Complications of Preterm Birth—The Importance of Care for the Outcome: A Narrative Review
Abstract
:1. Introduction
2. Material and Methods
3. Complications of Preterm Birth
3.1. Early Neonatal Complications of Preterm Neonates
3.2. Late Complications of Preterm Birth
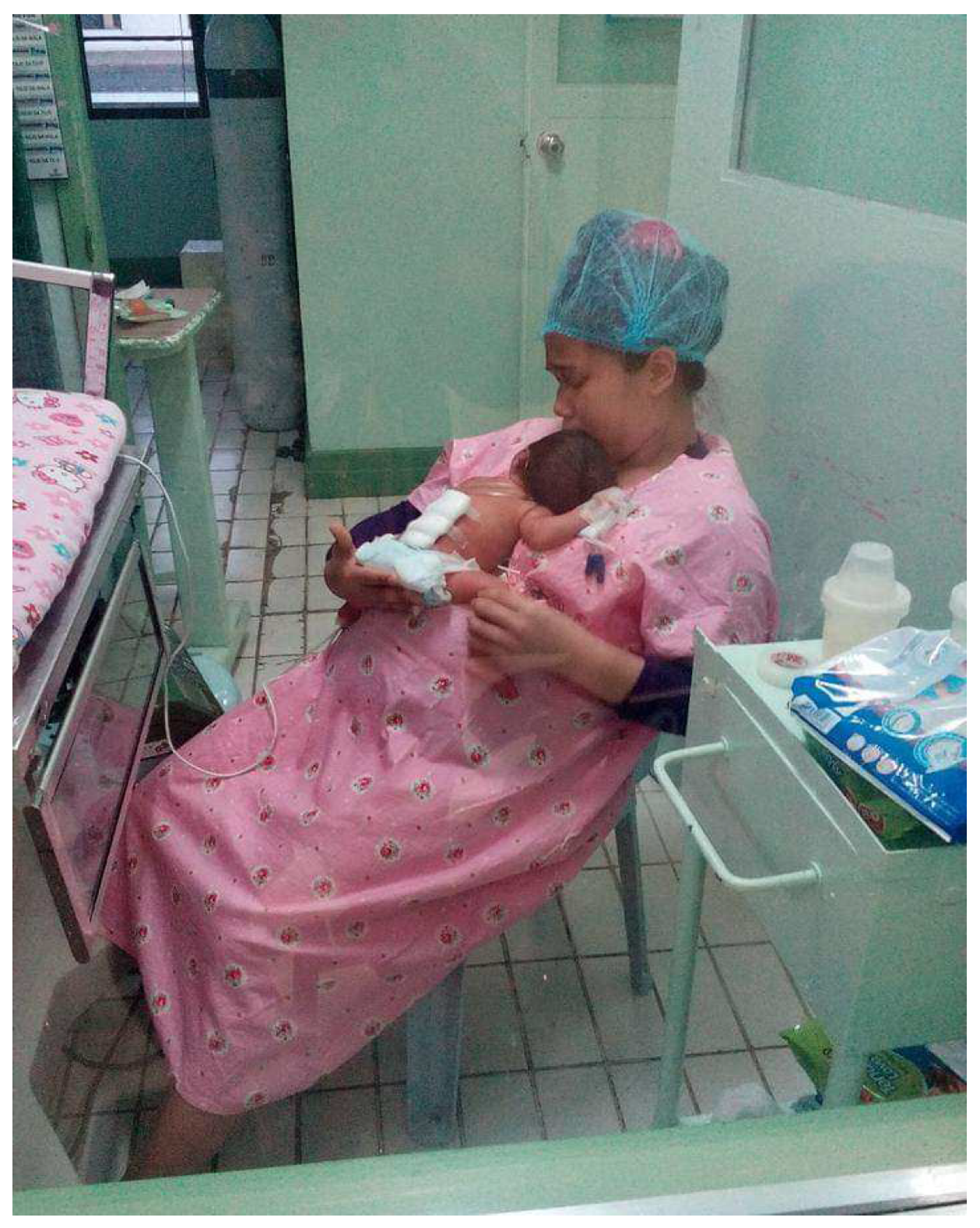
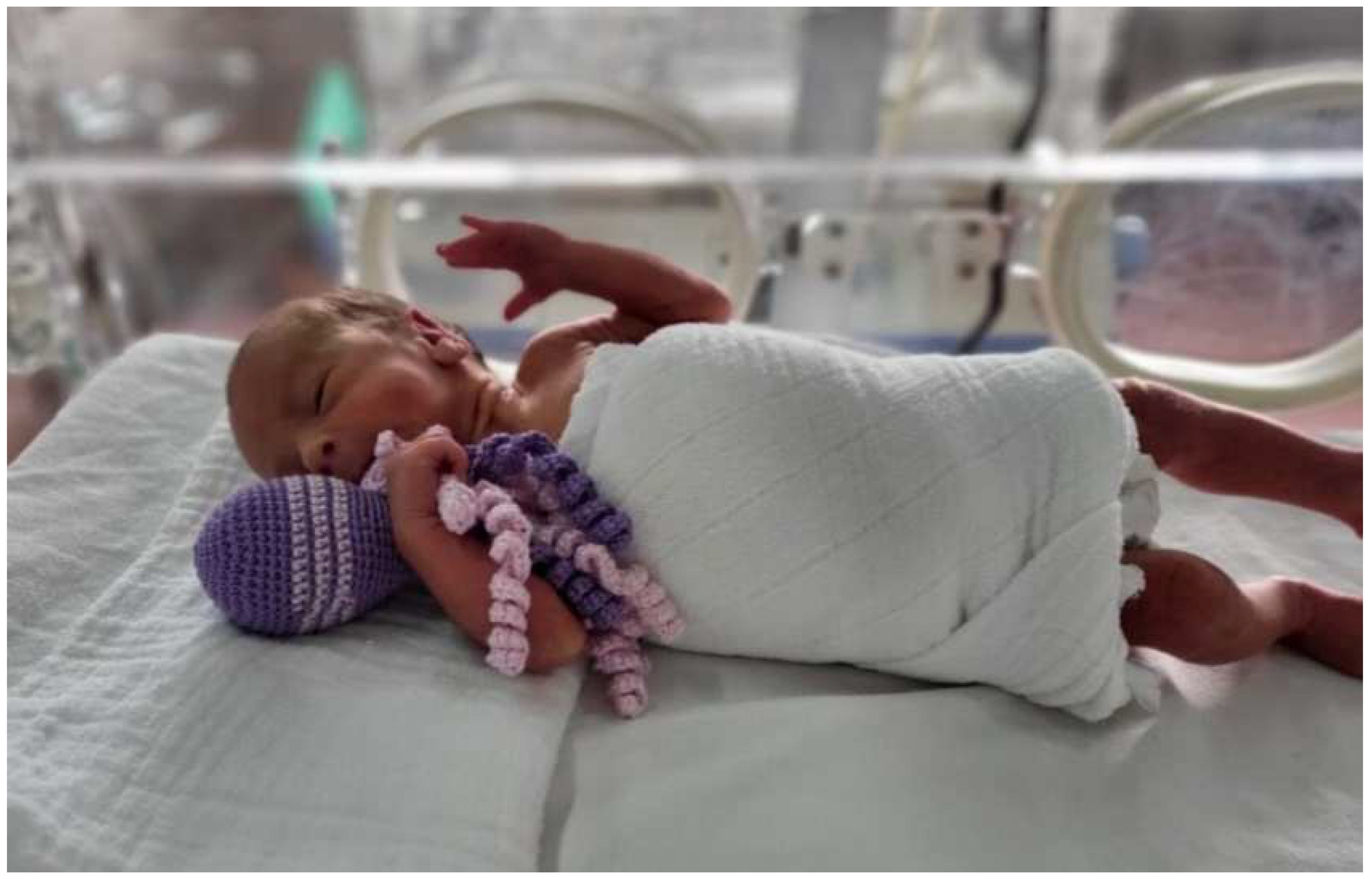
4. Care and Treatment of a Preterm Child
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hedderich, D.M.; Menegaux, A.; Schmitz-Koep, B.; Nuttall, R.; Zimmermann, J.; Schneider, S.C.; Bäuml, J.G.; Daamen, M.; Boecker, H.; Wilke, M.; et al. Increased Brain Age Gap Estimate (BrainAGE) in Young Adults After Premature Birth. Front. Aging Neurosci. 2021, 13, 653365. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome–2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.; Ment, L.R. Long-term Outcome of Preterm Infants and the Role of Neuroimaging. Clin. Perinatol. 2009, 36, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.W.; Kim, J.W.; Chang, Y.P. Transient intubation for surfactant administration in the treatment of respiratory distress syndrome in extremely premature infants. Korean J. Pediatr. 2018, 61, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Babovic, I.R.; Dotlic, J.R.; Jovandaric, M.Z.; Sparic, R.M.; Bila, J.S.; Nejkovic, L.V.; Stulic, J.M.; Tinelli, A. Neurological outcomes of antenatal corticosteroid therapy. Int. J. Clin. Pract. 2021, 75, e14936. [Google Scholar] [CrossRef]
- Briceño-Pérez, C.; Reyna-Villasmil, E.; Vigil-De-Gracia, P. Antenatal corticosteroid therapy: Historical and scientific basis to improve preterm birth management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 32–37. [Google Scholar] [CrossRef]
- Guralnick, M.J. Preventive interventions for preterm children: Effectiveness and developmental mechanisms. J. Dev. Behav. Pediatr. 2012, 33, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Gamarra-Oca, L.; Ojeda, N.; Gómez-Gastiasoro, A.; Peña, J.; Ibarretxe-Bilbao, N.; García-Guerrero, M.A.; Loureiro, B.; Zubiaurre-Elorza, L. Long-Term Neurodevelopmental Outcomes after Moderate and Late Preterm Birth: A Systematic Review. J. Pediatr. 2021, 237, 168–176.e11. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.E.; Vohr, B.R. Neurodevelopmental Outcomes of Preterm Infants Fed Human Milk: A Systematic Review. Clin. Perinatol. 2017, 44, 69–83. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Burnett, A.C.; Treyvaud, K.; Spittle, A.J. Early environment and long-term outcomes of preterm infants. J. Neural Transm. 2020, 127, 1–8. [Google Scholar] [CrossRef]
