BCG Vaccination Suppresses Glucose Intolerance Progression in High-Fat-Diet-Fed C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
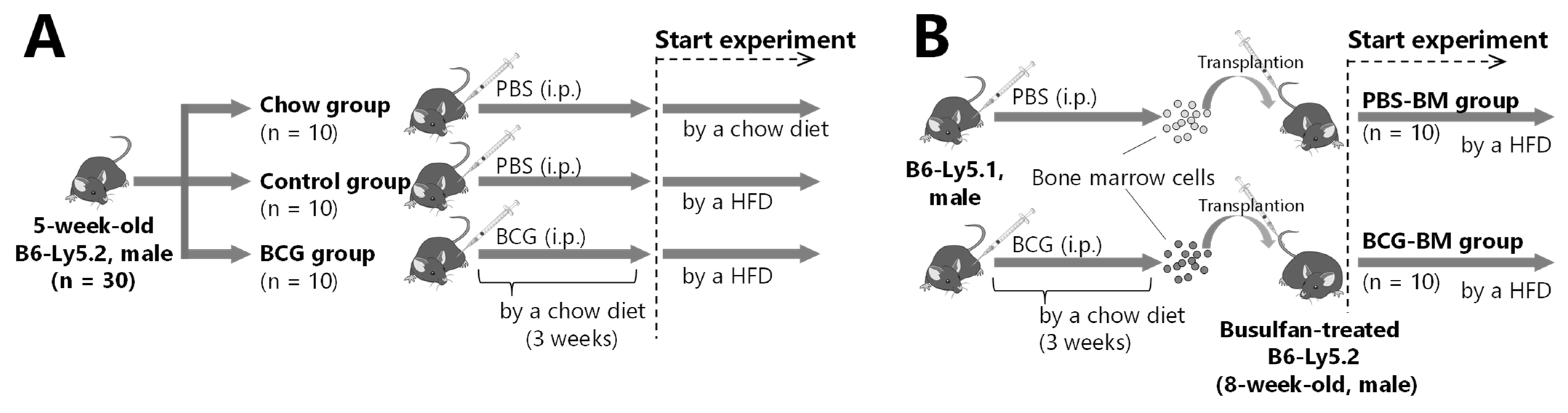
2.1. Animals, Diet, and Microorganisms
2.2. BCG Vaccination in HFD-Fed Mice
2.3. Bone Marrow Transplantation from BCG-Vaccinated Mice
2.4. Intraperitoneal Glucose Tolerance Test and Insulin Tolerance Test
2.5. Measurement of Biochemical Parameters in Serum
2.6. Measurement of Hepatic Lipid Levels
2.7. Histopathological Examination
2.8. Flow Cytometry
2.9. Statistical Analyses
3. Results
3.1. Effect of BCG Vaccination on Glucose Intolerance in HFD-Fed Mice
3.2. Effect of BCG Vaccination on Growth Parameters, Blood Parameters, and Hepatic Lipid Content
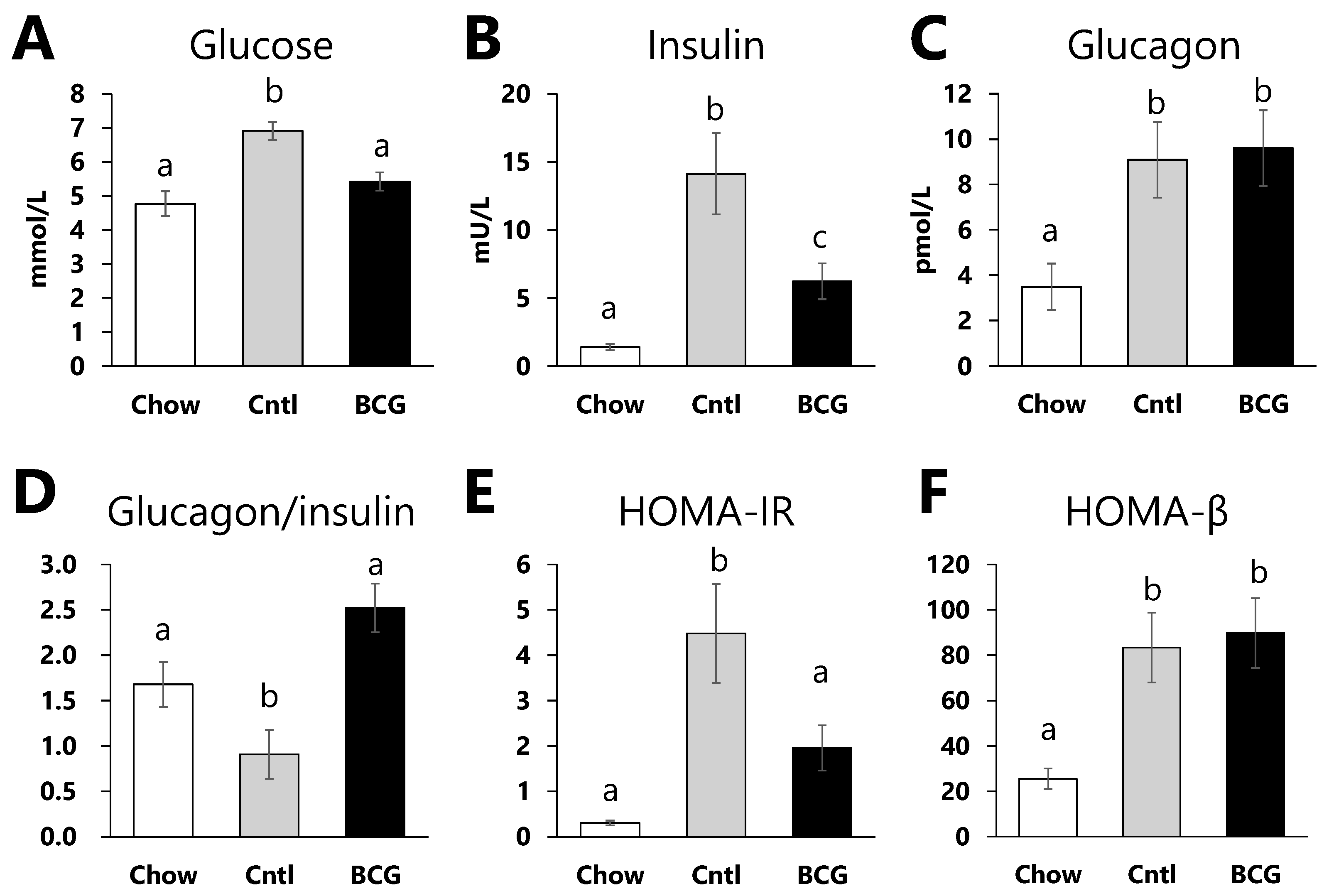
3.3. Effect of BCG Vaccination on Glucose Metabolism Parameters
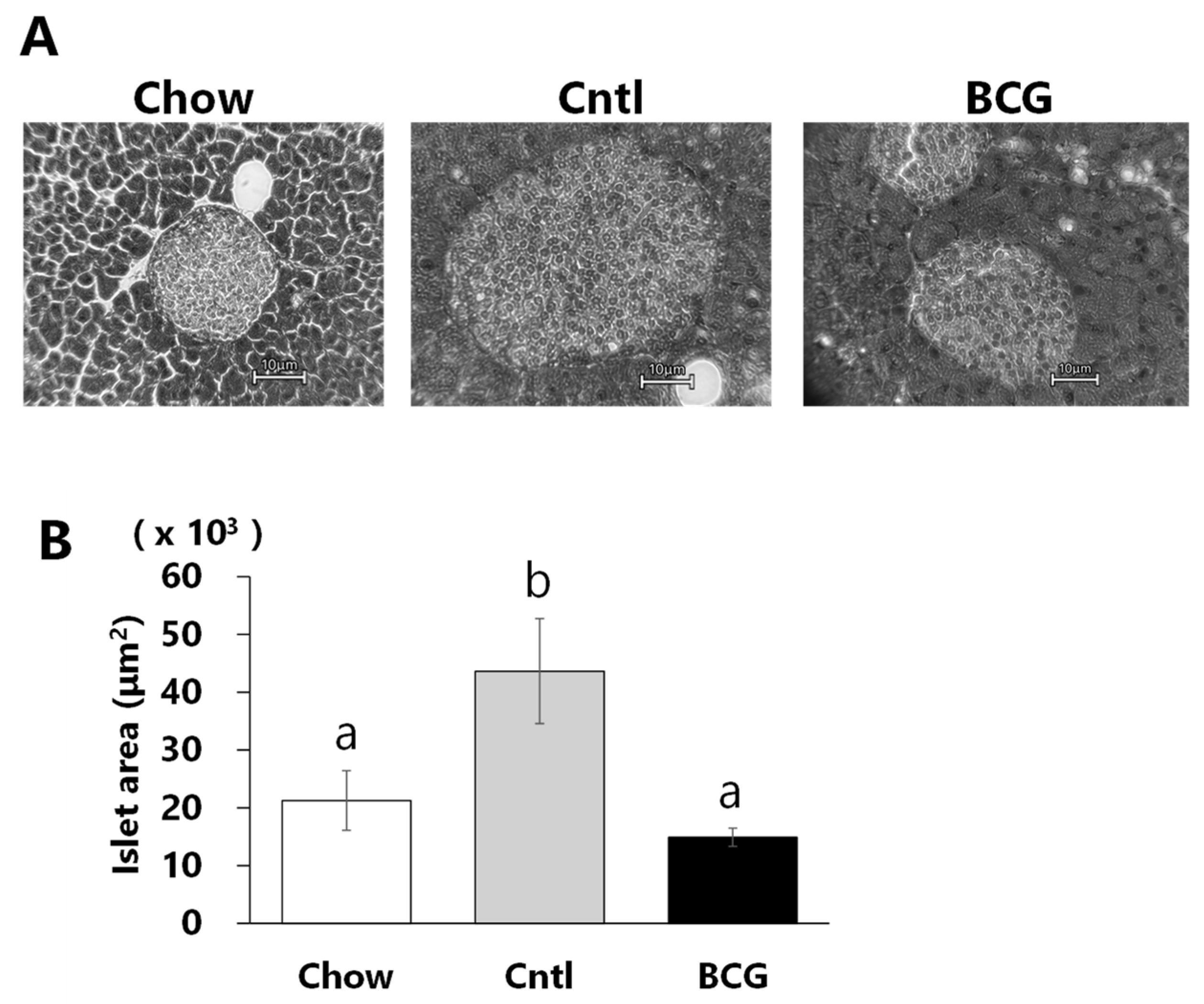
3.4. Effect of BCG Vaccination on Pancreatic Islet Size
3.5. Effect of Bone Marrow Transplantation from BCG-Vaccinated Mice on Glucose Intolerance Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Formiguera, X.; Canton, A. Obesity: Epidemiology and clinical aspects. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 1125–1146. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Buras, E.D.; Balasubramanyam, A. The Role of the Immune System in Obesity and Insulin Resistance. J. Obes. 2013, 616193. [Google Scholar] [CrossRef] [PubMed]
- Pedicino, D.; Francesca, A.; Alessandro, V.; Trotta, F.; Liuzzo, G. Type 2 Diabetes, Immunity and Cardiovascular Risk: A Complex Relationship; InTech: Houston, TX, USA, 2012. [Google Scholar]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 7457269. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R. Diabetes and global ageing among 65-99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2020, 162, 108078. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.W.; Cantó, C.; Houtkooper, R.H. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol. Med. 2014, 6, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Lachmandas, E.; Van Den Heuvel, C.N.A.M.; Damen, M.S.M.A.; Cleophas, M.C.P.; Netea, M.G.; Van Crevel, R. Diabetes Mellitus and Increased Tuberculosis Susceptibility: The Role of Short-Chain Fatty Acids. J. Diabetes Res. 2016, 6014631. [Google Scholar] [CrossRef]
