The Dysregulation of Essential Fatty Acid (EFA) Metabolism May Be a Factor in the Pathogenesis of Sepsis
Abstract
1. Introduction
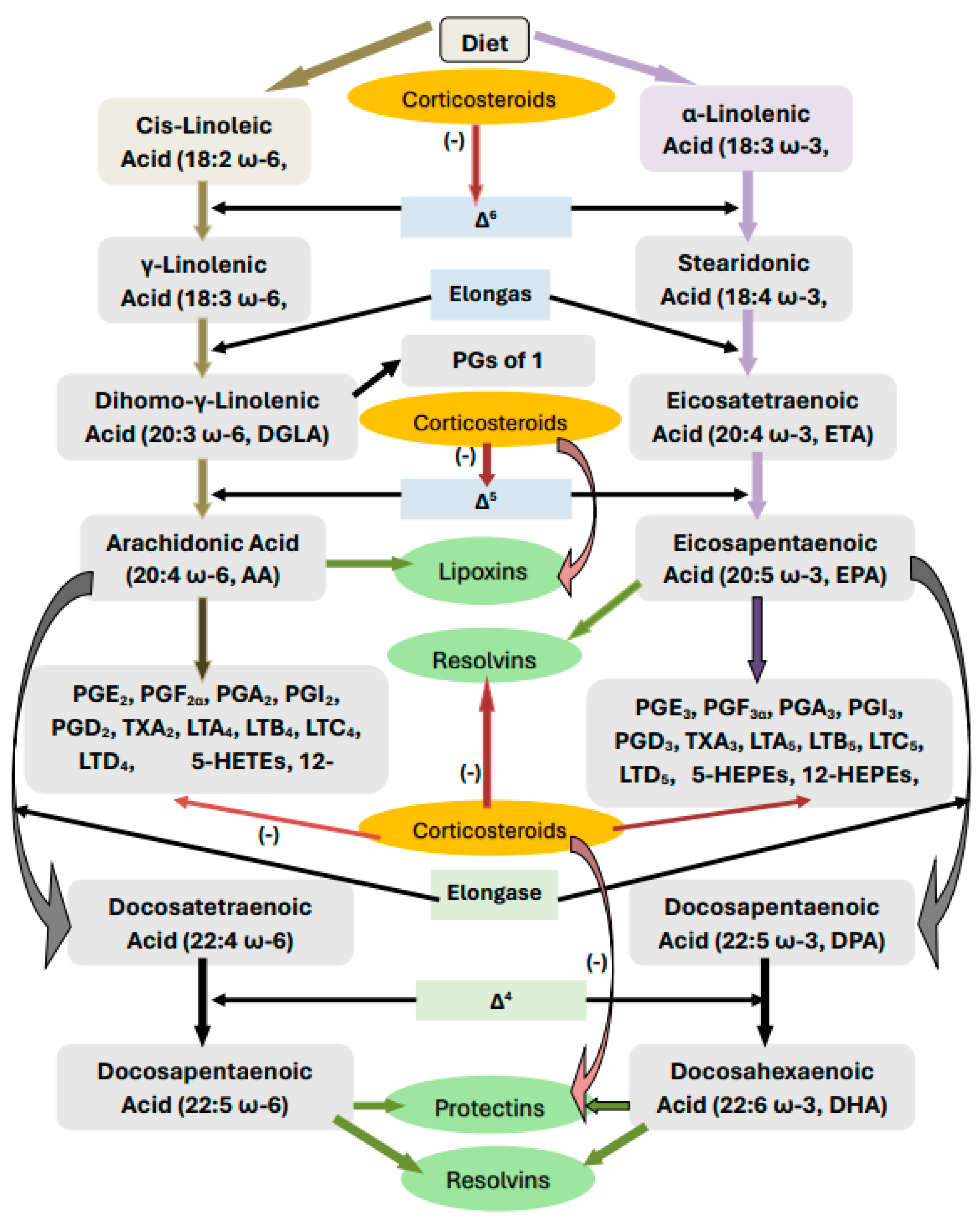
2. Metabolism of Essential Fatty Acids
3. EFAs and Cytokines
4. Corticosteroids and EFAs
5. Antimicrobial Action of EFAs
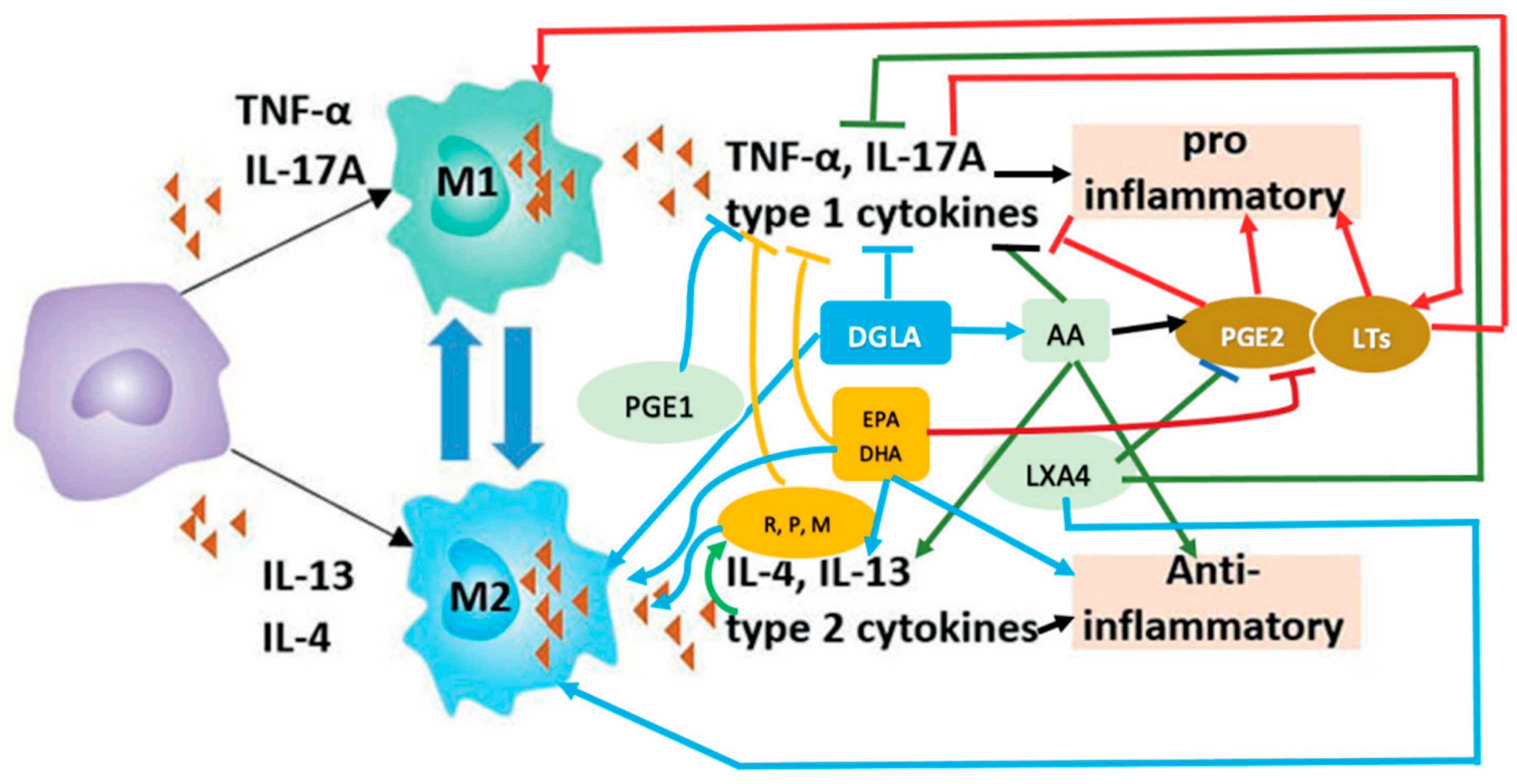
6. M1 and M2 Macrophages and EFAs
7. PGE2 and LXA4 Interact to Initiate Resolution of Inflammation
8. Brain–Body Immune Regulation—A Role for Bioactive Lipids
9. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Rhee, C.; Klompas, M. Sepsis trends: Increasing incidence and decreasing mortality, or changing denominator? J. Thorac. Dis. 2020, 12 (Suppl. S1), S89. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Anti-cytokine therapies in response to systemic infection. J. Investig. Dermatol. Symp. Proc. 2001, 6, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.S.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Infection, Inflammation, and Immunity in Sepsis. Biomolecules 2023, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, F.; Liu, S.; Jin, Y.; Zhang, X.; Yang, T.; Dai, Y.; Li, X.; Zhao, A.Z. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J. Clin. Investig. 2017, 127, 1757–1771. [Google Scholar] [CrossRef]
- Tai, C.C.; Ding, S.T. N-3 polyunsaturated fatty acids regulate lipid metabolism through several inflammation mediators: Mechanisms and implications for obesity prevention. J. Nutr. Biochem. 2010, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Sugimoto, Y.; Tanaka, S.; Ichikawa, A. Prostaglandin E(2) receptors, EP2 and EP4, differentially modulate TNF-alpha and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem. Biophys. Res. Commun. 2000, 278, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot Essent Fat. Acids 2013, 88, 201–210. [Google Scholar] [CrossRef]