- Almadhoob, A.; Ohlsson, A. Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2020, 1, CD010333. [Google Scholar] [CrossRef]
- Cook, K.M.; De Asis-Cruz, J.; Kim, J.-H.; Basu, S.K.; Andescavage, N.; Murnick, J.; Spoehr, E.; Liggett, M.; du Plessis, A.J.; Limperopoulos, C. Experience of early-life pain in premature infants is associated with atypical cerebellar development and later neurodevelopmental deficits. BMC Med. 2023, 21, 435. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Fabrizi, L.; Fitzgerald, M. Early Life Pain Experience Changes Adult Functional Pain Connectivity in the Rat Somatosensory and the Medial Prefrontal Cortex. J. Neurosci. 2022, 42, 8284–8296. [Google Scholar] [CrossRef]
- Ding, L.; Chen, Y.; Zhang, W.; Song, J.; Yao, X.; Wan, Y.; Huang, R. Effect of family integrated care on breastfeeding of preterm infants: A scoping review. Nurs. Open 2023, 10, 5950–5960. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Manley, B.J.; Doyle, L.W.; Davies, M.W.; Davis, P.G. Fifty years in neonatology. J. Paediatr. Child Health 2015, 51, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.N.K.; Buist, A.S.; Blaisdell, C.J.; Moxey-Mims, M.; Saigal, S. Adults born preterm: A review of general health and system-specific outcomes. Acta Paediatr. 2017, 106, 1409–1437. [Google Scholar] [CrossRef]
- Raju, T.N.; Pemberton, V.L.; Saigal, S.; Blaisdell, C.J.; Moxey-Mims, M.; Buist, S. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health. J. Pediatr. 2017, 181, 309–318.e1. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.S.; Elbarbary, M.; Embabi, S.N.; Zaki, M.A.; Awad, H.; Al-Feky, M. Screening and Risk Factors for Retinopathy of Prematurity in a Tertiary Care Hospital in Cairo, Egypt. Clin. Ophthalmol. 2022, 16, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Mwita, S.; Jande, M.; Katabalo, D.; Kamala, B.; Dewey, D. Reducing neonatal mortality and respiratory distress syndrome associated with preterm birth: A scoping review on the impact of antenatal corticosteroids in low- and middle-income countries. World J. Pediatr. 2021, 17, 131–140. [Google Scholar] [CrossRef]
