The Role of Epithelial Cell Adhesion Molecule Cancer Stem Cell Marker in Evaluation of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Immunohistochemistry
2.3. Interpretation of the Staining
2.4. Positive and Negative Controls
2.5. Statistical Analysis
3. Results
3.1. EpCAM Expression in Different Studied Cases
3.2. Correlations between Survival and Different Clinicopathological Parameters
3.3. Correlations between Recurrence and Different Clinicopathological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Asafo-Agyei, K.O.; Samant, H. Hepatocellular Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK559177/ (accessed on 1 March 2024).
- Vasanthakumar, S.; Sasikala, P.; Padma, M.; Balachandar, V.; Venkatesh, B.; Ganesan, S. EpCAM as a novel therapeutic target for hepatocellular carcinoma. J. Oncol. Sci. 2017, 3, 71–76. [Google Scholar]
- Chen, Y.; Yu, D.; Zhang, H.; He, H.; Zhang, C.; Zhao, W.; Shao, R.G. CD133+EpCAM+ phenotype possesses more characteristics of tumor initiating cells in hepatocellular carcinoma Huh7 cells. Int. J. Biol. Sci. 2012, 8, 992–1004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nurrul, A.L.; Chow, E.K.-H. Mechanism of chemoresistance in cancer stem cell. Clin. Transl. Med. 2013, 2, 3. [Google Scholar]
- Lima, L.d.P.; Machado, C.J.; Rodrigues, J.B.S.R.; Vasconcellos, L.d.S.; Junior, E.P.; Vidigal, P.V.T.; Resende, V. Immunohistochemical Coexpression of Epithelial Cell Adhesion Molecule and Alpha-Fetoprotein in Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 5970852. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Q.; Zhao, Q.; Yang, F.; Liu, T.; Huang, X.; Yan, Q.; Yang, X. Deglycosylated EpCAM regulates proliferation by enhancing autophagy of breast cancer cells via PI3K/Akt/mTOR pathway. Aging 2022, 14, 316–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Yang, L.; Liu, X.; Gao, J.; Liu, T.; Yan, Q.; Yang, X. Hypoxia modulates stem cell properties and induces EMT through N-glycosylation of EpCAM in breast cancer cells. J. Cell. Physiol. 2020, 235, 3626–3633. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef]
