Can We Rely on Prophylactic Two-Level Vertebral Cement Augmentation in Long-Segment Adult Spinal Deformity Surgery to Reduce the Incidence of Proximal Junctional Complications?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Spine Imaging Studies
2.3. Surgical Techniques
- Anterior procedures (LLIF)
- 2.
- Posterior procedures, including prophylactic vertebral cement augmentation, pedicle screw placement, and S2-alar-iliac screw insertion
2.4. Statistical Analysis
3. Results
3.1. Changes in PJA
3.2. Changes in Sagittal Spinopelvic Parameters and Coronal Cobb Angles
3.3. PJK Group
3.4. PJF Group
3.5. Analysis of Risk Factors for the Development of PJK and PJF
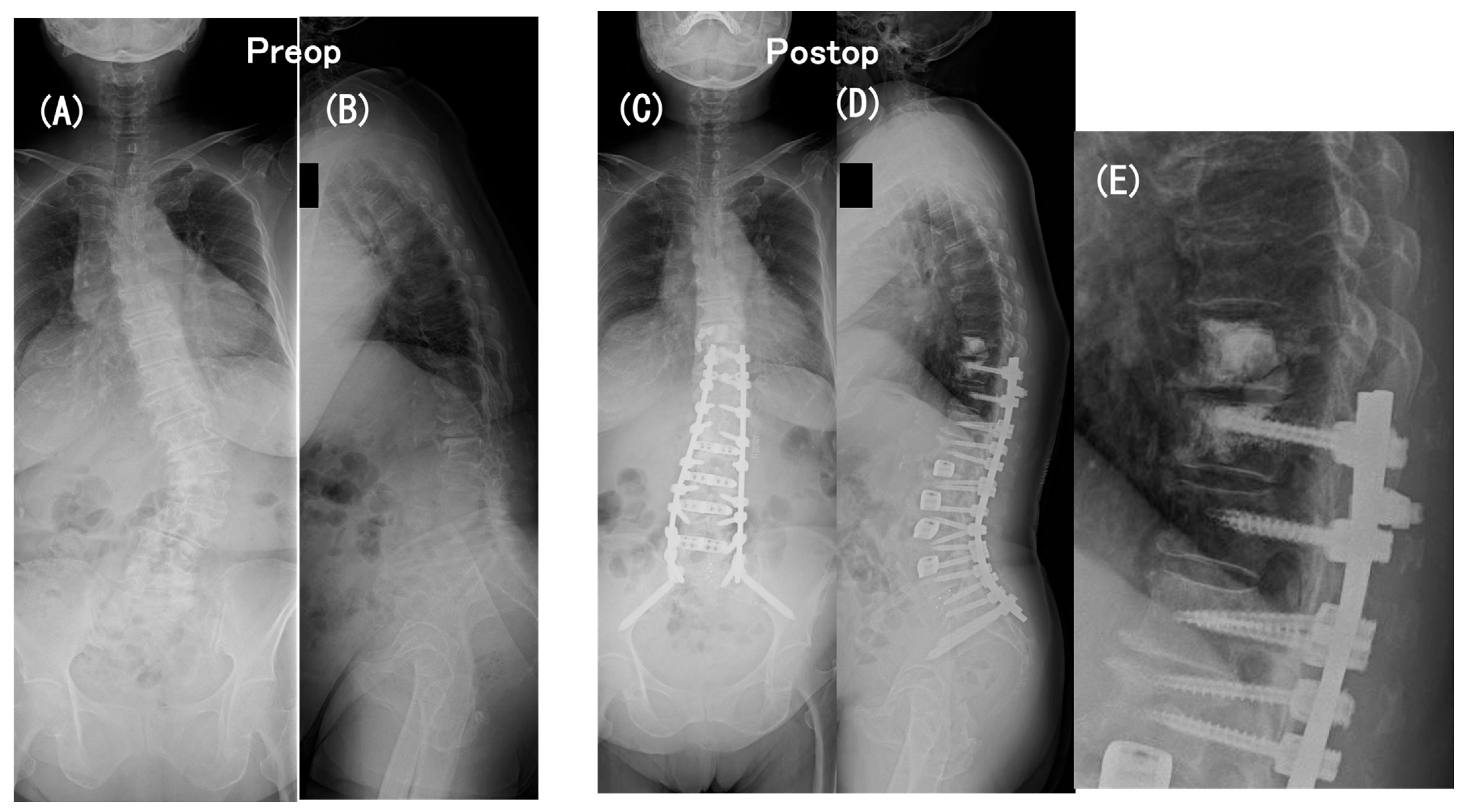
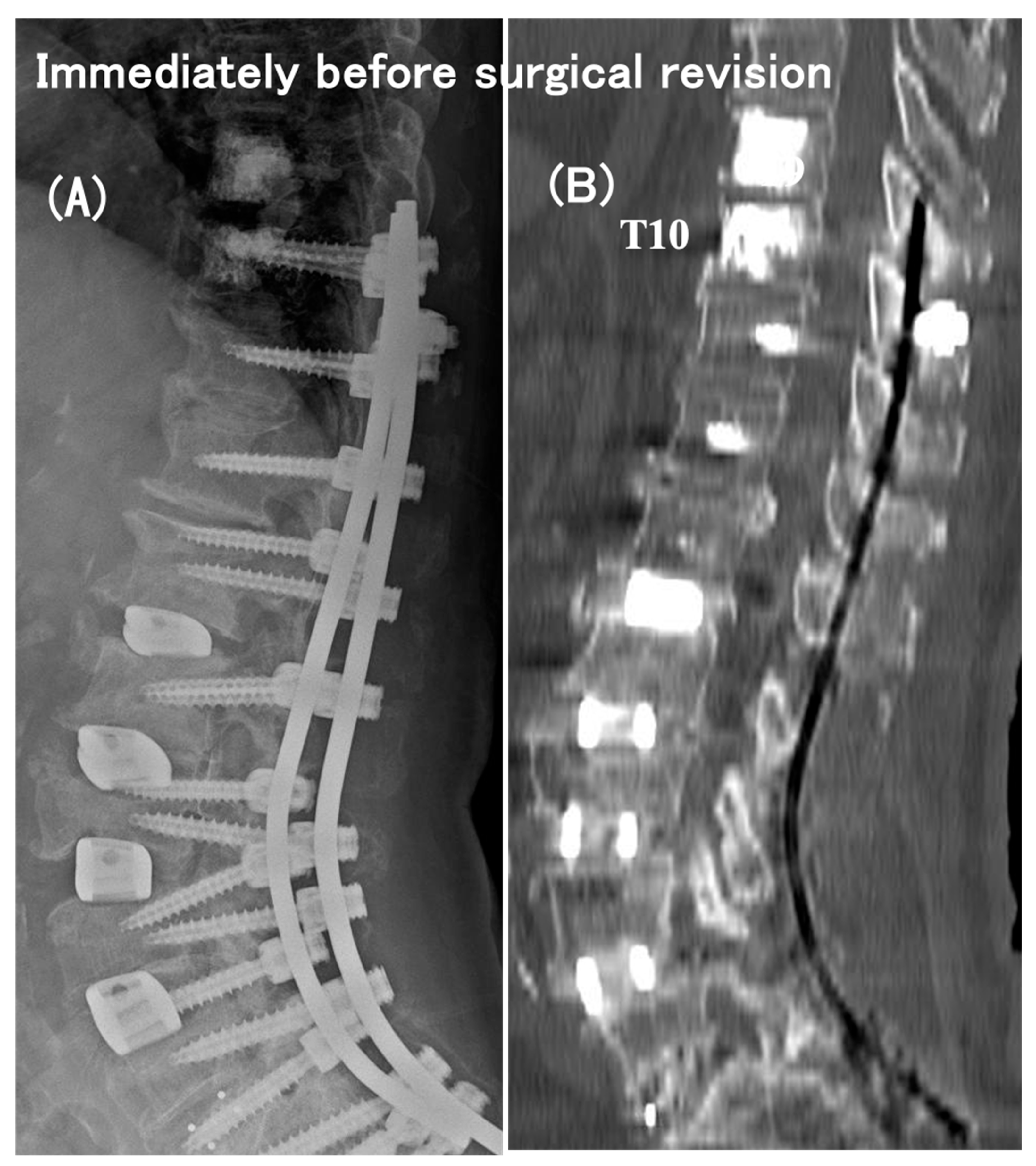
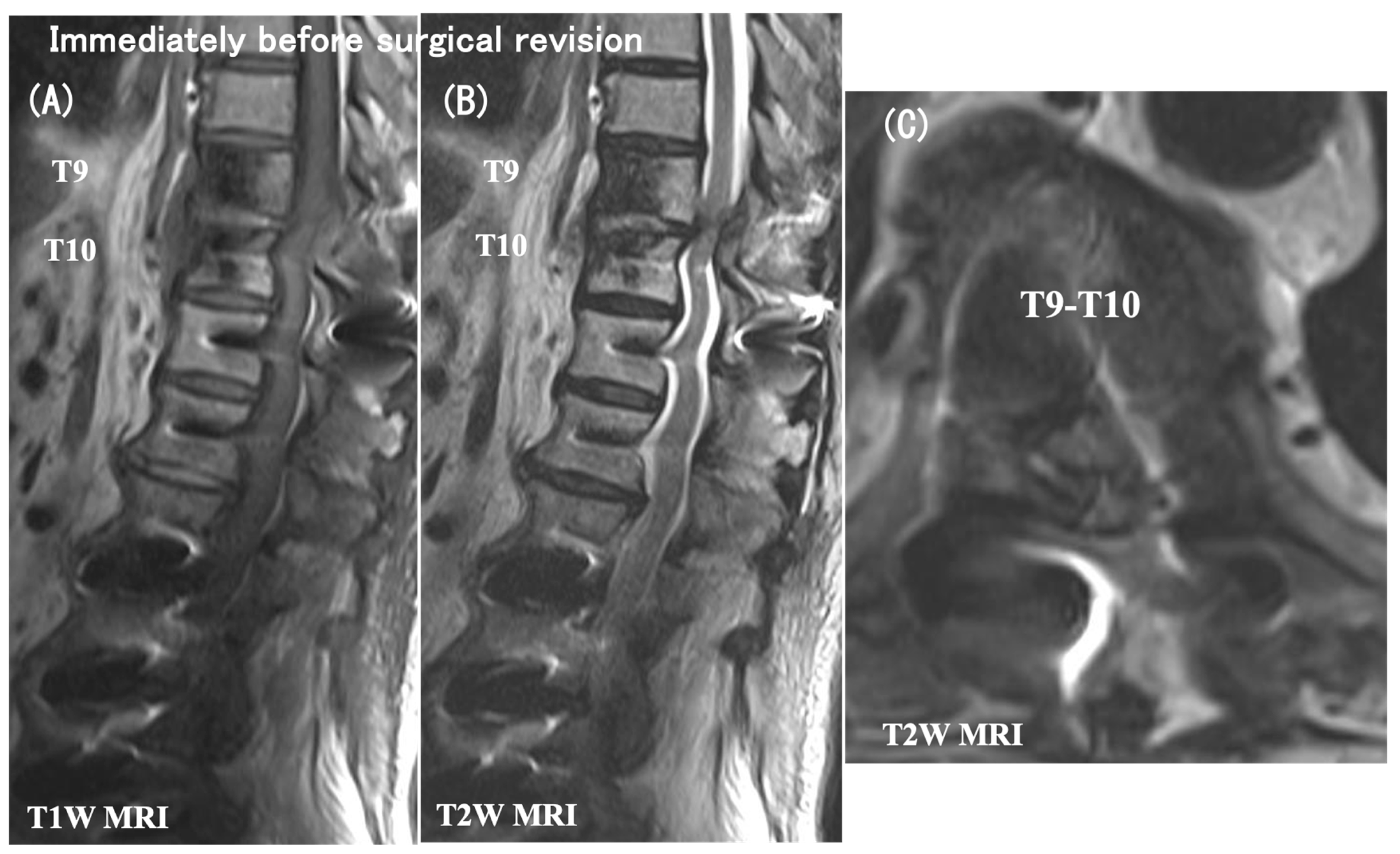
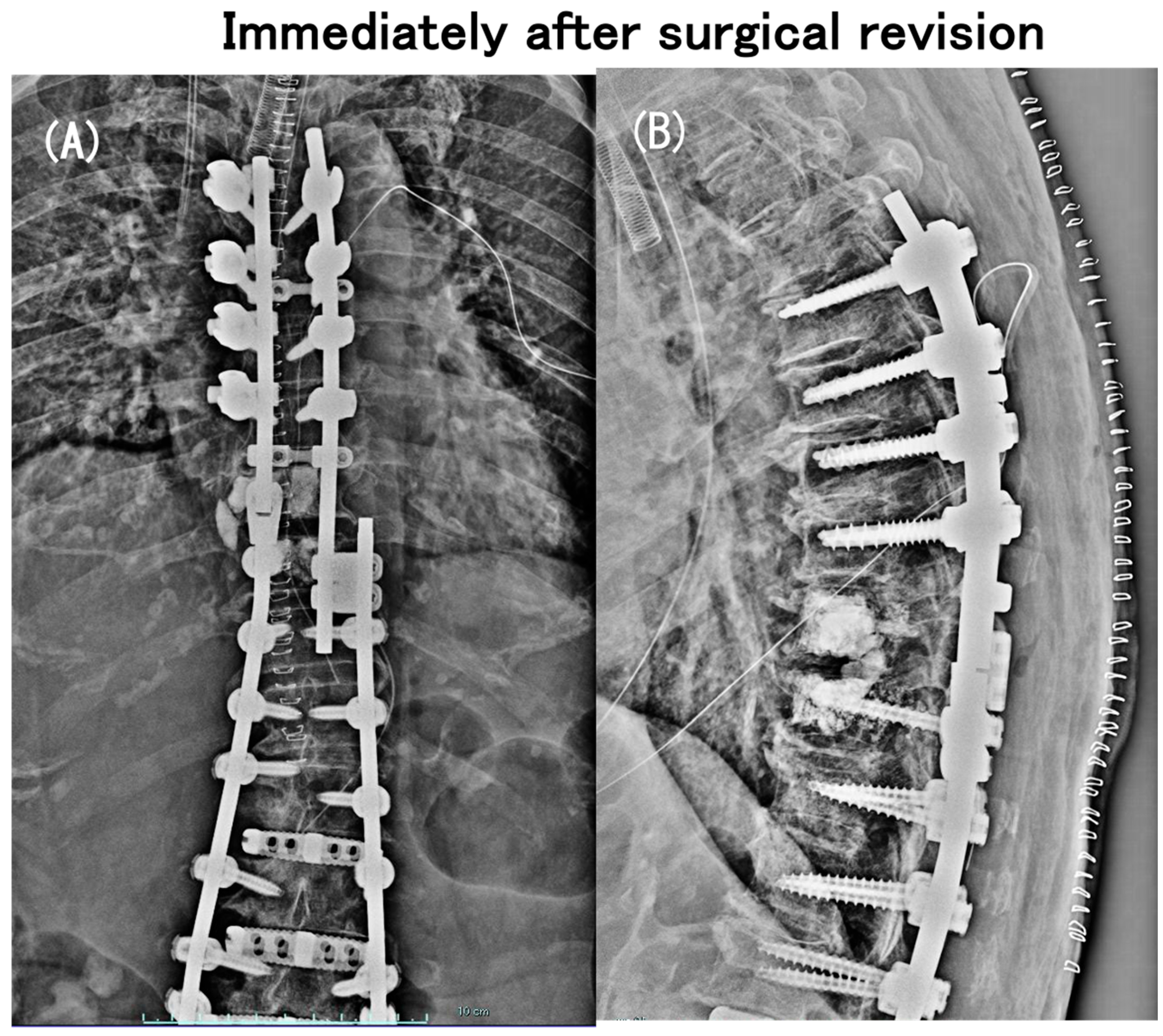