- Yu, S.; Xie, J.; Xiang, Y.; Dai, S.; Yu, D.; Sun, H.; Chen, B.; Zhou, M. Downregulation of TNF-α/TNF-R1 Signals by AT-Lipoxin A4 May Be a Significant Mechanism of Attenuation in SAP-Associated Lung Injury. Mediat. Inflamm. 2019, 2019, 9019404. [Google Scholar] [CrossRef]
- Mayer, K.; Schmidt, R.; Muhly-Reinholz, M.; Bögeholz, T.; Gokorsch, S.; Grimminger, F.; Seeger, W. In vitro mimicry of essential fatty acid deficiency in human endothelial cells by TNFalpha impact of omega-3 versus omega-6 fatty acids. J. Lipid Res. 2002, 43, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Manjari, V.; Das, U.N. Effect of polyunsaturated fatty acids on dexamethasone-induced gastric mucosal damage. Prostaglandins Leukot Essent Fat. Acids 2000, 62, 85–96. [Google Scholar] [CrossRef]
- Huang, Y.S.; Das UNHorrobin, D.F. Effect of dexamethasone on the distribution of essential fatty acids in plasma and liver phospholipids. IRCS Med. Sci. 1986, 14, 180. [Google Scholar]
- Gundala, N.K.V.; Naidu, V.G.M.; Das, U.N. Arachidonic acid and lipoxinA4 attenuate streptozotocin-induced cytotoxicity to RIN5 F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition 2017, 35, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Siresha, B.; Naveen, K.V.G.; Poorani, R.; Sailaja, P.; Devi, H.D.; Monika, S.; Das, U.N. Resolvin D1 ameliorates nicotinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/mTOR pathway in the brain. Arch. Med. Res. 2020, 51, 492–503. [Google Scholar]