- Dong, K.; Chen, Y.; Yang, G.; Liao, Z.; Zhang, H.; Liang, H.; Chen, X.; Dong, H. TGF-β1 accelerates the hepatitis B virus X-induced malignant transformation of hepatic progenitor cells by upregulating miR-199a-3p. Oncogene 2020, 39, 1807–1820. [Google Scholar] [CrossRef]
- Gramantieri, L.; Pollutri, D.; Gagliardi, M.; Giovannini, C.; Quarta, S.; Ferracin, M.; Casadei-Gardini, A.; Callegari, E.; De Carolis, S.; Marinelli, S.; et al. MiR-30e-3p influence tumor phenotype through MDM2/TP53 axis and predicts sorafenib resistance in hepatocellular carcinoma. Cancer Res. 2020, 80, 1720–1734. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Jia, H.; Ye, Q.; Qin, L.X.; Wauthier, E.; et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Felden, J.; Schulze, K.; Krech, T.; Ewald, F.; Nashan, B.; Pantel, K.; Lohse, A.W.; Riethdorf, S.; Wege, H. Circulating tumor cells as liquid biomarker for high HCC recurrence risk after curative liver resection. Oncotarget 2017, 8, 89978–89987. [Google Scholar] [CrossRef] [PubMed]
- Martins-Filho, S.N.; Paiva, C.; Azevedo, R.S.; Alves, V.A.F. Histological Grading of Hepatocellular Carcinoma-A Systematic Review of Literature. Front. Med. 2017, 4, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noh, C.-K.; Wang, H.J.; Kim, C.M.; Kim, J.; Yoon, S.Y.; Lee, G.H.; Cho, H.J.; Yang, M.J.; Kim, S.S.; Hwang, J.C.; et al. EpCAM as a Predictive Marker of Tumor Recurrence and Survival in Patients Who Underwent Surgical Resection for Hepatocellular Carcinoma. Anticancer. Res. 2018, 38, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhu, Y. The EpCAM overexpression is associated with clinicopathological significance and prognosis in hepatocellular carcinoma patients: A systematic review and meta-analysis. Int. J. Surg. 2018, 56, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Farmer, R.W.; Yang, Y.; Martin, R.C.G. Epithelial cell adhesion molecule in human hepatocellular carcinoma cell lines: A target of chemoresistance. BMC Cancer 2016, 16, 228. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.M. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef]
- Chan, A.W.H.; Tong, J.H.M.; Chan, S.L.; Lai, P.B.S.; To, K.F. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology 2014, 64, 935–950. [Google Scholar] [CrossRef]