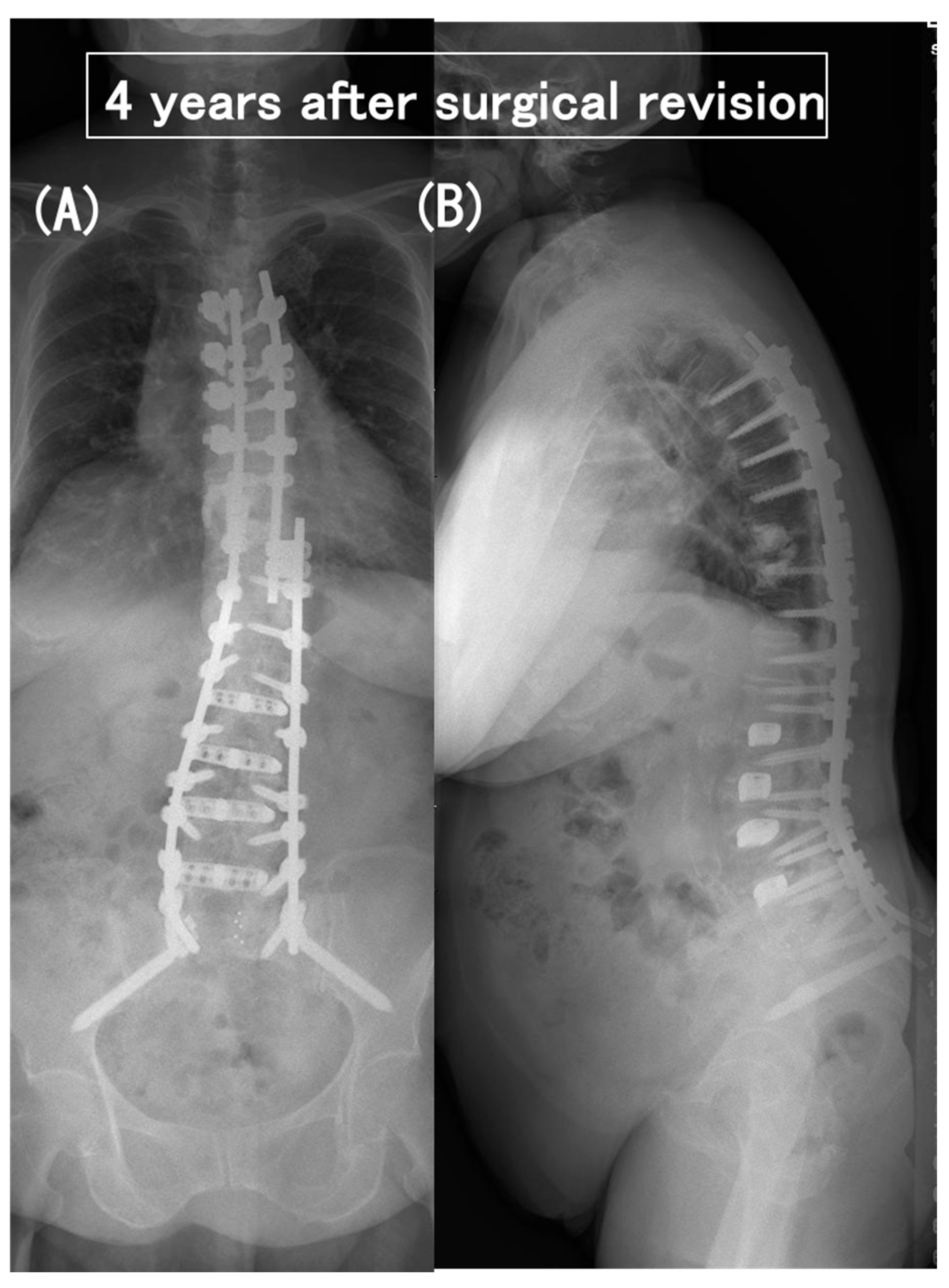
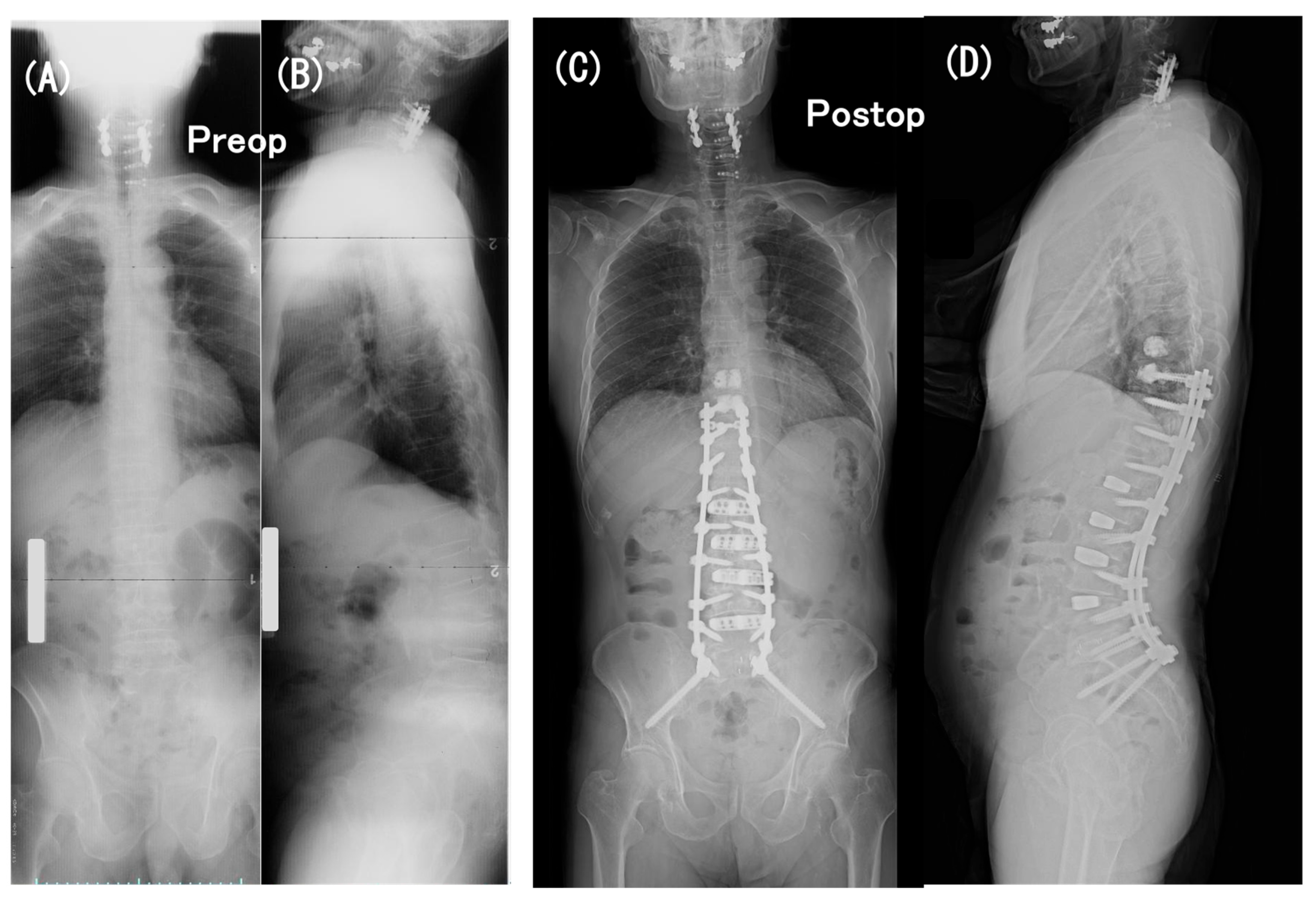
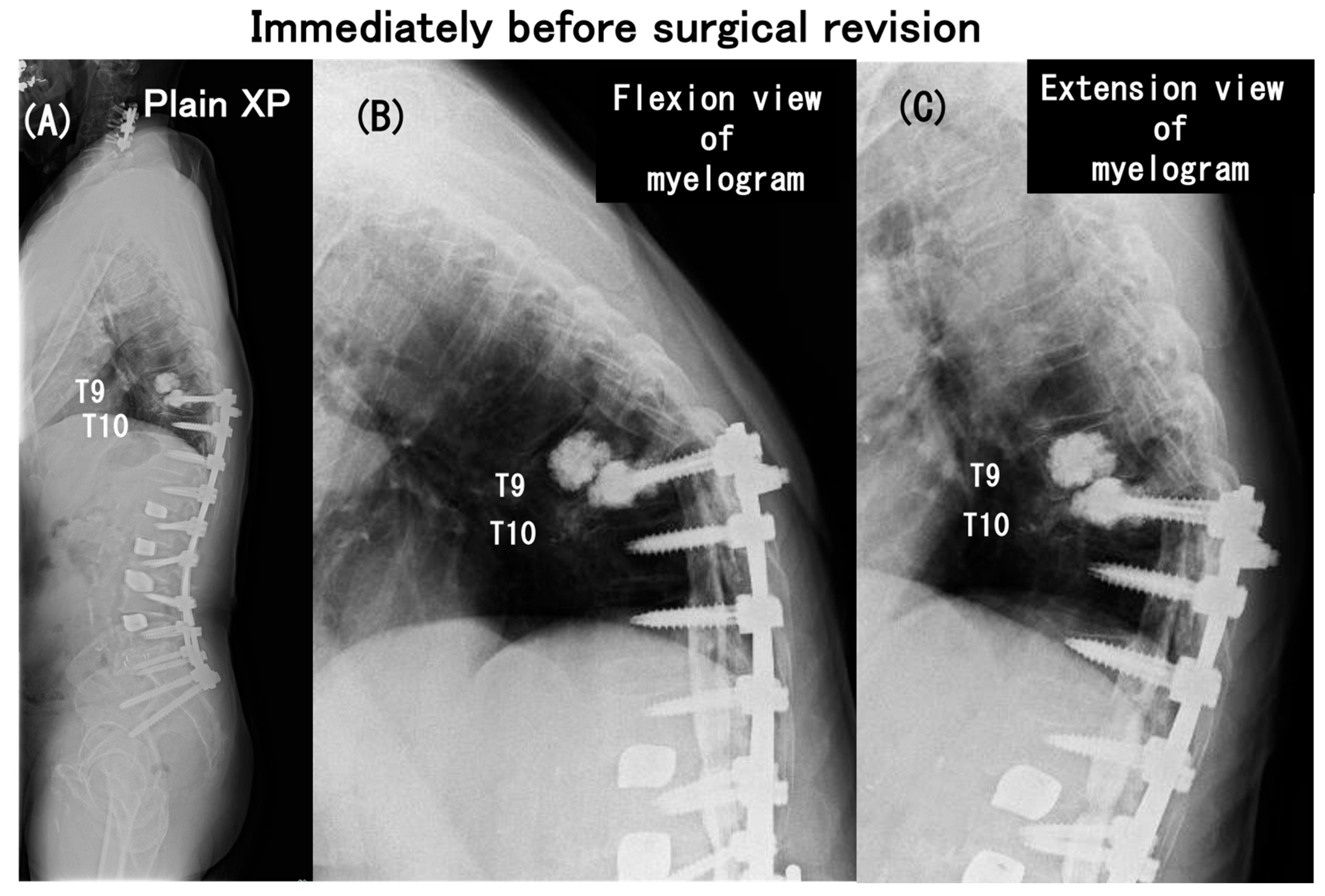
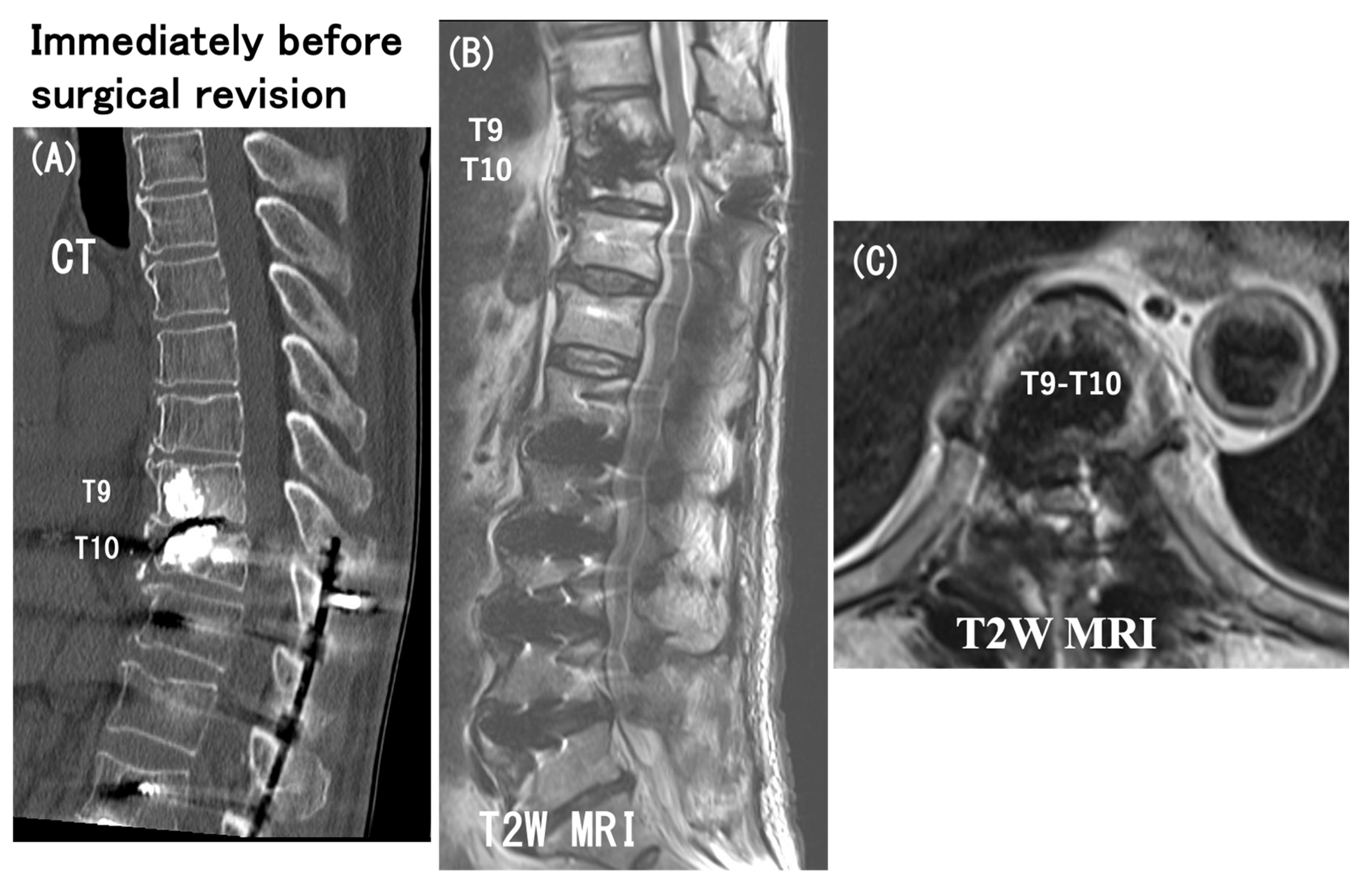
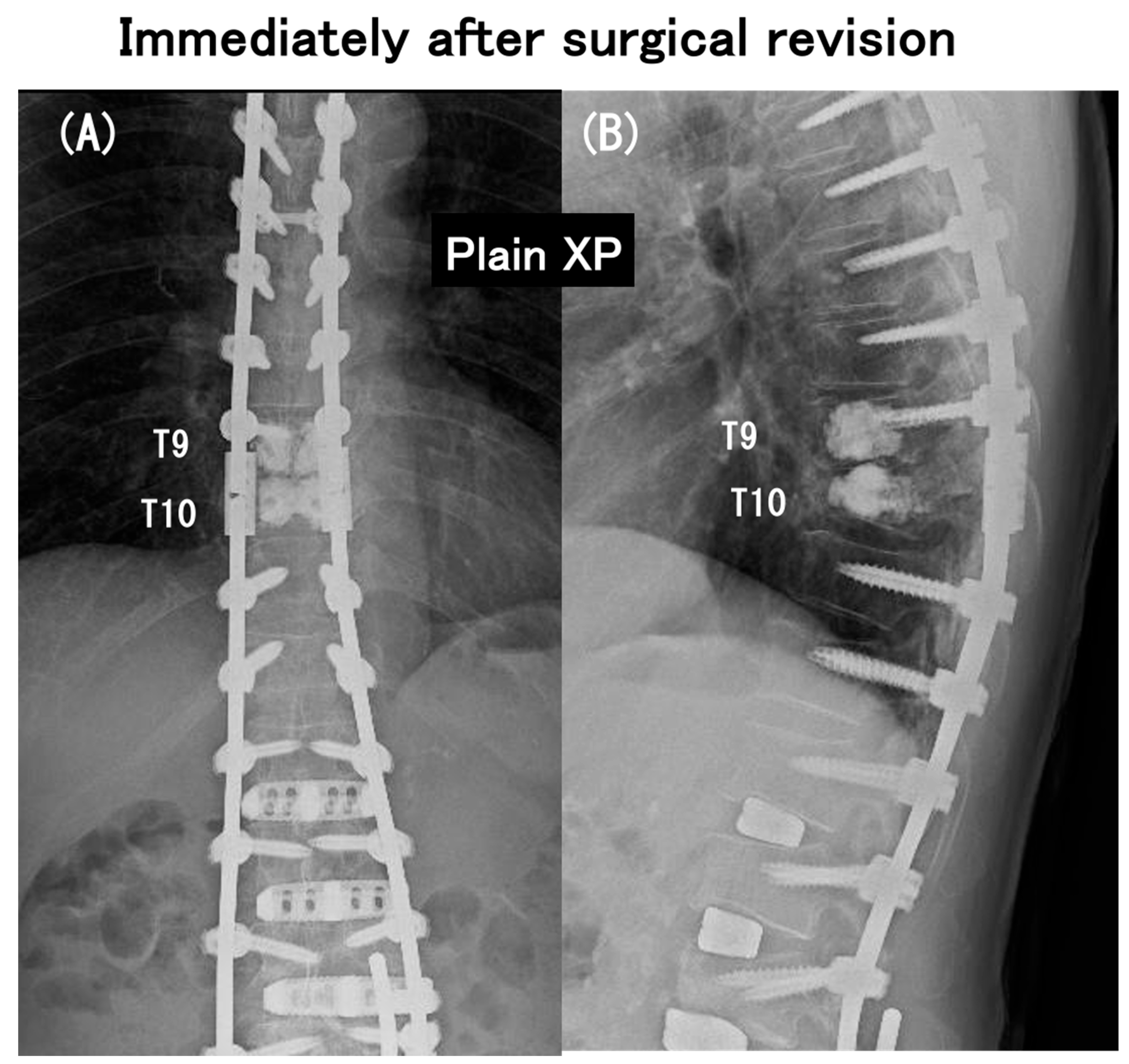
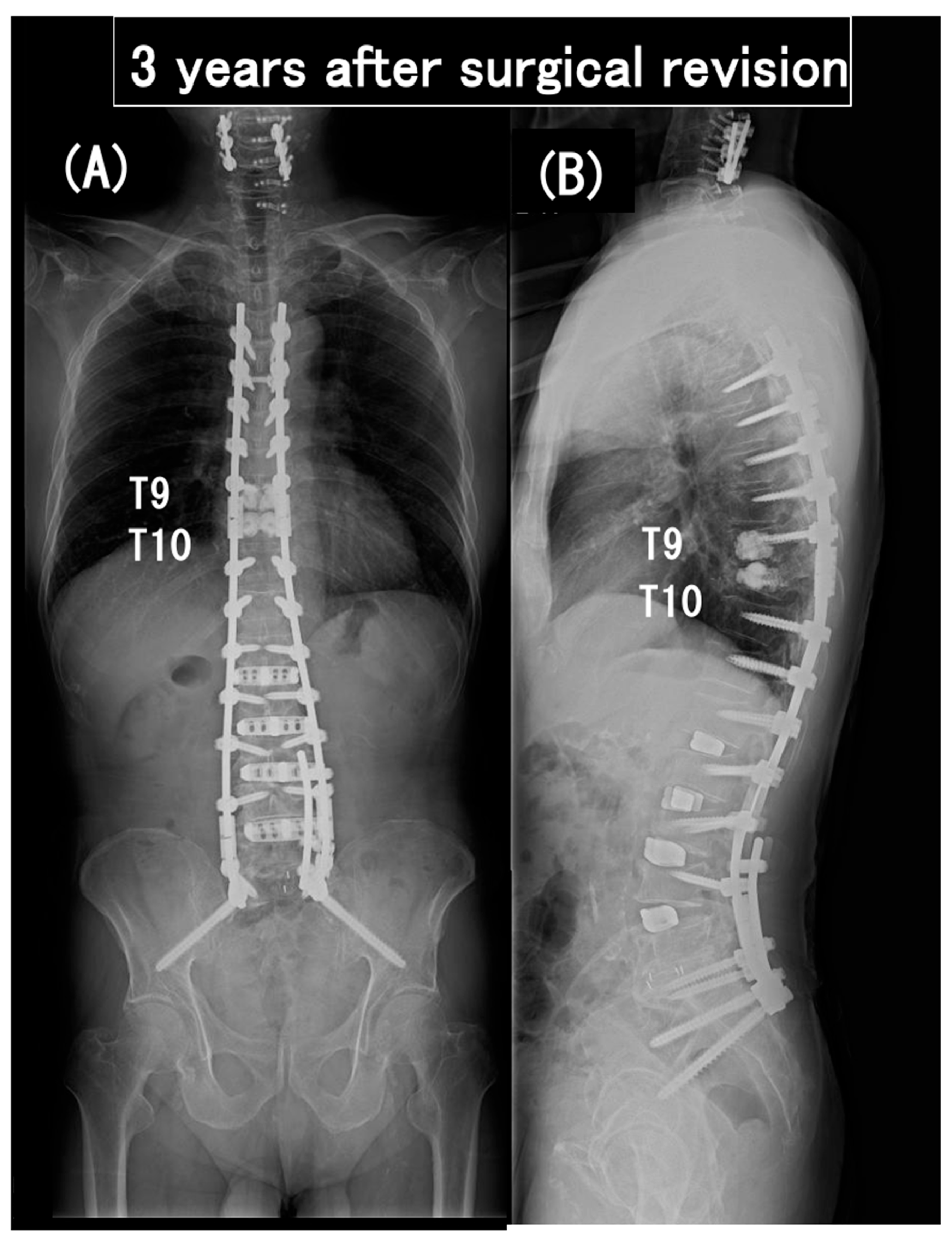
3.6. Case Presentation
- Case no. 6
- 2.