- Radhakrishnan, R.K.; Thandi, R.S.; Tripathi, D.; Paidipally, P.; McAllister, M.K.; Mulik, S.; Samten, B.; Vankayalapati, R. BCG vaccination reduces the mortality of Mycobacterium tuberculosis–infected type 2 diabetes mellitus mice. JCI Insight 2020, 5, e133788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, W.; Wei, M.; Yin, D.; Tang, Y.; Jia, W.; Wang, C.; Guo, J.; Li, A.; Gong, Y. Associations between type 1 diabetes and pulmonary tuberculosis: A bidirectional mendelian randomization study. Diabetol. Amp; Metab. Syndr. 2024, 16, 60. [Google Scholar] [CrossRef]
- Sugawara, I.; Mizuno, S. Higher Susceptibility of Type 1 Diabetic Rats to Mycobacterium tuberculosis Infection. Tohoku J. Exp. Med. 2008, 216, 363–370. [Google Scholar] [CrossRef]
- Jamshidi, P.; Danaei, B.; Mohammadzadeh, B.; Arbabi, M.; Nayebzade, A.; Sechi, L.A.; Nasiri, M.J. BCG Vaccination and the Risk of Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Pathogens 2023, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100 e105. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.A.; Arts, R.J.W.; Van Crevel, R.; Benn, C.S.; Netea, M.G. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J. Leukoc. Biol. 2015, 98, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Vaziri, F.; Wan, Y.-J.Y. Effects of Bacillus Calmette-Gu erin on immunometabolism, microbiome and liver diseases. Liver Res. 2023, 7, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, A.; Pittet, L.F.; Faustman, D.; Curtis, N.; Levy, O. BCG vaccine’s off-target effects on allergic, inflammatory, and autoimmune diseases: Worth another shot? J. Allergy Clin. Immunol. 2022, 149, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Kühtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Kühtreiber, W.M.; Faustman, D.L. BCG Therapy for Type 1 Diabetes: Restoration of Balanced Immunity and Metabolism. Trends Endocrinol. Metab. 