- Fouse, S.D.; Costello, J.F. Cancer stem cells activate STAT3 the EZ way. Cancer Cell 2013, 23, 711–713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, M.; Qian, G.; Xie, F.; Shi, C.; Yan, L.; Yu, L.; Zheng, T.; Wei, L.; Yang, J. Expression of epithelial cell adhesion molecule associated with elevated ductular reactions in hepatocellar carcinoma. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, I.A. Epithelial Cell Adhesion Molecule mRNA Can be a Potential Marker to Predict Metastasis in Hepatocellular Carcinoma Patients. Asian Pac. J. Cancer Prev. 2020, 21, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Gerasimidou, D.; Kuwahara, R.; Hytiroglou, P.; Yoo, J.E.; Park, Y.N.; Theise, N.D. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011, 53, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; von Felden, J.; Casar, C.; Fründt, T.W.; Galaski, J.; Schmidt, C.; Jung, C.; Ittrich, H.; Weidemann, S.A.; Krech, T.; et al. Hepatocellular carcinoma: Intratumoral EpCAM-positive cancer stem cell heterogeneity identifies high-risk tumor subtype. BMC Cancer 2020, 20, 1130. [Google Scholar] [CrossRef] [PubMed]
- Gires, O.; Pan, M.; Schinke, H.; Canis, M.; Baeuerle, P.A. Expression and function of epithelial cell adhesion molecule EpCAM: Where are we after 40 years? Cancer Metastasis Rev. 2020, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, R.; Negm, M.S.; Amer, I.S. EpCAM Expression in Epithelial Ovarian Cancer: Immunohistochemical and Histopathological Study. Med. J. Cairo Univ. 2022, 90, 113–119. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Chen, T.; Shen, T.; Zhang, X.; Song, P.; Liu, L.; Liu, J.; Jiang, T.; Liang, X. Clinical verification of vimentin/EpCAM immunolipid magnetic sorting system in monitoring CTCs in arterial and venous blood of advanced tumor. J. Nanobiotechnol. 2021, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, G.H.; Na DC Ahn, E.Y.; Kim, G.I.; Lee, J.E.; Cho, J.Y.; Yoo, J.E.; Choi, J.S.; Park, Y.N. Human hepatocellular carcinomas with ‘stemness’-related marker expression: Keratin 19 expression and a poor prognosis. Hepatology 2011, 54, 1707–1717. [Google Scholar] [CrossRef]