- Case no. 4
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tani, Y.; Saito, T.; Taniguchi, S.; Ishihara, M.; Paku, M.; Adachi, T.; Ando, M. Radiographic and MRI evidence of indirect neural decompression after the anterior column realignment procedure for adult spinal deformity. J. Neurosurg. Spine 2022, 37, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Naka, N.; Ono, N.; Kawashima, K.; Paku, M.; Ishihara, M.; Adachi, T.; Taniguchi, S.; Ando, M.; Saito, T. Lumbar lordosis restoration by minimally invasive short-segment fusion with anterior column realignment for adult spinal deformity: Minimum 2-year follow-up. J. Neurosurg. Spine 2024, 40, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Glattes, R.C.; Bridwell, K.H.; Lenke, L.G.; Kim, Y.J.; Rinella, A.; Edwards, I.I.C. Proximal Junctional Kyphosis in Adult Spinal Deformity Following Long Instrumented Posterior Spinal Fusion Incidence, Outcomes, and Risk Factor Analysis. Spine 2005, 30, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.G.; Kasten, M.D. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann’s disease. A review of 32 patients. Spine 1994, 19, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Betz, R.; Clements, D.H.; Huss, G.K. Proximal kyphosis after posterior spinal fusion in patients with idiopathic scoliosis. Spine 1999, 24, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.M.; Bridwell, K.H.; Won, D.S.; Lenke, L.G.; Chotigavanichaya, C.; Hanson, D.S. Sagittal plane analysis of adolescent idiopathic scoliosis: The effect of anterior versus posterior instrumentation. Spine 2002, 27, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Hostin, R.; McCarthy, I.; O’Brien, M.; Bess, S.; Line, B.; Boachie-Adjei, O.; Burton, D.; Gupta, M.; Ames, C.; Deviren, V.; et al. International Spine Study Group. Incidence, mode, and location of acute proximal junctional failures after surgical treatment of adult spinal deformity. Spine 2013, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Maruo, K.; Racine, L.; Schairer, W.W.; Hu, S.S.; Deviren, V.; Burch, S.; Tay, B.; Chou, D.; Mummaneni, P.V.; et al. Proximal junctional kyphosis and clinical outcomes in adult spinal deformity surgery with fusion from the thoracic spine to the sacrum: A comparison of proximal and distal upper instrumented vertebrae. J. Neurosurg. Spine 2013, 19, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Rahm, M.; Gaines, R.; Maziad, A.; Ross, T.; Kim, H.J.; Kebaish, K.; Boachie-Adjei, O. Complex Spine Study Group. Characterization and surgical outcomes of proximal junctional failure in surgically treated patients with adult spinal deformity. Spine 2014, 39, E607–E614. [Google Scholar] [CrossRef] [PubMed]
- Bess, S.; Harris, J.E.; Turner, A.W.L.; LaFage, V.; Smith, J.S.; Shaffrey, C.I.; Schwab, F.J.; Haid, R.W., Jr. The effect of posterior polyester tethers on the biomechanics of proximal junctional kyphosis: A finite element analysis. J. Neurosurg. Spine 2017, 26, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.A.; Prendergast, M.A.; Roberts, W.G.; Nesbit, G.M.; Barnwell, S.L. Proximal junctional acute collapse cranial to multi-level lumbar fusion: A cost analysis of prophylactic vertebral augmentation. Spine J. 2008, 8, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Lattig, F. Bone cement augmentation in the prevention of adjacent segment failure after multilevel adult deformity fusion. J. Spinal Disord. Tech. 2009, 22, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.T.; Skolasky, R.L.; Mohamed, A.S.; Kebaish, K.M. Preliminary results of the effect of prophylactic vertebroplasty on the incidence of proximal junctional complications after posterior spinal fusion to the low thoracic spine. Spine Deform. 2013, 1, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Theologis, A.A.; Burch, S. Prevention of acute proximal junctional fractures after long thoracolumbar posterior fusions for adult spinal deformity using 2-level cement augmentation at the upper instrumented vertebra and the vertebra 1 level proximal to the upper instrumented vertebra. Spine 2015, 40, 1516–1526. [Google Scholar] [PubMed]
- Raman, T.; Miller, E.; Martin, C.T.; Kebaish, K.M. The effect of prophylactic vertebroplasty on the incidence of proximal junctional kyphosis and proximal junctional failure following posterior spinal fusion in adult spinal deformity: A 5-year follow-up study. Spine J. 2017, 17, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, G.M.; Eichberg, D.G.; Kolcun, J.P.G.; Madhavan, K.; Lebwohl, N.H.; Green, B.A.; Gjolaj, J.P. Prophylactic vertebral cement augmentation at the uppermost instrumented vertebra and rostral adjacent vertebra for the prevention of proximal junctional kyphosis and failure following long-segment fusion for adult spinal deformity. Spine J. 2017, 17, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Sharfman, Z.T.; Clark, A.J.; Theologis, A.A. Proximal junctional kyphosis and failure: Strategies for prevention. Neurosurg. Clin. N. Am. 2023, 34, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Kebaish, K.M.; Martin, C.T.; O’Brien, J.R.; LaMotta, I.E.; Voros, G.D.; Belkoff, S.M. Use of vertebroplasty to prevent proximal junctional fractures in adult deformity surgery: A biomechanical cadaveric study. Spine J. 2013, 13, 1897–1903. [Google Scholar] [CrossRef]
- Duan, P.G.; Mummaneni, P.V.; Rivera, J.; Guinn, J.M.V.; Wang, M.; Xi, Z.; Li, B.; Wu, H.H.; Ames, C.P.; Burch, S.; et al. The association between lower Hounsfield units of the upper instrumented vertebra and proximal junctional kyphosis in adult spinal deformity surgery with a minimum 2-year follow-up. Neurosurg. Focus. 2022, 49, E7. [Google Scholar] [CrossRef]
- Yao, Y.C.; Elysee, J.; Lafage, R.; McCarthy, M.; Louie, P.K.; Alshabab, B.S.; Weissmann, K.; Lafage, V.; Schwab, F.; Kim, H.J. Preoperative Hounsfield Units at the Planned Upper Instrumented Vertebrae May Predict Proximal Junctional Kyphosis in Adult Spinal Deformity. Spine 2020, 46, E174–E180. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, N.; Maruo, K.; Arizumi, F.; Kishima, K.; Kusukawa, T.; Tachibana, T. The Relationship between the Hounsfield Units Value of the Upper Instrumented Vertebra and the Severity of Proximal Junctional Fracture after Adult Spinal Deformity Surgery. Medicina 2023, 59, 1086. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.W.; Annis, P.; Lawrence, B.D.; Daubs Brodke, D.S. Early Proximal Junctional Failure in Patients with Preoperative Sagittal Imbalance. Evid. Based Spine Care J. 2013, 4, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Safaee, M.M.; Deviren, V.; Ore, C.D.; Scheer, J.K.; Lau, D.; Osorio, J.A.; Nicholls, F.; Ames, C.P. Ligament augmentation for prevention of proximal junctional kyphosis and proximal junctional failure in adult spinal deformity. J. Neurosurg. Spine 2018, 28, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Sardar, Z.M.; Kim, Y.; Lafage, V.; Rand, F.; Lenke, L.; Klineberg, E. SRS Adult Spinal Deformity Committee. State of the art: Proximal junctional kyphosis—Diagnosis, management and prevention. Spine Deform. 2021, 9, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Annis, P.; Lawrence, B.D.; Spiker, W.R.; Zhang, Y.; Chen, W.; Daubs, M.D.; Brodke, D.S. Predictive factors for acute proximal junctional failure after adult deformity surgery with upper instrumented vertebrae in the thoracolumbar spine. Evid. Based Spine Care J. 2014, 5, 160–162. [Google Scholar] [PubMed]