- Bathina, S.; Das, U.N. Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation. Int. J. Mol. Sci. 2021, 22, 1516. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, L.; Wang, Y.; Chen, Z.; Ma, J.; Fang, X.; Das, U.N.; Yao, K. Beneficial Actions of Essential Fatty Acids in Streptozotocin-Induced Type 1 Diabetes Mellitus. Front. Nutr. 2022, 9, 890277. [Google Scholar] [CrossRef] [PubMed]
- Rengachar, P.; Polavarapu, S.; Das, U.N. Insights in diabetes: Molecular mechanisms-Protectin DX, an anti-inflammatory and a stimulator of inflammation resolution metabolite of docosahexaenoic acid, protects against the development of streptozotocin-induced type 1 and type 2 diabetes mellitus in male Swiss albino mice. Front. Endocrinol. 2023, 13, 1053879. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, L.B.; Bombassaro, B.; Ramalho, A.F.; Coope, A.; Moura, R.F.; Correa-da-Silva, F.; Ignacio-Souza, L.; Razolli, D.; de Oliveira, D.; Catharino, R.; et al. Resolvin RvD2 reduces hypothalamic inflammation and rescues mice from diet-induced obesity. J. Neuroinflamm. 2017, 14, 5. [Google Scholar] [CrossRef]
- Gemperle, C.; Tran, S.; Schmid, M.; Rimann, N.; Marti-Jaun, J.; Hartling, I.; Wawrzyniak, P.; Hersberger, M. Resolvin D1 reduces inflammation in co-cultures of primary human macrophages and adipocytes by triggering macrophages. Prostaglandins Leukot Essent Fat. Acids 2021, 174, 102363. [Google Scholar] [CrossRef]
- Sima, C.; Paster, B.; Van Dyke, T.E. Function of Pro-Resolving Lipid Mediator Resolvin E1 in Type 2 Diabetes. Crit. Rev. Immunol. 2018, 38, 343–365. [Google Scholar] [CrossRef]