- Merlocco, A. Fetal hemodymanic effects on ductus arteriosus development and influences on postnatal management in infants with ductal-dependent pulmonary blood flow. Congenit. Hear. Dis. 2019, 14, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.E.G.; Sallmon, H.; Rose, A.T.; Porras, D.; Shelton, E.L.; Reese, J.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2020, 146, e20201209. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, I.O.; Bulbul, A. Neonatal Sepsis. Sisli Etfal Hastan. Tip Bul. 2020, 54, 142–158. [Google Scholar] [PubMed]
- Yee, W.H.; Soraisham, A.S.; Shah, V.S.; Aziz, K.; Yoon, W.; Lee, S.K. Canadian Neonatal Network Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012, 129, e298–e304. [Google Scholar] [CrossRef] [PubMed]
- Özek, E.; Kersin, S.G. Intraventricular hemorrhage in preterm babies. Turk. Pediatri. Ars. 2020, 55, 215–221. [Google Scholar] [PubMed]
- Hong, E.H.; Shin, Y.U.; Cho, H. Retinopathy of prematurity: A review of epidemiology and current treatment strategies. Clin. Exp. Pediatr. 2022, 65, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Lawn, J.E.; Vazquez, T.; Fielder, A.; Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res. 2013, 74, 35–49. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Ramamoorthy, C. Bronchopulmonary dysplasia. Pediatr. Anesth. 2022, 32, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Pascal, A.; de Bruyn, N.; Naulaers, G.; Ortibus, E.; Hanssen, B.; Oostra, A.; de Coen, K.; Sonnaert, M.; Cloet, E.; Casaer, A.; et al. The Impact of Intraventricular Hemorrhage and Periventricular Leukomalacia on Mortality and Neurodevelopmental Outcome in Very Preterm and Very Low Birthweight Infants: A Prospective Population-based Cohort Study. J. Pediatr. 2023, 262, 113600. [Google Scholar] [CrossRef]
- Zelellw, D.A.; Dessie, G.; Mengesha, E.W.; Shiferaw, M.B.; Merhaba, M.M.; Emishaw, S. A Systemic Review and Meta-analysis of the Leading Pathogens Causing Neonatal Sepsis in Developing Countries. BioMed Res. Int. 2021, 2021, 6626983. [Google Scholar] [CrossRef]
- Dong, Y.; Speer, C.P. Late-onset neonatal sepsis: Recent developments. Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F257–F263. [Google Scholar] [CrossRef] [PubMed]
- Adams-Chapman, I.; Bann, C.; Carter, S.L.; Stoll, B.J.; NICHD Neonatal Research Network. Language outcomes among ELBW infants in early childhood. Early Hum. Dev. 2015, 91, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Das, S.; Bhat, V.B.; Plakkal, N. Early Neurodevelopmental Outcome of Very Low Birthweight Neonates with Culture-positive Blood Stream Infection: A Prospective Cohort Study. Cureus 2018, 10, e3492. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; Winkleby, M.A.; Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: National cohort study. BMJ 2019, 365, l1346. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; Sundquist, K. Preterm birth and risk of type 1 and type 2 diabetes: A national cohort study. Diabetologia 2020, 63, 508–518. [Google Scholar] [CrossRef]