- Kuo, C.C.; Soliman, M.A.R.; Aguirre, A.O.; Ruggiero, N.; Kruk, M.; Asham Khan, A.; Ghannam, M.M.; Almeida, N.D.; Jowdy, P.K.; Smolar, D.E.; et al. Vertebral Bone Quality Score Independently Predicts Proximal Junctional Kyphosis and/or Failure After Adult Spinal Deformity Surgery. Neurosurgery 2023, 92, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Abbas, H.; Chua, S.; Bacon, S.; Bronstein, Y.; Goldstein, S.; Magana, S.; Sullivan, K.; Dold, A.P.; Bodrogi, A. Upper instrumented vertebral fractures in long lumbar fusions: What are the associated risk factors? Spine 2012, 37, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Badlami, N.; Phillips, F.M. Lateral lumbar interbody fusion. In The Textbook of Spinal Surgery, 3rd ed.; Bridwell, K.H., DeWald, R.L., Eds.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 357–372. [Google Scholar]
- Tani, Y.; Tanaka, T.; Kawashima, K.; Masada Paku, M.; Ishihara, M.; Adachi, T.; Taniguchi, S.; Ando, M.; Saito, T. A triple minimally invasive surgery combination for subacute osteoporotic lower lumbar vertebral collapse with neurological compromise: A potential alternative to the vertebral corpectomy/expandable cage strategy. Neurosurg. Focus. 2023, 54, E10. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme lateral interbody fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.J.; Hunter, W.D.; Moldavsky, M.; Salloum, K.; Bucklen, B. Indirect decompression and vertebral body endplate strength after lateral interbody spacer impaction: Cadaveric and foamblock models. J. Neurosurg. Spine 2016, 24, 727–733. [Google Scholar] [CrossRef]
- Salzmann, S.N.; Shue, J.; Hughes, A.P. Lateral lumbar interbody fusion–outcomes and complications. Curr. Rev. Musculoskelet. Med. 2017, 10, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Le, T.V.; Baaj, A.A.; Dakwar, E.; Burkett, C.J.; Murray, G.; Smith, D.A.; Uribe, J.S. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine 2012, 37, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Marchi, L.; Abdala, N.; Oliveira, L.; Amaral, R.; Coutinho, E.; Pimenta, L. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J. Neurosurg. Spine 2013, 19, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Fogel, G.; Martin, N.; Williams, G.M.; Unger, J.; Yee-Yanagishita, C.; Pelletier, M.; Walsh, W.; Peng, Y.; Jekir, M. Choice of spinal interbody fusion cage material and design influences subsidence and osseointegration performance. World Neurosug. 2022, 162, E626–E634. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Hynes, R.A.; Otsuki, B.; Kimura, H.; Takemoto, M.; Matsuda, S. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine 2015, 40, E175–E182. [Google Scholar] [CrossRef]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Sotome, S.; Ae, K.; Okawa, A.; Ishizuki, M.; Morioka, H.; Matsumoto, S.; Nakamura, T.; Abe, S.; Beppu, Y.; Shinomiya, K. Efficacy and safety of porous hydroxyapatite/type 1 collagen composite implantation for bone regeneration: A randomized controlled study. J. Orthop. Sci. 2016, 21, 373–380. [Google Scholar] [CrossRef]
- Tani, Y.; Saito, T.; Taniguchi, S.; Ishihara, M.; Paku, M.; Adachi, T.; Ando, M. A new technique useful for lumbosacral percutaneous pedicle screw placement without fluoroscopy or computer-aided navigation systems. J. Orthop. Sci. 2022, 27, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Saito, T.; Taniguchi, S.; Ishihara, M.; Paku, M.; Adachi, T.; Ando Kohara, N.; Kimura, J. Threshold-based monitoring of compound muscle action potentials for percutaneous pedicle screw placement in the lumbosacral spine: Can we rely on stimulation of the uninsulated screw to provide a valid safety warning? Spine 2021, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Tani, Y.; Saito, T.; Taniguchi, S.; Ishihara, M.; Paku, M.; Adachi, T.; Ando Mand Kotani, Y. A new treatment algorithm that incorporates minimally invasive surgery for pyogenic spondylodiscitis in the thoracic and lumbar spines: The results of Its clinical application to a series of 34 patients. Medicina 2022, 58, 478. [Google Scholar] [CrossRef]
- Kebaish, K.M. Sacropelvic Fixation Techniques and Complications. Spine 2010, 35, 2245–2251. [Google Scholar] [CrossRef]
- Jensen, M.E.; Evans, A.J.; Mathis, J.M.; Kallmes, D.F.; Cloft, H.J.; Dion, J.E. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: Technical aspects. Am. J. Neuroradiol. 1997, 18, 1897–1904. [Google Scholar] [PubMed]
- Ortiz, A.O. Vertebroplasty cement augmentation technique. In Vertebral Compression Fractures in Osteoporotic and Pathologic Bone; Razi, A.E., Hershman, S.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 115–135. [Google Scholar]
- Norton, R.P. Kyphoplasty cement augmentation technique. In Vertebral Compression Fractures in Osteoporotic and Pathologic Bone; Razi, A.E., Hershman, S.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 137–143. [Google Scholar]
- Schwab, F.; Ungar, B.; Blondel, B.; Buchowski, J.; Coe, J.; Deinlein, D.; DeWald, C.; Mehdian, H.; Shaffrey, C.; Tribus, C.; et al. Scoliosis Research Society-Schwab adult spinal deformity classification: A validation study. Spine 2012, 37, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Bradford, D.S.; Mcbride, G.G. Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin. Orthop. Relat. Res. 1987, 218, 201–216. [Google Scholar] [CrossRef]
- Watanabe, K.; Lenke, L.G.; Bridwell, K.H.; Kim, Y.J.; Koester, L.; Hensley, M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs analysis of morphological features. Spine 2010, 35, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Bridwell, K.H.; Lenke, L.G.; Glattes, C.R.; Rhim, S.; Cheh, G. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: Minimum five-year follow-up. Spine 2008, 33, 2179–2184. [Google Scholar] [CrossRef]
- O’Leary, P.T.; Bridwell, K.H.; Lenke, L.G.; Good, C.R.; Pichelmann, M.A.; Buchowski, J.M.; Kim, Y.J.; Flynn, J. Risk factors and outcomes for catastrophic failures at the top of long pedicle screw constructs: A matched cohort analysis performed at a single center. Spine 2009, 34, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bridwell, K.H.; Lenke, L.G.; Park, M.S.; Ahmad, A.; Kwang-Sup Song, K.S.; Piyaskulkaew, C.; Hershman, S.; Fogelson, J.; Mesfin, A. Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients. Spine 2013, 38, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, K.H.; Lenke, L.G.; Cho, S.K.; Pahys, J.M.; Zebala, L.P.; Dorward, I.G.; Cho, W.; Baldus, C.; Hill Kang, M.M. Proximal junctional kyphosis in primary adult deformity surgery: Evaluation of 20 degrees as a critical angle. Neurosurgery 2013, 72, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, J.D.; Wills, B.P.D.; McIntosh, T.C.; Balderston, R.A. Evaluation of spinal kinematics following lumbar total disc replacement and circumferential fusion using in vivo fluoroscopy. Spine 2007, 32, 527–536. [Google Scholar] [CrossRef]
- Yagi, M.; King, A.B.; Boachie-Adjei, O. Incidence, risk factors, and natural course of proximal junctional kyphosis: Surgical outcomes review of adult idiopathic scoliosis. minimum 5 years of follow-up. Spine 2012, 37, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Uei, H.; Tokuhashi, Y.; Maseda, M.; Nakahashi, M.; Sawada, H.; Matsumoto, K.; Miyakat, H. Exploratory analysis of predictors of revision surgery for proximal junctional kyphosis or additional postoperative vertebral fracture following adult spinal deformity surgery in elderly patients: A retrospective cohort study. J. Orthop. Surg. Res. 2018, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Fujita, N.; Tsuji, O.; Nagoshi, N.; Asazuma, T.; Ishii, K.; Nakamura, M.; Matsumoto, M.; Watanabe, K. Low bone-mineral density is a significant risk for proximal junctional failure after surgical correction of adult spinal deformity: A propensity score-matched analysis. Spine 2018, 43, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; King BAkilah Boachie-Adjei, O. Incidence, risk factors and classification of proximal junctional kyphosis: Surgical outcomes review of adult idiopathic scoliosis. Spine 2010, 36, E60–E68. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Lattes, S.; Ries, Z.; Gao, Y.; Weinstein, S.L. Proximal junctional kyphosis in adult reconstructive spine surgery results from incomplete restoration of the lumbar lordosis relative to the magnitude of the thoracic kyphosis. Iowa Orthop. J. 2011, 31, 199–206. [Google Scholar] [PubMed]