- Shan, Y.; Huang, Y.; Xie, Y.; Tan, Y.; Chen, B.; Zhou, M.; Shi, H.; Yu, Z.; Song, Q.; Zhang, Q. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and α-fetoprotein expression status. Med. Oncol. 2010, 28, 1012–1016. [Google Scholar] [CrossRef]
- Bae, J.S.; Noh, S.J.; Jang, K.Y.; Park, H.S.; Chung, M.J.; Park, C.K.; Moon, W.S. Expression and role of epithelial cell adhesion molecule in dysplastic nodule and hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 2150–2158. [Google Scholar] [CrossRef]
- Liu, R.; Shen, Y.; Nan, K.; Mi, B.; Wu, T.; Guo, J.; Li, M.; Lv, Y.; Guo, H. Association between expression of cancer stem cell markers and poor differentiation of hepatocellular carcinoma: A meta-analysis (PRISMA). Medicine 2015, 94, e1306. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Honda, M.; Nakamoto, Y.; Baba, M.; Nio, K.; Hara, Y.; Zeng, S.S.; Hayashi, T.; Kondo, M.; Takatori, H.; et al. Discrete nature of EpCAM+ and CD90+ cancer stem cells in human hepatocellular carcinoma. Hepatology 2013, 57, 1484–1497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelley, R.K.; Venook, A.P. Novel therapeutics in hepatocellular carcinoma: How can we make progress? Am. Soc. Clin. Oncol. Educ. Book 2013, 33, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Suda, T.; Oda, C.; Kimura, A.; Hosaka, K.; Kimura, N.; Tominaga, K.; Hayashi, K.; Takamura, M.; Terai, S. EpCAM- and/or NCAM-Expressing Hepatocellular Carcinoma in Which Behaviour of Hepatic Progenitor Cell Marker-Positive Cells Are Followed. Case Rep. Gastroenterol. 2019, 13, 118–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Wang, B.; Wu, J.; Zhang, C.; Zhou, Y.; Yang, X.; Zhou, J.; Guo, W.; Fan, J. Association of preoperative EpCAM Circulating Tumor Cells and peripheral Treg cell levels with early recurrence of hepatocellular carcinoma following radical hepatic resection. BMC Cancer 2016, 16, 506. [Google Scholar] [CrossRef]
- Schulze, K.; Gasch, C.; Staufer, K.; Nashan, B.; Lohse, A.W.; Pantel, K.; Riethdorf, S.; Wege, H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int. J. Cancer 2013, 133, 2165–2171. [Google Scholar] [CrossRef]
