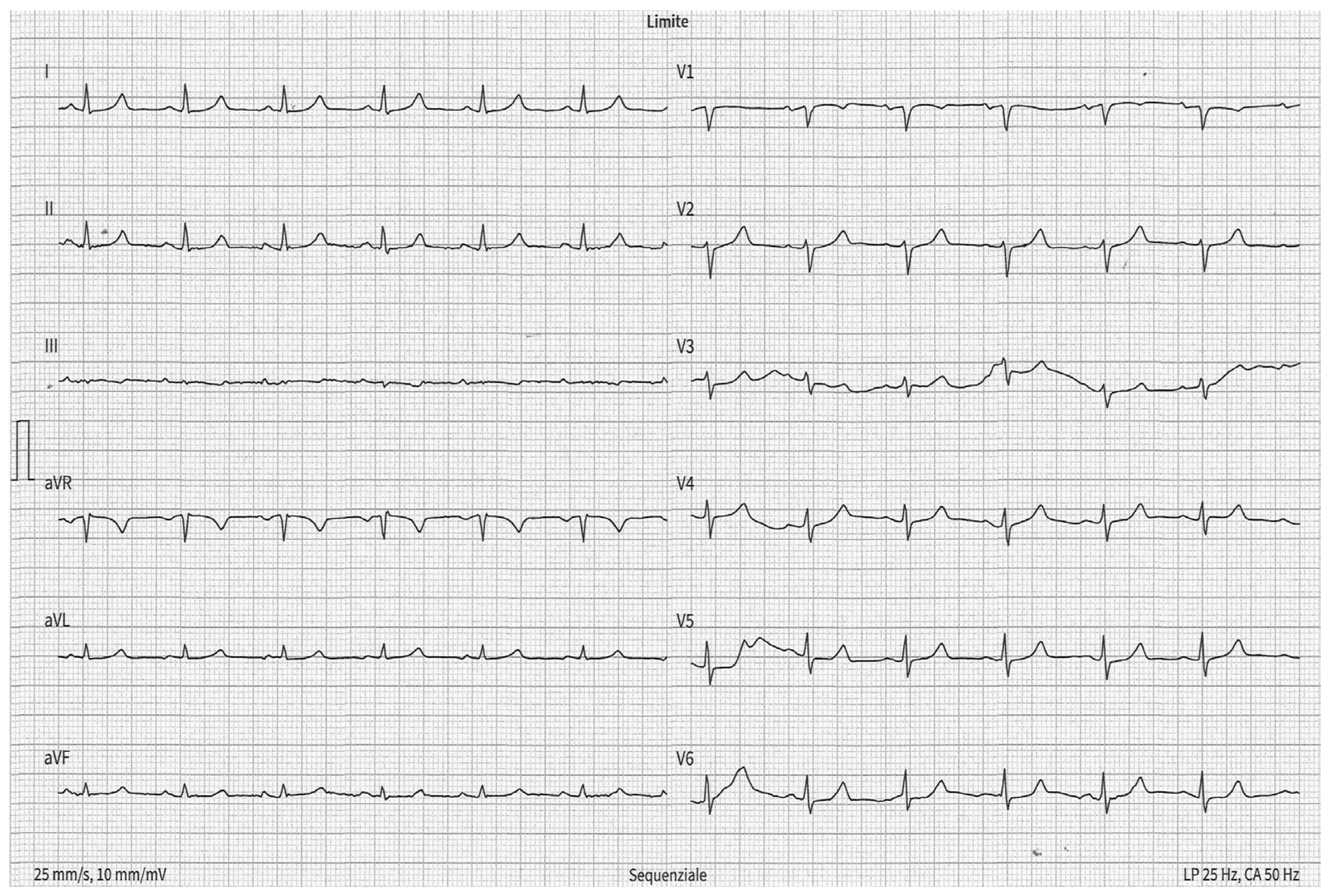
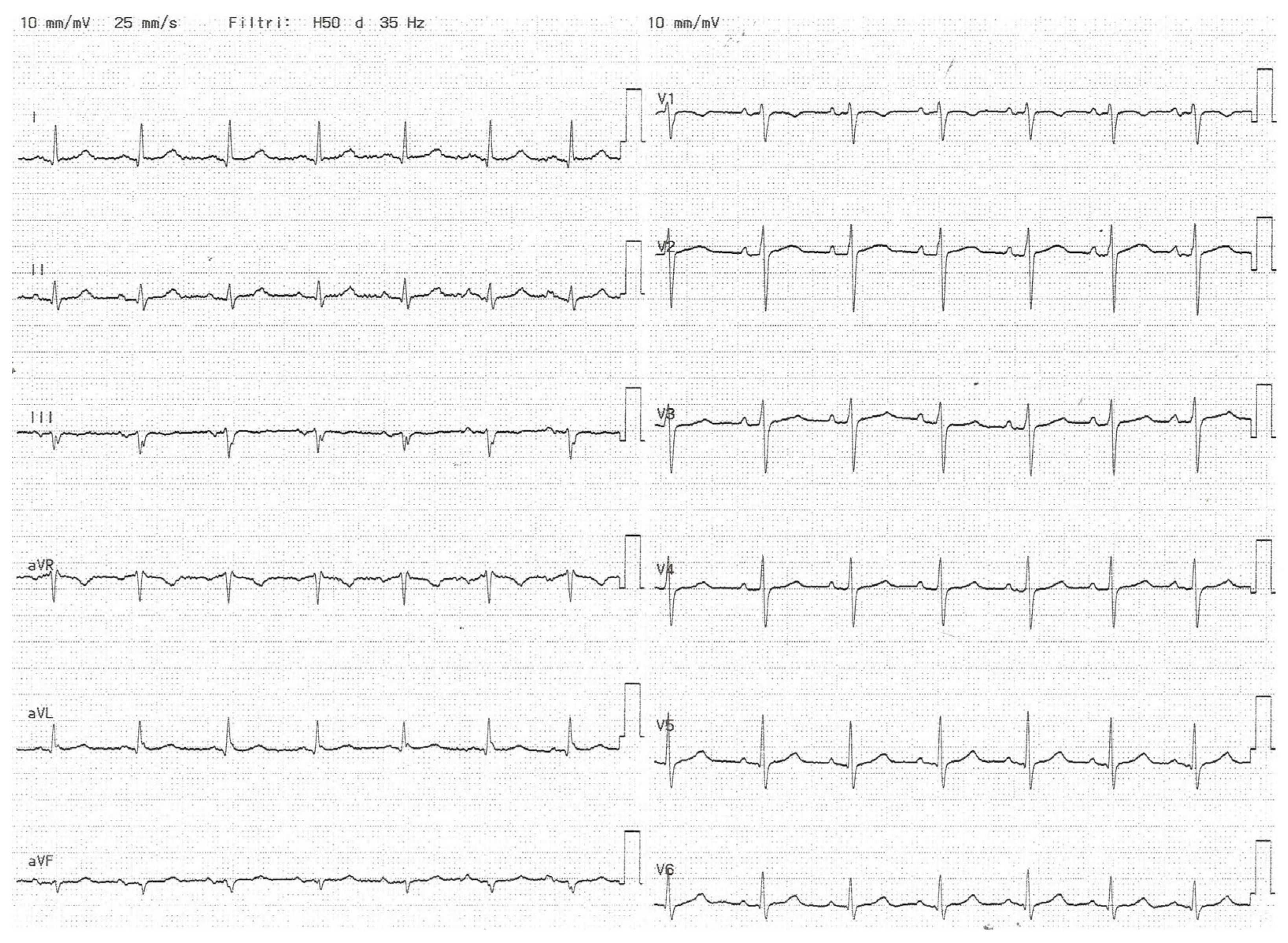
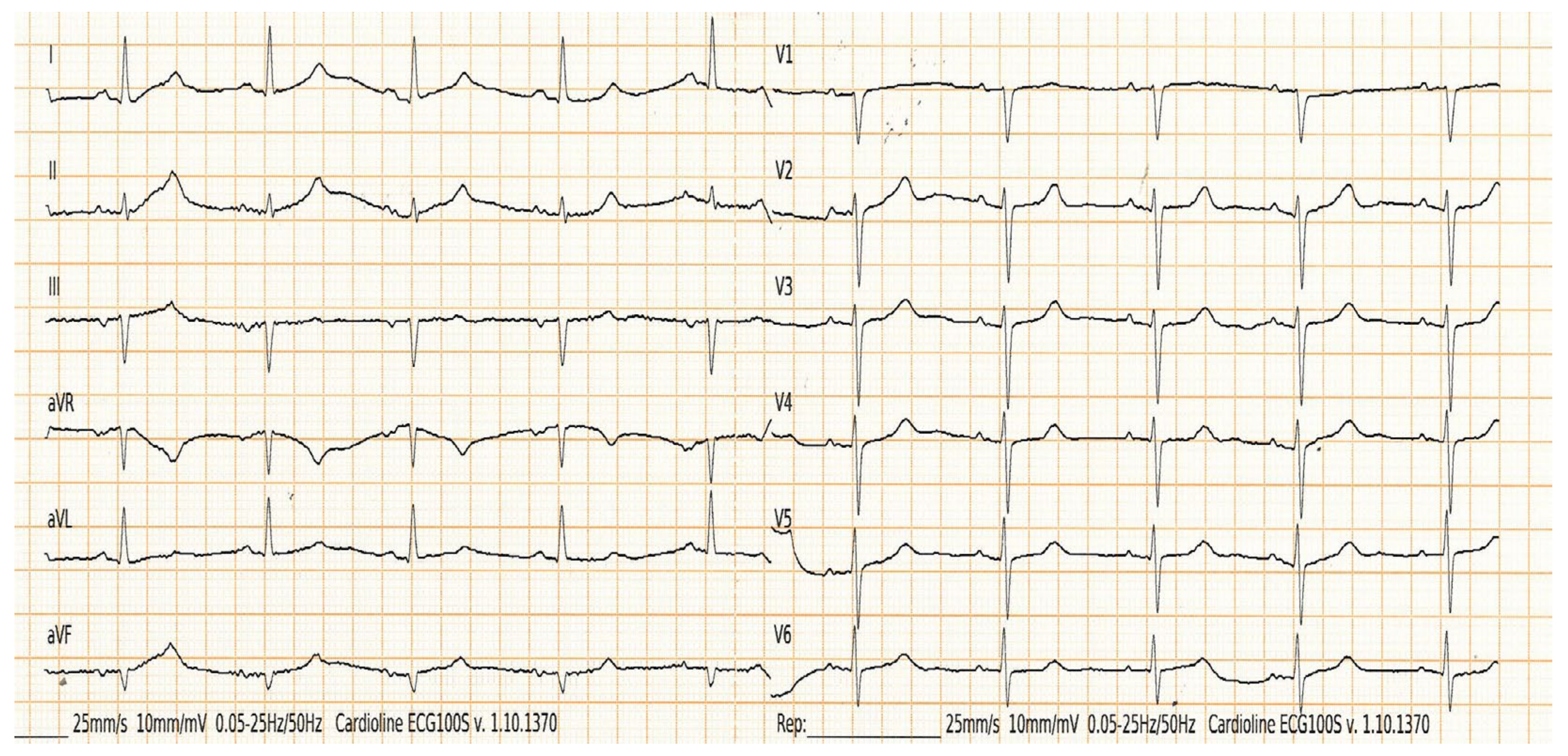
| Control Group n (57) | CKD Group n (45) | ESKD Group n (49) | p (CKD vs. Control) | p (ESKD vs. Control) | |
|---|---|---|---|---|---|
| Male gender, n (%) | 31 (54.4) | 21 (46.7) | 30 (61.2) | 0.4 | 0.5 |
| Age, mean ± SD, years | 71.3 ± 9.9 | 74.5 ± 10.4 | 65.7 ± 12.3 | 0.11 | 0.01 |
| Diabetes mellitus, n (%) | 11 (19.3%) | 18 (40%) | 15 (30.6%) | 0.4 | 0.2 |
| Hypertension, n (%) | 32 (56.1%) | 29 (64.4%) | 25 (51%) | 0.4 | 0.6 |
| CAD, n (%) | 10 (17.5%) | 7 (15.6%) | 13 (26.5%) | 0.8 | 0.3 |
| AF, n (%) | 1 (1.8%) | 2 (4.4%) | 1 (2%) | 0.4 | 0.9 |
| LA diameter, n (%) | 39 ± 3.1 | 40.5 ± 1.6 | 39.2 ± 3.3 | 0.004 | 0.75 |
| LVEF (%), mean ± SD | 60.3 ± 2.7 | 60 ± 1.4 | 54 ± 5 | 0.5 | <0.0001 |
| Mild AR, n (%) | 4 (7.1) | 0 | 1 (2) | 0.07 | 0.22 |