2019, 30, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Shpilsky, G.F.; Takahashi, H.; Aristarkhova, A.; Weil, M.; Ng, N.; Nelson, K.J.; Lee, A.; Zheng, H.; Kühtreiber, W.M.; Faustman, D.L. Bacillus Calmette-Guerin ‘s beneficial impact on glucose metabolism: Evidence for broad based applications. iScience 2021, 24, 103150. [Google Scholar] [CrossRef] [PubMed]
- Inafuku, M.; Matsuzaki, G.; Oku, H. Intravenous Mycobacterium Bovis Bacillus Calmette-Guérin Ameliorates Nonalcoholic Fatty Liver Disease in Obese, Diabetic ob/ob Mice. PLoS ONE 2015, 10, e0128676. [Google Scholar] [CrossRef]
- Akbarian, F.; Rahmani, M.; Tavalaee, M.; Abedpoor, N.; Taki, M.; Ghaedi, K.; Nasr-Esfahani, M.H. Effect of Different High-Fat and Advanced Glycation End-Products Diets in Obesity and Diabetes-Prone C57BL/6 Mice on Sperm Function. Int. J. Fertil. Steril. 2021, 15, 226–233. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Harada, M.; Kishimoto, Y.; Makino, S. Prevention of overt diabetes and insulitis in NOD mice by a single BCG vaccination. Diabetes Res. Clin. Pract. 1990, 8, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, N.; Etzioni, A.; Cahana, A.; Teninboum, G.; Gorodetsky, B.; Barzilai, D.; Karnieli, E. Repeated BCG vaccination is more effective than a single dose in preventing diabetes in non-obese diabetic (NOD) mice. Isr. J. Med. Sci. 1997, 33, 711–715. [Google Scholar]
- Doupis, J.; Kolokathis, K.; Markopoulou, E.; Efthymiou, V.; Festas, G.; Papandreopoulou, V.; Kallinikou, C.; Antikidou, D.; Gemistou, G.; Angelopoulos, T. The Role of Pediatric BCG Vaccine in Type 1 Diabetes Onset. Diabetes Ther. 2021, 12, 2971–2976. [Google Scholar] [CrossRef] [PubMed]
- Faustman, D.; Faustman, D. ScienceDirect. In The Value of BCG and TNF in Autoimmunity, 2nd ed.; Academic Press: London, UK; San Diego, CA, USA,, 2018. [Google Scholar]