- Maruo, K.; Ha, Y.; Inoue, S.; Samuel, S.; Okada, E.; Hu, S.S.; Deviren, V.; Burch, S.; William, S.; Ames, C.P.; et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine 2013, 38, E1469–E1476. [Google Scholar] [CrossRef] [PubMed]
- Line, B.G.; Bess, S.; Lafage, R.; Lafage, V.; Schwab, F.; Ames, C.; Kim, H.J.; Kelly, M.; Gupta, M.; Burton, D.; et al. Effective prevention of proximal junctional failure in adult spinal deformity surgery requires a combination of surgical implant prophylaxis and avoidance of sagittal alignment overcorrection. Spine 2020, 45, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bridwell, K.H.; Lenke, L.G.; Park, M.S.; Song, K.S.; Piyaskulkaew, C.; Chuntarapas, T. Patients with proximal junctional kyphosis requiring revision surgery have higher postoperative lumbar lordosis and larger sagittal balance corrections. Spine 2014, 39, E576–E580. [Google Scholar] [CrossRef] [PubMed]
- Eck, J.C.; Humphreys, S.C.; Lim, T.H.; Jeong, S.T.; Kim, J.G.; Hodges, S.D.; Howard, S. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine 2002, 27, 2431–2434. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.E.; Cunningham, B.W.; Hu, N.; Sell, G.; Vigna, F.; McAfee, P.C. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: An in vitro human cadaveric model. Spine 2005, 30, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Ingalhalikar, A.V.; Reddy, C.G.; Lim, T.H.; Torner, J.C.; Hitchon, P.W. Effect of lumbar total disc arthroplasty on the segmental motion and intradiscal pressure at the adjacent level: An in vitro biomechanical study: Presented at the 2008 Joint Spine Section Meeting Laboratory investigation. J. Neurosurg. Spine. 2009, 11, 715–723. [Google Scholar] [CrossRef]
- Bernhardt, M. Adjacent segmental disease. In The Textbook of Spinal Surgery, 3rd ed.; Bridwell, K.H., DeWald, R.L., Eds.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 509–511. [Google Scholar]
- Shufflebarger, H.; Suk Se-Il Mardjetko, S. Debate: Determining the upper instrumented vertebra in the management of adult degenerative dcoliosis stopping at T10 versus L1. Spine 2006, 31, S185–S194. [Google Scholar] [CrossRef] [PubMed]
- Alshabab, B.S.; Lafage, R.; Smith, J.S.; Kim, H.J.; Mundis, G.; Klineberg, E.; Shaffrey, C.; Daniels, A.; Ames, C.; Gupta, M.; et al. Evolution of Proximal Junctional Kyphosis and Proximal Junctional Failure Rates Over 10 Years of Enrollment in a Prospective Multicenter Adult Spinal Deformity Database. Spine 2022, 47, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lenke, L.G.; Shaffrey, C.I.; Van Alstyne, E.M.; Skelly, A.C. Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: A systematic review. Spine 2012, 37 (Suppl. 22), S144–S164. [Google Scholar] [CrossRef] [PubMed]
- Helgeson, M.D.; Shah, S.A.; Newton, P.O.; Clements, D.H.; Betz, R.R.; Marks, M.C.; Bastrom, T.; The Harms Study Group. Evaluation of proximal junctional kyphosis in adolescent idiopathic scoliosis following pedicle screw, hook, or hybrid instrumentation. Spine 2010, 35, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cahill, P.J.; Wang, W.; Asghar, J.; Booker, R.; Betz, R.R.; Ramsey, C.; Baran, G. The use of a transition rod may prevent proximal junctional kyphosis in the thoracic spine after scoliosis surgery: A finite element analysis. Spine 2012, 37, E687–E695. [Google Scholar] [CrossRef]
- Anderson, A.L.; McIff, T.E.; Asher, M.A.; Burton, D.C.; Glattes, R.C. The effect of posterior thoracic spine anatomical structures on motion segment flexion stiffness. Spine 2009, 34, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Baíllo, N.; Márquez, J.M.S.; Pérez-Grueso, F.J.S.; Fernández, A.G. Proximal junctional vertebral fracture-subluxation after adult spine deformity surgery. Does vertebral augmentation avoid this complication? A case report. Scoliosis 2012, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Kayanja, M.M.; Schlenk, R.; Togawa, D.; Ferrara, L.; Lieberman, I. The biomechanics of 1, 2, and 3 levels of vertebral augmentation with polymethylmethacrylate in multilevel spinal segments. Spine 2006, 31, 769–774. [Google Scholar] [CrossRef]
- Kim, H.J.; Yagi, M.; Nyugen, J.; Cunningham, M.E.; Boachie-Adjei, O. Combined Anterior-Posterior Surgery is the Most Important Risk Factor for Developing Proximal Junctional Kyphosis in Idiopathic Scoliosis. Clin. Orthop. Relat. Res. 2012, 470, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, M.; Aubin, C.-É.; Wang, X.; Mac-Thiong, J.-M. Biomechanical risk factors for proximal junctional kyphosis. Spine 2014, 39, E500–E507. [Google Scholar] [CrossRef] [PubMed]
- Safaee, M.M.; Osorio, J.A.; Verma, K.; Bess, S.; Shaffrey, C.I.; Smith, J.S.; Hart, R.; Deviren, V.; Ames, C.P. Proximal junctional kyphosis prevention strategies: A video technique guide. Oper. Neurosurg. 2017, 13, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, H.; Gupta, S.; Jain, A.; Dafrawy, M.H.E.I.; Skolasky RLKebaish, K.M. Type of anchor at the proximal fusion level has a significant effect on the incidence of proximal junctional kyphosis and outcome in adults after long posterior spinal fusion. Spine Deform. 2013, 1, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Taniguchi, S.; Adachi, T.; Kushida, T.; Paku, M.; Muneharu Ando, M.; Saito, T.; Kotani, Y.; Tani, Y. Rod contour and overcorrection are risk factors of proximal junctional kyphosis after adult spinal deformity correction surgery. Eur. Spine J. 2021, 30, 1208–1214. [Google Scholar] [CrossRef]
| 29 | |
|---|---|
| Mean age, yrs (range) | 72.6 (57–81) |
| Female sex, n (%) | 25 (86.2) |
| Mean hip BMD, g/cm2 (range) § | 0.73 (0.42–1.1) |
| T-score | −1.6 (−3.8 ~ 1.6) |
| Mean HUs (±SE) ǂ | |
| UIV | 125.6 ± 7.5 |
| UIV + 1 | 127.1 ± 7.4 |
| UIV + 2 | 128.9 ± 7.6 |
| Medical comorbidities, n | |
| Hypertension | 13 |
| Coronary heart disease | 7 |
| Hyperlipidemia | 6 |
| Diabetes | 3 |
| Rheumatoid arthritis | 2 |
| Arteriosclerosis obliterans | 1 |
| Autoimmune hepatitis | 1 |
| Colorectal cancer | 1 |
| No. of levels fused (motion segment) | 8 or 9 |
| UIV, n (%) | |
| T9 | 1 (0.4) |
| T10 | 28 (96.6) |
| LIV, n (%) | |
| S2 alar-iliac fixation | 29 (100) |
| Anterior–posterior procedure, n (%) | |
| One-stage operation | 9 (31.0) |
| Two-stage operation | 20 (69.0) |
| PJA §, ° | PJA §, ° | PJA §, ° | |||||
|---|---|---|---|---|---|---|---|
| Preop | Early Postop | p Value | 3 yrs Postop | p Value (Early vs. 3 yrs) | Immediately before Re-Operation | p Value (Early vs. Before Re-Operation) | |
| Non-PJK/PJF group (n = 15) | 3.9 ± 0.6 | 9.4 ± 1.4 | 0.0118 * | 13.3 | 0.0883 | NA | NA |
| PJK group (n = 8) | 4.9 ± 1.0 | 10.6 ± 1.6 | 0.1649 | 28.1 | <0.0001 * | NA | NA |
| PJF group (n = 6) | 6.5 ± 1.2 | 11.7 ± 1.2 | 0.2465 | NA | NA | 37.6 | <0.0001 * |
| Non-PJK/PJF Group (n = 15) | PJK Group (n = 8) | PJF Group (n = 6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop | Postop | Preop | Postop | Preop | Postop | ||||||
| Parameters | Early | 1 yr | 3 yrs | Early | 1 yr | 3 yrs | Early | Immediately before the Re-Operation | |||
| PI (°) | 49.4 ± 3.4 | 48.1 ± 2.8 | 48.0 ± 3.0 | 48.9 ± 2.9 | 49.1 ± 3.9 | 47.5 ± 3.7 | 49.5 ± 3.2 | 50.0 ± 3.2 | 49.3 ± 2.3 | 49.6 ± 2.0 | 50.4 ± 1.9 |
| LL (°) | 5.3 ± 4.8 | 44.5 ± 2.6 ** | 43.0 ± 2.8 ** | 38.1 ± 2.4 ** | 16.1 ± 4.8 | 47.6 ± 2.4 ** | 46.4 ± 2.7 ** | 43.0 ± 2.5 ** | 7.5 ± 5.9 | 51.0 ± 4.3 ** | 47.6 ± 3.3 ** |
| PI–LL (°) | 44.1 ± 4.0 | 3.4 ± 2.2 ** | 5.1 ± 2.4 ** | 10.7 ± 2.7 ** | 32.7 ± 5.3 | 0.4 ± 4.5 ** | 3.1 ± 3.9 §§ | 7.0 ± 4.2 ** | 41.9 ± 3.8 | −1.4 ± 5.0 ** | 2.8 ± 3.3 ** |