- Hollanders, J.J.; Schaëfer, N.; Van Der Pal, S.M.; Oosterlaan, J.; Rotteveel, J.; Finken, M.J.J. Long-Term Neurodevelopmental and Functional Outcomes of Infants Born Very Preterm and/or with a Very Low Birth Weight. Neonatology 2019, 115, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Aita, M.; De Clifford Faugère, G.; Lavallée, A.; Feeley, N.; Stremler, R.; Rioux, É.; Proulx, M.H. Effectiveness of interventions on early neurodevelopment of preterm infants: A systematic review and meta-analysis. BMC Pediatr. 2021, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Klein, V.; Zores-Koenig, C.; Dillenseger, L.; Langlet, C.; Escande, B.; Astruc, D.; Le Ray, I.; Kuhn, P. Strasbourg NIDCAP Study group Changes of Infant- and Family-Centered Care Practices Administered to Extremely Preterm Infants During Implementation of the NIDCAP Program. Front. Pediatr. 2021, 9, 718813. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.C.; Mendes, E.W.; Fuentefria, R.N.; Valentini, N.C.; Procianoy, R.S. Early intervention program for very low birth weight preterm infants and their parents: A study protocol. BMC Pediatr. 2018, 18, 268. [Google Scholar] [CrossRef]
- Flacking, R.; Tandberg, B.S.; Niela-Vilén, H.; Jónsdóttir, R.B.; Jonas, W.; Ewald, U.; Thomson, G. Positive breastfeeding experiences and facilitators in mothers of preterm and low birthweight infants: A meta-ethnographic review. Int. Breastfeed. J. 2021, 16, 88. [Google Scholar] [CrossRef]
- Yue, G.; Wang, J.; Li, H.; Li, B.; Ju, R. Risk Factors of Mechanical Ventilation in Premature Infants During Hospitalization. Ther. Clin. Risk Manag. 2021, 17, 777–787. [Google Scholar] [CrossRef]
- Luz, S.C.L.; Backes, M.T.S.; Rosa, R.D.; Schmit, E.L.; Santos, E.K.A.D. Kangaroo Method: Potentialities, barriers and difficulties in humanized care for newborns in the Neonatal ICU. Rev. Bras. Enferm. 2021, 75, e20201121. [Google Scholar] [PubMed]
- Kurt, F.Y.; Kucukoglu, S.; Ozdemir, A.A.; Ozcan, Z. The effect of kangaroo care on maternal attachment in preterm infants. Niger. J. Clin. Pract. 2020, 23, 26–32. [Google Scholar] [CrossRef] [PubMed]