| Mild MR, n(%) | 4(7.1) | 2 (4.4) | 5 (10.2) | 0.57 | 0.57 |
| 1st-degree AV block, n (%) | 2 (3.5%) | 6 (13.3%) | 4 (8.2%) | 0.07 | 0.3 |
| RBBB, n (%) | 6 (10.5%) | 3 (6.7%) | 3 (6.1%) | 0.5 | 0.4 |
| LBBB, n (%) | 0 (0%) | 0 (0%) | 1 (2%) | / | / |
| LAFB, n (%) | 6 (10.5%) | 2 (4.4%) | 7 (14.3%) | 0.3 | 0.5 |
| Partial IAB, n (%) | 34 (59.6%) | 20 (44.4%) | 18 (36.7%) | 0.1 | 0.02 |
| Advanced IAB, n (%) | 3 (5.3%) | 8 (17.8%) | 12 (24.5%) | 0.04 | 0.005 |
| Oral Anticoagulant Drugs, n (%) | 4 (7%) | 3 (6.7%) | 1 (2%) | 0.9 | 0.2 |
| ACE-I/ARB, n (%) | 22 (38.6%) | 24 (53.3%) | 11 (22.4%) | 0.1 | 0.07 |
| β-blockers, n (%) | 11 (19.3%) | 17 (37.8%) | 21 (42.9%) | 0.04 | 0.009 |
| Antiarrhythmic drugs, n (%) | 2 (3.5%) | 3 (6.7%) | 4 (8.2%) | 0.5 | 0.3 |
| Univariable Analysis | ||
|---|---|---|
| OR (95% CI) | p | |
| Age | 0.99 (0.96–1.03) | 0.74 |
| Male gender | 0.76 (3.1–1.8) | 0.54 |