| PT (°) | 35.5 ± 2.6 | 18.9 ± 2.5 ** | 21.9 ± 2.4 ** | 24.4 ± 1.8 ** | 25.8 ± 4.5 | 17.7 ± 3.0 | 20.6 ± 1.6 | 24.8 ± 2.6 | 30.3 ± 3.7 | 17.7 ± 3.2 † | 23.3 ± 2.3 |
| TK (°) | 16.9 ± 2.5 | 34.9 ± 2.7 ** | 37.2 ± 2.5 ** | 39.5 ± 2.4 ** | 18.1 ± 3.2 | 31.9 ± 3.8 † | 37.3 ± 3.1 §§ | 50.3 ± 2.6 ** | 18.7 ± 4.7 | 41.4 ± 4.9 § | 46.0 ± 3.7 § |
| SVA (mm) | 110.2 ± 13.8 | 20.7 ± 6.1 ** | 39.1 ± 10.7 * | 65.1 ± 16.9 † | 130.9 ± 20.0 | 37.1 ± 9.8 ** | 36.8 ± 7.2 ** | 83.9 ± 13.0 † | 105.6 ± 21.8 | 27.6 ± 13.4 †† | 40.3 ± 12.6 †† |
| CA (°) | 25.0 ± 4.9 | 5.0 ± 1.0 ** | 5.2 ± 1.0 ** | 7.0 ± 1.3 ** | 30.8 ± 7.5 | 4.2 ± 0.9 ** | 5.0 ± 1.3 ** | 5.2 ± 0.9 ** | 24.1 ± 8.5 | 5.8 ± 0.9 † | 4.7 ± 1.3 † |
| No. of Patients, n (%) | |||
|---|---|---|---|
| PJK (n = 8) | PJF (n = 6) | ||
| Pathogenesis | Bony failure (vertebral fracture) | 2 (25) | 5 (83) |
| Proximal disc or ligament failure | 8 (100) | 5 (83) | |
| Implant/bone interface failure | 0 | 0 | |
| Disability for revision | Neurologic compromise | 0 | 5 (83) |
| Severe pain | 0 | 1 (17) | |
| (A) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before the Initial Op | Initial Op | Early Postop | |||||||||||||||
| Case No. | Age (yrs)/ Sex | BMD (g/cm2)/ T-Score | HU (UIV/UIV + 1/UIV + 2) | LL (°) | PI–LL (°) | PT (°) | TK (°) | SVA (mm) | CA (°) | UIV | 1 or 2-Satge Op | LL (°) | PI–LL (°) | PT (°) | TK (°) | SVA (mm) | CA (°) |
| 1 | 73/F | 0.525/−3.8 | 100.7/104.4/114.3 | 2 | 43 | 38 | 22 | 157 | 55 | T10 | 2 | 46 | 7 | 27 | 60 | 30 | 8 |
| 2 | 75/F | 0.670/−2.2 | 95.6/104.0/110.0 | 13 | 39 | 35 | 13 | 100 | 15 | T10 | 2 | 35 | 17 | 28 | 24 | 45 | 8 |
| 3 | 76/F | 0.718/−1.8 | 116.1/108.9/135.4 | 20 | 31 | 25 | 15 | 63 | 25 | T10 | 2 | 63 | −14 | 14 | 43 | 14 | 3 |
| 4 | 73/M | 0.423/−3.1 | 113.6/109.2/110.2 | −19 | 59 | 38 | 5 | 118 | 4 | T10 | 2 | 47 | −6 | 9 | 35 | −8 | 5 |
| 5 | 69/M | 0.733/−1.6 | 106.3/113.9/123.5 | 10 | 42 | 15 | 39 | 29 | 4 | T10 | 1 | 62 | −14 | 13 | 41 | 3 | 3 |
| 6 | 78/F | 0.728/−1.4 | 206.1/174.4/168.5 | 19 | 37 | 31 | 18 | 167 | 42 | T10 | 2 | 53 | 2 | 16 | 46 | 82 | 7 |
| Mean | 74/NA | 0.633/−2.3 | 123.0/119.1/127.0 | 7.5 | 41.8 | 30.3 | 18.7 | 105.7 | 24.2 | NA | NA | 51 | −1.3 | 17.8 | 41.5 | 27.7 | 5.7 |
| (B) | |||||||||||||||||
| PJF | Re-Operation | ||||||||||||||||
| Case No. | Modified Frankel Grade § | Vertebral Collapse | Segmental Instability | Time Interval Between the Initial Op and PJF (month) | Proximal Extension of Fusion | Decompression | Modified Frankel Grade | FU (months) | |||||||||
| 1 | D3 | T8, T10 | – | 13 | T5 | − | D3 | 79 | |||||||||
| 2 | D1 | T10 | UIV–UIV + 1 | 20 | T5 | - | D3 | 37 | |||||||||
| 3 | C | T9, T10 | UIV–UIV + 1 | 4 | T3 | + | D2 | 77 | |||||||||
| 4 | D1 | T10 | UIV–UIV + 1 | 6 | T5 | + | D2 | 40 | |||||||||
| 5 | C | T9 | UIV–UIV + 1 | 14 | T5 | + | D3 | 74 | |||||||||
| 6 | C | – | UIV–UIV + 1 | 2 | T5 | + | D2 | 96 | |||||||||
| Group | p Value | |||||
|---|---|---|---|---|---|---|
| Poteatial Risk Factors | Non-PJK/PJF Group (n = 15) | PJK Group (n = 8) | PJF Group (n = 6) | Non-PJK/PJF vs. PJK | Non-PJK/PJF vs. PJF | PJK vs. PJF |
| Mean age, yrs (range) | 72.7 (57–81) | 71.5 (62–75) | 74.0 (69–78) | 0.8530 | 0.8712 | 0.6542 |
| Mean hip BMD, g/cm2 (range) § | 0.74 (0.65–1.1) | 0.78 (0.66–0.88) | 0.63 (0.42–0.73) | 0.8366 | 0.3858 | 0.2384 |
| T-score | −1.5 (−3.6 ~ 1.6) | −1.3 (−0.5 ~ −2.4) | −2.3 (−3.8 ~ −1.4) | 0.9156 | 0.2461 | 0.1912 |
| Mean HUs (±SE) ‡ | ||||||
| UIV | 127.4 ± 10.2 | 124 ± 14.5 | 123.1 ± 16.9 | 0.9789 | 0.9725 | 0.9990 |
| UV + 1 | 126.5 ± 11.6 | 134.2 ± 12.6 | 119.1 ± 11.2 | 0.8964 | 0.9216 | 0.7617 |
| UIV + 2 | 130.3 ± 12.1 | 127.7 ± 13.7 | 127 ± 9.2 | 0.9883 | 0.9847 | 0.9995 |
| Mean PJA (±SE), ° | ||||||
| Preop | 3.9 ± 0.6 | 4.9 ± 1.0 | 6.5 ± 1.2 | 0.6889 | 0.1159 | 0.4756 |
| Early Postop | 9.4 ± 1.4 | 10.6 ± 1.6 | 11.7 ± 1.2 | 0.8367 | 0.5722 | 0.8956 |
| Difference | 5.4 ± 1.5 | 5.7 ± 2.1 | 5.2 ± 1.6 | 0.9947 | 0.9941 | 0.9838 |
| Mean PI–LL (±SE), ° | ||||||
| Preop | 44.1 ± 4.0 | 32.7 ± 5.3 | 41.9 ± 3.8 | 0.1844 | 0.9425 | 0.4765 |
| Early Postop | 3.4 ± 2.2 | 0.4 ± 4.5 | −1.4 ± 5.0 | 0.8017 | 0.6314 | 0.9488 |
| Difference | −40.7 ± 4.7 | −32.3 ± 6.2 | −43.2 ± 6.3 | 0.5247 | 0.9536 | 0.4919 |
| Mean TK (±SE), ° | ||||||
| Preop | 16.9 ± 2.5 | 18.1 ± 3.2 | 18.7 ± 4.7 | 0.9626 | 0.9329 | 0.9940 |
| Early Postop | 34.9 ± 2.7 | 31.9 ± 3.8 | 41.4 ± 4.9 | 0.7909 | 0.4341 | 0.2427 |
| Difference | 18.0 ± 3.2 | 13.8 ± 4.8 | 22.8 ± 5.5 | 0.7399 | 0.7283 | 0.4155 |
| Mean SVA (±SE), mm | ||||||
| Preop | 110.2 ± 13.8 | 130.9 ± 19.7 | 105.6 ± 21.8 | 0.6603 | 0.9831 | 0.6655 |
| Early Postop | 20.7 ± 6.1 | 37.1 ± 9.8 | 27.6 ± 13.4 | 0.3562 | 0.8552 | 0.7908 |
| Difference | −82.9 ± 12.5 | −93.8 ± 20.0 | −78.0 ± 17.1 | 0.8699 | 0.9779 | 0.8273 |
| Mean UIV angle (±SE) †, ° | ||||||
| Early Postop | 7.5 ± 1.9 | 10.5 ± 2.6 | 13.1 ± 3.0 | 0.6309 | 0.2690 | 0.7822 |
| Parameters | Two Groups (2-Level Cement Group /Control Group) | Current Study | Previous Reports in Favor of Prophylatic Two-Level Vertebral Cement Augmentation | |||
|---|---|---|---|---|---|---|
| Martin CT, et al. (2013) [13] | Theologis AA. and Burch S. (2015) [14] | Raman T, et al. (2017) [15] | Ghobrial GM, et al. (2017) [16] | |||
| No of patients, n | 2-level cement No cement | 29 0 | 38 0 | 19 23 | 39 0 | 38 47 |
| Surgical technique, (n) | NA | MIS-LLIF, PPS, PSF with PS at T10 –T12 | PSF with PS (27) + ALIF (11) | PSF with PS (27) + 3-CO (15) | PSF with PS (28) + ALIF (11) | PSF with PS (56) + ALIF (29) Sublaminar hooks at UIV (21) |
| Age at operation, yrs [mean (range)] | 2-level cement No cement | 72.6 (57–81) NA | 64.4 (41–80) NA | 68.2 (55–79) 59.8 (33–77) | 65.6 (41–87) NA | 71.0 58.3 |
| Follow-up, mos [min or mean (range)] | 2-level cement No cement | >36 NA | 32.3 (24–80) NA | 14.8 (6.6–32.8) 24.9 (8.3–55.3) | >60 NA | 24.2 27.9 |
| No. of levels fused, n [mean (range or min)] | 2-level cement No cement | (8–9) NA | (approx 8–9) NA | 9 7.7 (5–10) | (approx 8–9) NA | 9 (>5) 9 (>5) |
| Pelvic fixation, n (%) | 2-level cement No cement | 29 (100) NA | 32 (84) NA | 19 (100) 23 (100) | 33 (85) NA | 36 (95) 45 (96) |
| PJF, n (%) | 2-level cement No cement | 6 (21) NA | 2 (5) NA | 0 (0) 5 (22) | 2 (5) NA | 0 (0) 6 (13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, Y.; Naka, N.; Ono, N.; Kawashima, K.; Paku, M.; Ishihara, M.; Adachi, T.; Ando, M.; Taniguchi, S.; Saito, T. Can We Rely on Prophylactic Two-Level Vertebral Cement Augmentation in Long-Segment Adult Spinal Deformity Surgery to Reduce the Incidence of Proximal Junctional Complications? Medicina 2024, 60, 860. https://doi.org/10.3390/medicina60060860
Tani Y, Naka N, Ono N, Kawashima K, Paku M, Ishihara M, Adachi T, Ando M, Taniguchi S, Saito T. Can We Rely on Prophylactic Two-Level Vertebral Cement Augmentation in Long-Segment Adult Spinal Deformity Surgery to Reduce the Incidence of Proximal Junctional Complications? Medicina. 2024; 60(6):860. https://doi.org/10.3390/medicina60060860
Chicago/Turabian StyleTani, Yoichi, Nobuhiro Naka, Naoto Ono, Koki Kawashima, Masaaki Paku, Masayuki Ishihara, Takashi Adachi, Muneharu Ando, Shinichirou Taniguchi, and Takanori Saito. 2024. "Can We Rely on Prophylactic Two-Level Vertebral Cement Augmentation in Long-Segment Adult Spinal Deformity Surgery to Reduce the Incidence of Proximal Junctional Complications?" Medicina 60, no. 6: 860. https://doi.org/10.3390/medicina60060860
APA StyleTani, Y., Naka, N., Ono, N., Kawashima, K., Paku, M., Ishihara, M., Adachi, T., Ando, M., Taniguchi, S., & Saito, T. (2024). Can We Rely on Prophylactic Two-Level Vertebral Cement Augmentation in Long-Segment Adult Spinal Deformity Surgery to Reduce the Incidence of Proximal Junctional Complications? Medicina, 60(6), 860. https://doi.org/10.3390/medicina60060860