- Salehi, M.; Lavasani, Z.M.; Keshavarz Alikhani, H.; Shokouhian, B.; Hassan, M.; Najimi, M.; Vosough, M. Circulating Tumor Cells as a Promising Tool for Early Detection of Hepatocellular Carcinoma. Cells 2023, 12, 2260. [Google Scholar] [CrossRef]
- Kim, M.; Bakyt, L.; Akhmetkaliyev, A.; Toktarkhanova, D.; Bulanin, D. Re-Sensitizing Cancer Stem Cells to Conventional Chemotherapy Agents. Int. J. Mol. Sci. 2023, 24, 2122. [Google Scholar] [CrossRef]
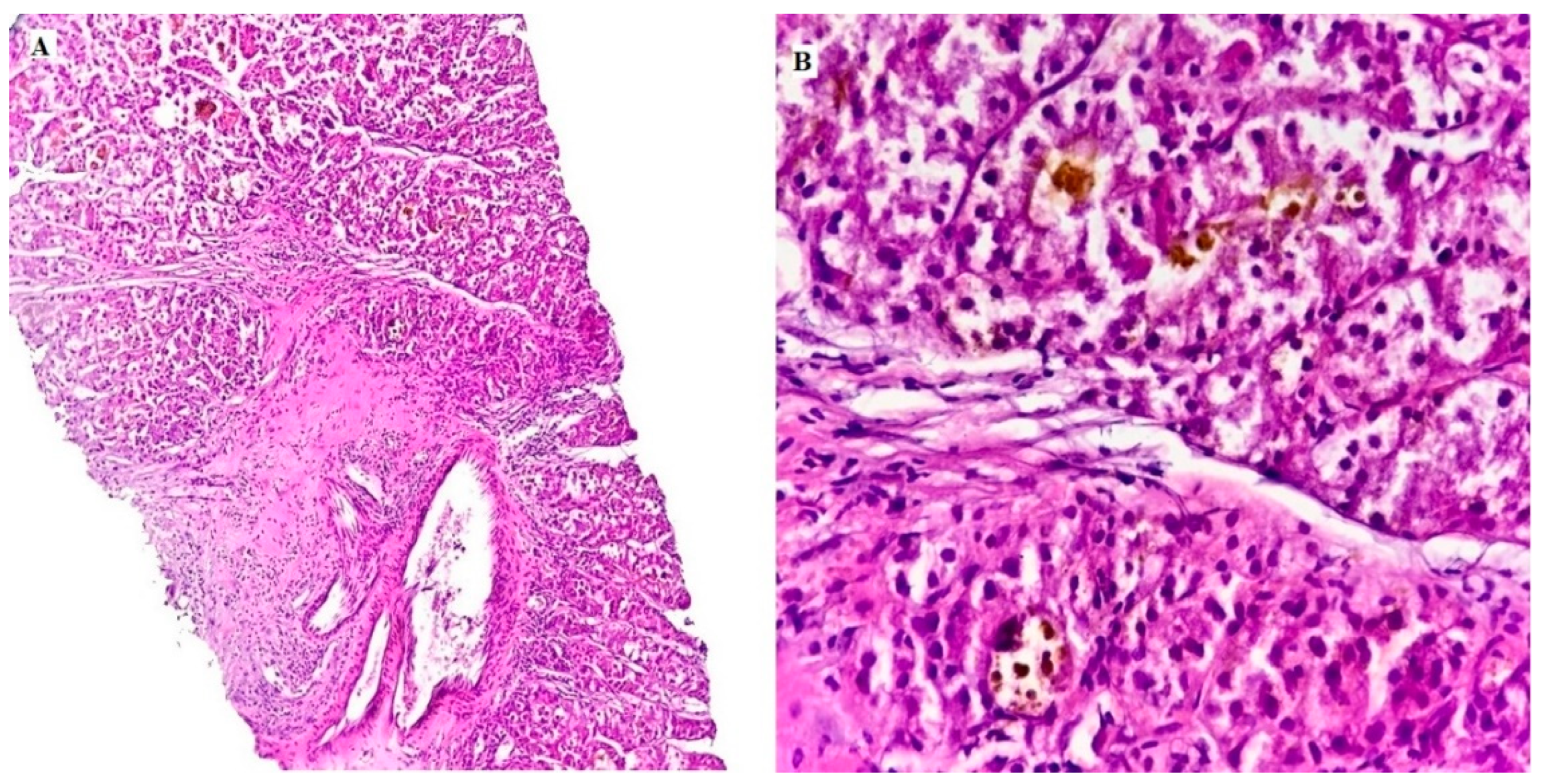
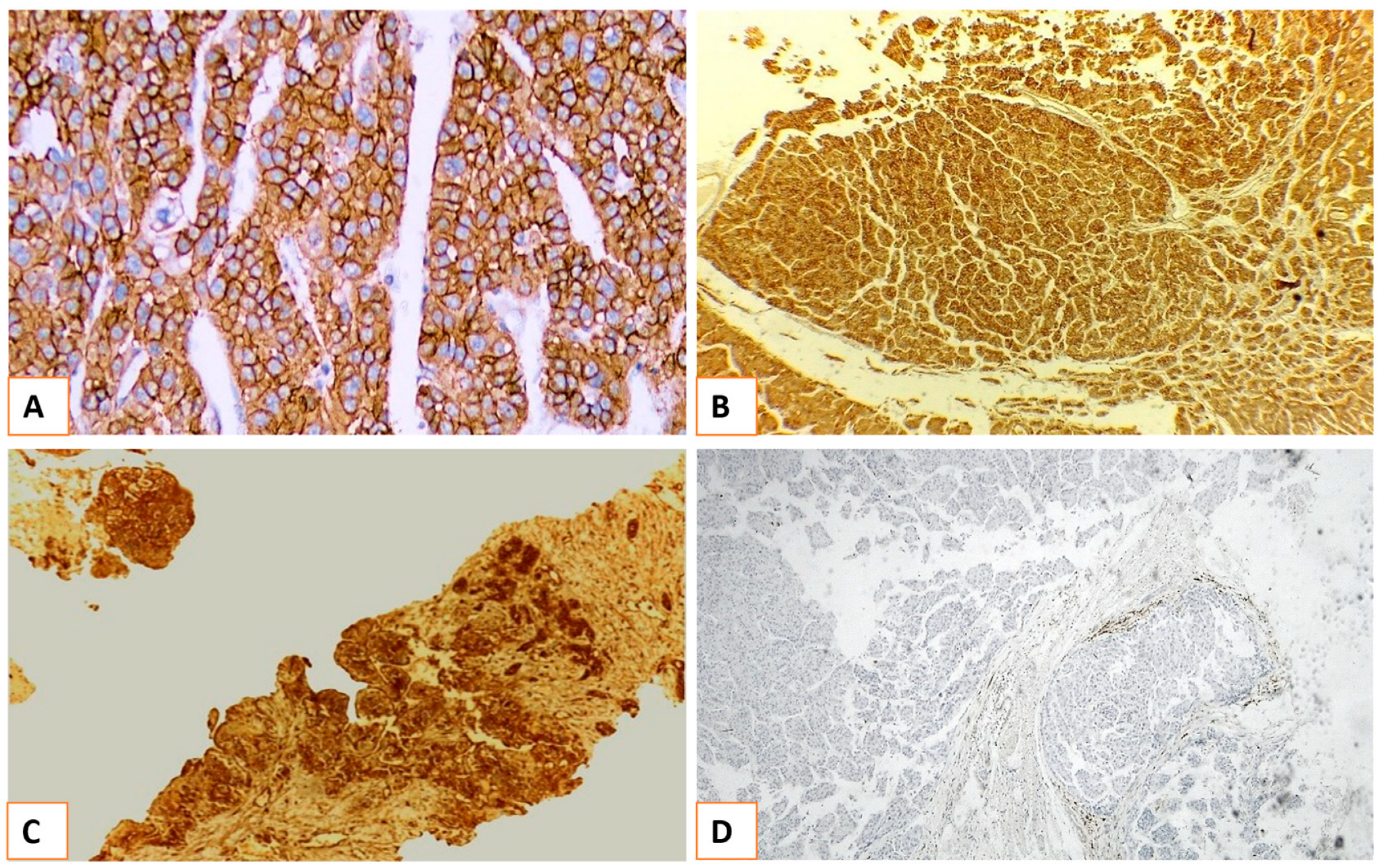
| Characteristics | N (%) |
| N = 42 | |
| Age, mean (SD) | 50.1 (13) |
| Tumor Size, median (IQR) | 5.75 (3.38) |
| Size | |
| >5 | 24 (57%) |
| ≤5 | 18 (43%) |
| Sex | |
| Male | 16 (38%) |
| Female | 26 (62%) |
| Tumor Multiplicity | |
| Yes | 8 (19%) |
| No | 34 (81%) |
| Grade | |
| Low | 17 (40.5%) |
| High | 25 (59.5%) |
| Stage | |
| I | 19 (45.2%) |
| II | 9 (21.4%) |
| III | 14 (33.3%) |
| Vascular Invasion | |
| Present | 11 (26%) |
| Absent | 31 (74%) |
| Associated Cirrhosis | |
| Yes | 31 (74%) |
| No | 11 (26%) |
| AFP (ng/mL) | |
| >100 | 27 (64%) |
| ≤100 | 15 (36%) |
| EpCAM expression | |
| Positive | 22 (52.5%) |
| Negative | 20 (47.5%) |
| Characteristics | EpCAM Positive | EpCAM Negative | Total | p-Value |
| N | 22 | 20 | 42 (100%) | |
| Age, mean (SD) | 52 (12.7) | 47.4 (13.2) | 0.202 | |
| Tumor size, median (IQR) | 6 (1.88) | 5 (3.25) | 0.061 | |
| Size | 0.006 | |||
| >5 | 17 (71%) | 7 (29%) | 24 (100%) | |
| ≤5 | 5 (28%) | 13 (72%) | 18 (100%) | |
| Sex | 0.694 | |||
| Male | 9 (56%) | 7 (44%) | 16 (100%) | |
| Female | 13 (50%) | 13 (50%) | 26 (100%) | |
| Tumor Multiplicity | 0.004 | |||
| Yes | 8 (100%) | 0 (0%) | 8 (100%) | |
| No | 14 (41%) | 20 (59%) | 34 (100%) | |
| Grade | 0.002 | |||
| Low | 4 (23.5%) | 13 (76.5%) | 17 (100%) | |
| High | 18 (72%) | 7 (28%) | 25 (100%) | |
| Stage | 0.003 | |||
| I | 5 (26%) | 14 (74%) | 19 (100%) | |
| II | 5 (55.5%) | 4 (44.5%) | 9 (100%) | |
| III | 12 (86%) | 2 (14%) | 14 (100%) | |
| Vascular Invasion | 0.023 | |||
| Present | 9 (82%) | 2 (18%) | 11 (100%) | |
| Absent | 13 (42%) | 18 (58%) | 31 (100%) | |
| Associated Cirrhosis | 0.052 | |||
| Yes | 19 (61%) | 12 (39%) | 31 (100%) | |