| Creatinine | 1.18 (0.91–1.53) | 0.22 |
| Diabetes Mellitus | 1.36 (0.53–3.49) | 0.52 |
| Hypertension | 1.90 (0.73–4.92) | 0.19 |
| CAD | 0.83 (0.26–2.64) | 0.75 |
| AF | 0.51(0.43–0.59) | 0.99 |
| LA diameter | 1.12 (0.81–1.53) | 0.09 |
| LVEF | 1.00 (0.84–1.19) | 0.99 |
| Mild MR | 0.45 (0.03–5.84) | 0.53 |
| Mild AR | 1.87 (0.13–26.3) | 0.65 |
| 1st-degree AV block | 0.48 (0.06–3.94) | 0.46 |
| RBBB | 1.98 (0.49–7.96) | 0.33 |
| LBBB | 1.01 (0.43–0.60) | 0.99 |
| LAFB | 2.24 (0.64–7.76) | 0.20 |
| GFR > 60 mL/min/1.73 m2 | 0.20 (0.06–0.72) | 0.01 |
| GFR < 60 mL/min/1.73 m2 | 1.31 (0.51–3.36) | 0.57 |
| ESKD | 2.68 (1.09–6.62) | 0.03 |
| Oral Anticoagulant Drugs | 1.36 (0.53–3.49) | 0.52 |
| ACE-I/ARB | 1.33 (0.54–3.26) | 0.54 |
| β-blockers | 0.53 (0.18–1.52) | 0.22 |
| Antiarrhythmic drugs | 3.05 (0.70–13.2) | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marano, M.; Senigalliesi, L.; Cocola, R.; Fontana, M.; Parente, E.; Russo, V. Advanced Interatrial Block across the Spectrum of Renal Function. Medicina 2024, 60, 1001. https://doi.org/10.3390/medicina60061001
Marano M, Senigalliesi L, Cocola R, Fontana M, Parente E, Russo V. Advanced Interatrial Block across the Spectrum of Renal Function. Medicina. 2024; 60(6):1001. https://doi.org/10.3390/medicina60061001
Chicago/Turabian StyleMarano, Marco, Luigi Senigalliesi, Rossella Cocola, Mariarosaria Fontana, Erika Parente, and Vincenzo Russo. 2024. "Advanced Interatrial Block across the Spectrum of Renal Function" Medicina 60, no. 6: 1001. https://doi.org/10.3390/medicina60061001
APA StyleMarano, M., Senigalliesi, L., Cocola, R., Fontana, M., Parente, E., & Russo, V. (2024). Advanced Interatrial Block across the Spectrum of Renal Function. Medicina, 60(6), 1001. https://doi.org/10.3390/medicina60061001