- Dias, H.F.; Mochizuki, Y.; Kühtreiber, W.M.; Takahashi, H.; Zheng, H.; Faustman, D.L. Bacille Calmette Guerin (BCG) and prevention of types 1 and 2 diabetes: Results of two observational studies. PLoS ONE 2023, 18, e0276423. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Lin, C.-J.; Hsiao, Y.-H.; Chang, Y.-H.; Liu, S.-J.; Hsu, H.-Y. Therapeutic Effects of BCG Vaccination on Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2020, 8954125. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Zarei, P.; Shakerian, B.; Babaei, M.; Mostafavi, A.; Modaressi, M. The Non-Significant Benefit of BCG Vaccination for the Treatment of Iranian Patients with Type 1 Diabetes up to 48 Weeks: A Controversial Result. Med. J. Islam. Repub. Iran 2021, 35, 161. [Google Scholar] [CrossRef]
- Allen, H.F.; Klingensmith, G.J.; Jensen, P.; Simoes, E.; Hayward, A.; Chase, H.P. Effect of Bacillus Calmette-Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care 1999, 22, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Faustman, D.L. Benefits of BCG-induced metabolic switch from oxidative phosphorylation to aerobic glycolysis in autoimmune and nervous system diseases. J. Intern. Med. 2020, 288, 641–650. [Google Scholar] [CrossRef]
- Faustman, D.L.; Wang, L.; Okubo, Y.; Burger, D.; Ban, L.; Man, G.; Zheng, H.; Schoenfeld, D.; Pompei, R.; Avruch, J.; et al. Proof-of-Concept, Randomized, Controlled Clinical Trial of Bacillus-Calmette-Guerin for Treatment of Long-Term Type 1 Diabetes. PLoS ONE 2012, 7, e41756. [Google Scholar] [CrossRef]
- Keefe, R.C.; Takahashi, H.; Tran, L.; Nelson, K.; Ng, N.; Kühtreiber, W.M.; Faustman, D.L. BCG therapy is associated with long-term, durable induction of Treg signature genes by epigenetic modulation. Sci. Rep. 2021, 11, 14933. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Utzschneider, K.M.; Kahn, S.E. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia 2020, 63, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Tabak, A.G.; Jokela, M.; Akbaraly, T.N.; Brunner, E.J.; Kivimaki, M.; Witte, D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: An analysis from the Whitehall II study. Lancet 2009, 373, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Matveyenko, A.V.; Gurlo, T.; Daval, M.; Butler, A.E.; Butler, P.C. Successful Versus Failed Adaptation to High-Fat Diet–Induced Insulin Resistance. Diabetes 2009, 58, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-W.; Son, M.; Choi, J.; Oh, S.; Jeon, Y.