| No | 3 (27%) | 8 (73%) | 11 (100%) | |
| AFP (ng/mL) | 0.013 | |||
| >100 | 18 (67%) | 9 (33%) | 27 (100%) | |
| ≤100 | 4 (27%) | 11 (73%) | 15 (100%) |
| Characteristic | Survivors | Non-Survivors | Total | p-Value |
| N | 33 | 5 | 38 (100%) | |
| Age, mean (SD) | 50.8 (14.1) | 53.4 (6.6) | 0.685 | |
| Tumor size, median (IQR) | 5.5 (4) | 6 (1.5) | 0.557 | |
| Size | 0.061 | |||
| >5 | 17 (77%) | 5 (23%) | 22 (100%) | |
| ≤5 | 16 (100%) | 0 (0%) | 16 (100%) | |
| Sex | 0.337 | |||
| Male | 11 (78.5%) | 3 (21.5%) | 14 (100%) | |
| Female | 22 (92%) | 2 (8%) | 24 (100%) | |
| Tumor Multiplicity | 0.279 | |||
| Yes | 6 (75%) | 2 (25%) | 8 (100%) | |
| No | 27 (90%) | 3 (10%) | 30 (100%) | |
| Grade | 0.136 | |||
| Low | 15 (100%) | 0 (0%) | 15 (100%) | |
| High | 18 (78%) | 5 (22%) | 23 (100%) | |
| Stage | 0.006 | |||
| I | 17 (100%) | 0 (0%) | 17 (100%) | |
| II | 7 (100%) | 0 (0%) | 7 (100%) | |
| III | 9 (64%) | 5 (36%) | 14 (100%) | |
| Vascular Invasion | 0.134 | |||
| Present | 8 (73%) | 3 (27%) | 11 (100%) | |
| Absent | 25 (92.5%) | 2 (7.5%) | 27 (100%) | |
| Associated Cirrhosis | 0.298 | |||
| Yes | 23 (82%) | 5 (18%) | 28 (100%) | |
| No | 10 (100%) | 0 (0%) | 10 (100%) | |
| AFP (ng/mL) | 0.144 | |||
| >100 | 20 (80%) | 5 (20%) | 25 (100%) | |
| ≤100 | 13 (100%) | 0 (0%) | 13 (100%) | |
| EpCAM expression | 0.355 | |||
| Positive | 17 (81%) | 4 (19%) | 21 (100%) | |
| Negative | 16 (94%) | 1 (6%) | 17 (100%) |
| Characteristic | Recurrent | Non-Recurrent | Total | p-Value |
| N | 9 | 29 | 38 (100%) | |
| Age, mean (SD) | 62.2 (8.4) | 40.7 (12.7) | 0.003 | |
| Tumor size, median (IQR) | 6 (1.25) | 5.25 (4.88) | 0.40 | |
| Size | 0.005 | |||
| >5 | 9 (41%) | 13 (59%) | 22 (100%) | |
| ≤5 | 0 (0%) | 16 (100%) | 16 (100%) | |
| Sex | 1.00 | |||
| Male | 3 (21.5%) | 11 (78.5%) | 14 (100%) | |
| Female | 6 (25%) | 18 (75%) | 24 (100%) | |
| Tumor Multiplicity | <0.001 | |||
| Yes | 8 (100%) | 0 (0%) | 8 (100%) | |
| No | 1 (3%) | 29 (97%) | 30 (100%) | |
| Grade | 0.006 | |||
| Low | 0 (0%) | 15 (100%) | 15 (100%) | |
| High | 9 (39%) | 14 (61%) | 23 (100%) | |
| Stage | <0.001 | |||
| I | 0 (0%) | 17 (100%) | 17 (100%) | |