- El-Farrash, R.A.; Shinkar, D.M.; Ragab, D.A.; Salem, R.M.; Saad, W.E.; Farag, A.S.; Salama, D.H.; Sakr, M.F. Longer duration of kangaroo care improves neurobehavioral performance and feeding in preterm infants: A randomized controlled trial. Pediatr. Res. 2020, 87, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, S.M.; Patel, V.T.; Patel, D.V.; Phatak, A.G. Impact of hypothermia alert device (BEMPU) on improvement of duration of Kangaroo Mother Care (KMC) provided at home: Parallel-group randomized control trial. Sci. Rep. 2023, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Herting, E.; Härtel, C.; Göpel, W. Less invasive surfactant administration: Best practices and unanswered questions. Curr. Opin. Pediatr. 2020, 32, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.; Dobson, N.R.; Hunt, C.E. Immature control of breathing and apnea of prematurity: The known and unknown. J. Perinatol. 2021, 41, 2111–2123. [Google Scholar] [CrossRef]
- Çövener Özçelik, Ç.; Eren, Ö.; Sabaz, N.; Bulut, M. Effect of using crochet octopus in reducing the pain: A randomized controlled study. J. Trop. Pediatr. 2022, 69, fmac107. [Google Scholar] [CrossRef] [PubMed]
- Cobo, M.M.; Moultrie, F.; Hauck, A.G.V.; Crankshaw, D.; Monk, V.; Hartley, C.; Fry, R.E.; Robinson, S.; van der Vaart, M.; Baxter, L.; et al. Multicentre, randomised controlled trial to investigate the effects of parental touch on relieving acute procedural pain in neonates (Petal). BMJ Open 2022, 12, e061841. [Google Scholar] [CrossRef]
- Als, H.; Duffy, F.H.; McAnulty, G.; Butler, S.C.; Lightbody, L.; Kosta, S.; Weisenfeld, N.I.; Robertson, R.; Parad, R.B.; Ringer, S.A.; et al. NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J. Perinatol. 2012, 32, 797–803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zivaljevic, J.; Jovandaric, M.Z.; Babic, S.; Raus, M. Complications of Preterm Birth—The Importance of Care for the Outcome: A Narrative Review. Medicina 2024, 60, 1014. https://doi.org/10.3390/medicina60061014
Zivaljevic J, Jovandaric MZ, Babic S, Raus M. Complications of Preterm Birth—The Importance of Care for the Outcome: A Narrative Review. Medicina. 2024; 60(6):1014. https://doi.org/10.3390/medicina60061014
Chicago/Turabian StyleZivaljevic, Jelica, Miljana Z. Jovandaric, Sandra Babic, and Misela Raus. 2024. "Complications of Preterm Birth—The Importance of Care for the Outcome: A Narrative Review" Medicina 60, no. 6: 1014. https://doi.org/10.3390/medicina60061014