-J.; Byun, K.; Ryu, B.M. Ishige okamurae reduces blood glucose levels in high-fat diet mice and improves glucose metabolism in the skeletal muscle and pancreas. Fish. Aquat. Sci. 2020, 23, 24. [Google Scholar] [CrossRef]
- Hull, R.L.; Kodama, K.; Utzschneider, K.M.; Carr, D.B.; Prigeon, R.L.; Kahn, S.E. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: Evidence for specificity of impaired beta cell adaptation. Diabetologia 2005, 48, 1350–1358. [Google Scholar] [CrossRef]
- Moh Moh, M.A.; Jung, C.H.; Lee, B.; Choi, D.; Kim, B.Y.; Kim, C.H.; Kang, S.K.; Mok, J.O. Association of glucagon-to-insulin ratio and nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2019, 16, 186–195. [Google Scholar] [CrossRef]
- Bang, J.; Lee, S.A.; Koh, G.; Yoo, S. Association of Glucagon to Insulin Ratio and Metabolic Syndrome in Patients with Type 2 Diabetes. J. Clin. Med. 2023, 12, 5806. [Google Scholar] [CrossRef]
- Lee, E.E.; Sears, D.D.; Liu, J.; Jin, H.; Tu, X.M.; Eyler, L.T.; Jeste, D.V. A novel biomarker of cardiometabolic pathology in schizophrenia? J. Psychiatr. Res. 2019, 117, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.; Franz, K. Innate defence: Evidence for memory in invertebrate immunity. Nature 2003, 425, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; Van Loenhout, J.; De Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322–334 e325. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Zhou, L.; Lu, J.; Zhu, F.; Li, J. Research progress in the off-target effects of Bacille Calmette-Guerin vaccine. Chin. Med. J. (Engl.) 2023. [Google Scholar] [CrossRef] [PubMed]