| II | 0 (0%) | 7 (100%) | 7 (100%) | |
| III | 9 (64%) | 5 (36%) | 14 (100%) | |
| Vascular Invasion | <0.001 | |||
| Present | 9 (82%) | 2 (18%) | 11 (100%) | |
| Absent | 0 (0%) | 27 (100%) | 27 (100%) | |
| Associated Cirrhosis | 0.079 | |||
| Yes | 9 (32%) | 19 (68%) | 28 (100%) | |
| No | 0 (0%) | 10 (100%) | 10 (100%) | |
| AFP (ng/mL) | 0.016 | |||
| >100 | 9 (36%) | 16 (64%) | 25 (100%) | |
| ≤100 | 0 (0%) | 13 (100%) | 13 (100%) | |
| EpCAM expression | 0.002 | |||
| Positive | 9 (43%) | 12 (57%) | 21 (100%) | |
| Negative | 0 (0%) | 17 (100%) | 17 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kholy, M.A.; Abu-Seadah, S.S.; Hasan, A.; Elhussiny, M.E.A.; Abdelwahed, M.S.; Hanbazazh, M.; Samman, A.; Alrashdi, S.A.; Rashed, Z.F.; Ashmawy, D.; et al. The Role of Epithelial Cell Adhesion Molecule Cancer Stem Cell Marker in Evaluation of Hepatocellular Carcinoma. Medicina 2024, 60, 915. https://doi.org/10.3390/medicina60060915
El-Kholy MA, Abu-Seadah SS, Hasan A, Elhussiny MEA, Abdelwahed MS, Hanbazazh M, Samman A, Alrashdi SA, Rashed ZF, Ashmawy D, et al. The Role of Epithelial Cell Adhesion Molecule Cancer Stem Cell Marker in Evaluation of Hepatocellular Carcinoma. Medicina. 2024; 60(6):915. https://doi.org/10.3390/medicina60060915
Chicago/Turabian StyleEl-Kholy, Marwa A., Shimaa S. Abu-Seadah, Abdulkarim Hasan, Mohammed E. A. Elhussiny, Mohammed S. Abdelwahed, Mehenaz Hanbazazh, Abdulhadi Samman, Saeed A. Alrashdi, Zaky F. Rashed, Diaa Ashmawy, and et al. 2024. "The Role of Epithelial Cell Adhesion Molecule Cancer Stem Cell Marker in Evaluation of Hepatocellular Carcinoma" Medicina 60, no. 6: 915. https://doi.org/10.3390/medicina60060915
APA StyleEl-Kholy, M. A., Abu-Seadah, S. S., Hasan, A., Elhussiny, M. E. A., Abdelwahed, M. S., Hanbazazh, M., Samman, A., Alrashdi, S. A., Rashed, Z. F., Ashmawy, D., Othman, A. E., Abdelaleem, M. F., Abo-Saif, A. I. A., Abdel-Maqsoud, R. R., Attiah, S. M., Assiri, E. S., Nasr, M., Ismail, K. A., Saad, D. Z., & El-Mosely, M. M. (2024). The Role of Epithelial Cell Adhesion Molecule Cancer Stem Cell Marker in Evaluation of Hepatocellular Carcinoma. Medicina, 60(6), 915. https://doi.org/10.3390/medicina60060915