- van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.B.; Vermeulen, S.H. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 2020, 17, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Atallah, A.; Grossman, A.; Nauman, R.W.; Paré, J.F.; Khan, A.; Siemens, D.R.; Cotechini, T.; Graham, C.H. Systemic versus localized Bacillus Calmette Guérin immunotherapy of bladder cancer promotes an anti-tumoral microenvironment: Novel role of trained immunity. Int. J. Cancer 2024, 155, 352–364. [Google Scholar] [CrossRef]
- Kühtreiber, W.M.; Takahashi, H.; Keefe, R.C.; Song, Y.; Tran, L.; Luck, T.G.; Shpilsky, G.; Moore, L.; Sinton, S.M.; Graham, J.C.; et al. BCG Vaccinations Upregulate Myc, a Central Switch for Improved Glucose Metabolism in Diabetes. iScience 2020, 23, 101085. [Google Scholar] [CrossRef]


| Parameters | Chow | Cntl | BCG |
|---|---|---|---|
| Growth | |||
| Final body weight (g) | 27.5 ± 0.6 a | 39.7 ± 1.8 b | 36.6 ± 1.5 b |
| Liver weight (g) | 0.95 ± 0.03 a | 1.70 ± 0.19 b | 1.30 ± 0.08 ab |
| Serum | |||
| Triglyceride (mg/dL) | 54.4 ± 4.9 a | 69.7 ± 3.3 b | 60.3 ± 6.1 b |
| Total cholesterol (mg/dL) | 160 ± 12 | 188 ± 8.6 | 161 ± 9.5 |
| AST (IU/L) | 29.3 ± 5.2 | 26.9 ± 5.6 | 21.8 ± 5.9 |
| ALT (IU/L) | 4.41 ± 1.24 | 4.02 ± 0.50 | 4.47 ± 0.44 |
| Hepatic | |||
| Triglyceride (mg/g liver) | 31.3 ± 0.6 a | 57.4 ± 6.6 b | 40.6 ± 5.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakawa, H.; Inafuku, M. BCG Vaccination Suppresses Glucose Intolerance Progression in High-Fat-Diet-Fed C57BL/6 Mice. Medicina 2024, 60, 866. https://doi.org/10.3390/medicina60060866
Arakawa H, Inafuku M. BCG Vaccination Suppresses Glucose Intolerance Progression in High-Fat-Diet-Fed C57BL/6 Mice. Medicina. 2024; 60(6):866. https://doi.org/10.3390/medicina60060866
Chicago/Turabian StyleArakawa, Haruna, and Masashi Inafuku. 2024. "BCG Vaccination Suppresses Glucose Intolerance Progression in High-Fat-Diet-Fed C57BL/6 Mice" Medicina 60, no. 6: 866. https://doi.org/10.3390/medicina60060866
APA StyleArakawa, H., & Inafuku, M. (2024). BCG Vaccination Suppresses Glucose Intolerance Progression in High-Fat-Diet-Fed C57BL/6 Mice. Medicina, 60(6), 866. https://doi.org/10.3390/medicina60060866