- Kwon, Y. Immuno-Resolving Ability of Resolvins, Protectins, and Maresins Derived from Omega-3 Fatty Acids in Metabolic Syndrome. Mol. Nutr. Food Res. 2020, 64, e1900824. [Google Scholar] [CrossRef]
- Rius, B.; Titos, E.; Morán-Salvador, E.; López-Vicario, C.; García-Alonso, V.; González-Périz, A.; Arroyo, V.; Clària, J. Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 2014, 28, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Cho, W.; Abd El-Aty, A.M.; Bayram, C.; Jeong, J.H.; Jung, T.W. Resolvin D3 improves the impairment of insulin signaling in skeletal muscle and nonalcoholic fatty liver disease through AMPK/autophagy-associated attenuation of ER stress. Biochem. Pharmacol. 2022, 203, 115203. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Dalli, J.; Yacoubian, S.; Gao, F.; Serhan, C.N. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 2012, 189, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Dobrian, A.D.; Huyck, R.W.; Glenn, L.; Gottipati, V.; Haynes, B.A.; Hansson, G.I.; Marley, A.; McPheat, W.L.; Nadler, J.L. Activation of the 12/15 lipoxygenase pathway accompanies metabolic decline in db/db pre-diabetic mice. Prostaglandins Other Lipid Mediat. 2018, 136, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, L.; González-Muniesa, P.; Laiglesia, L.M.; Sáinz, N.; Prieto-Hontoria, P.L.; Escoté, X.; Odriozola, L.; Corrales, F.J.; Arbones-Mainar, J.M.; Martínez, J.A.; et al. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 2017, 31, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
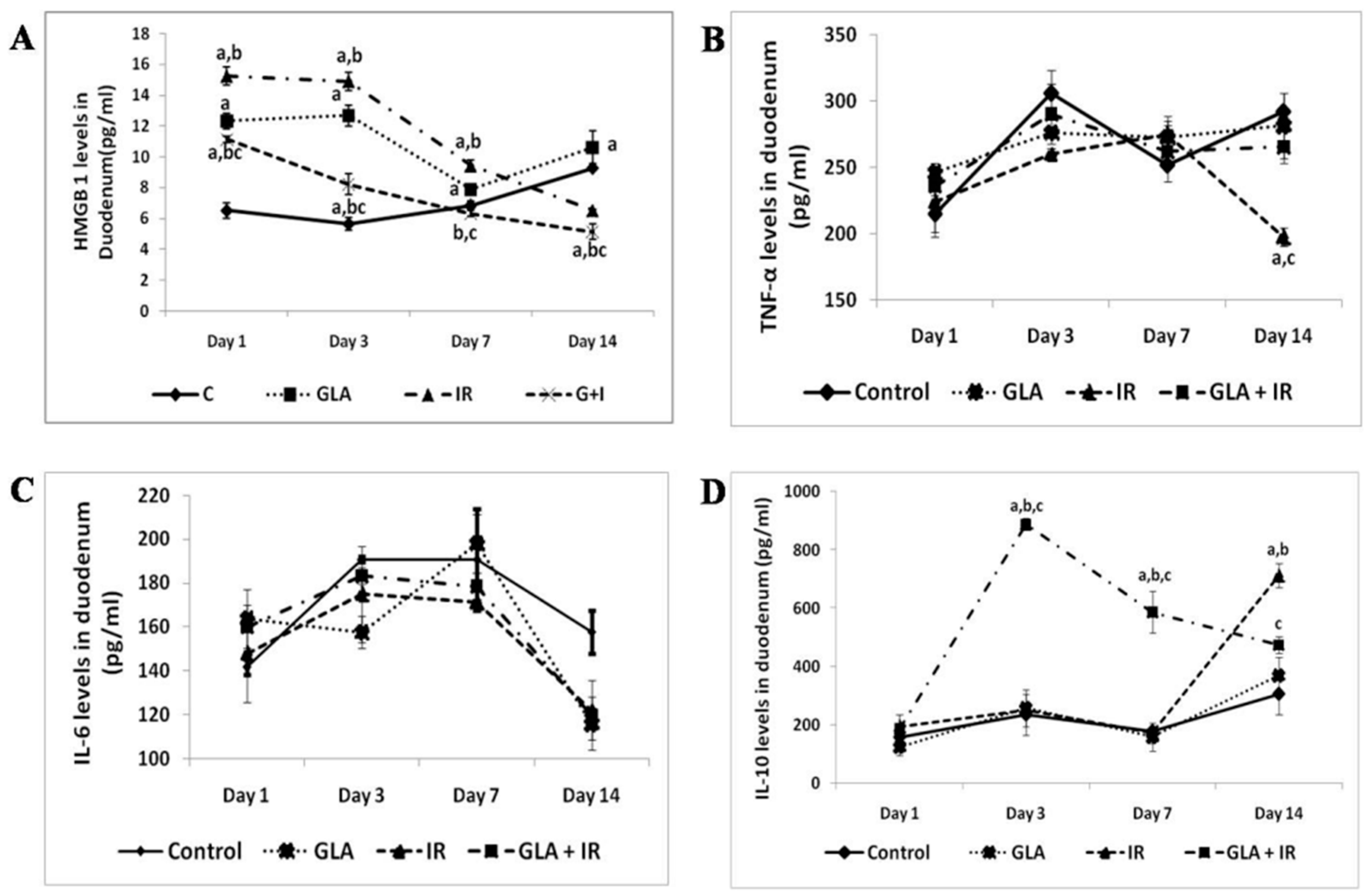
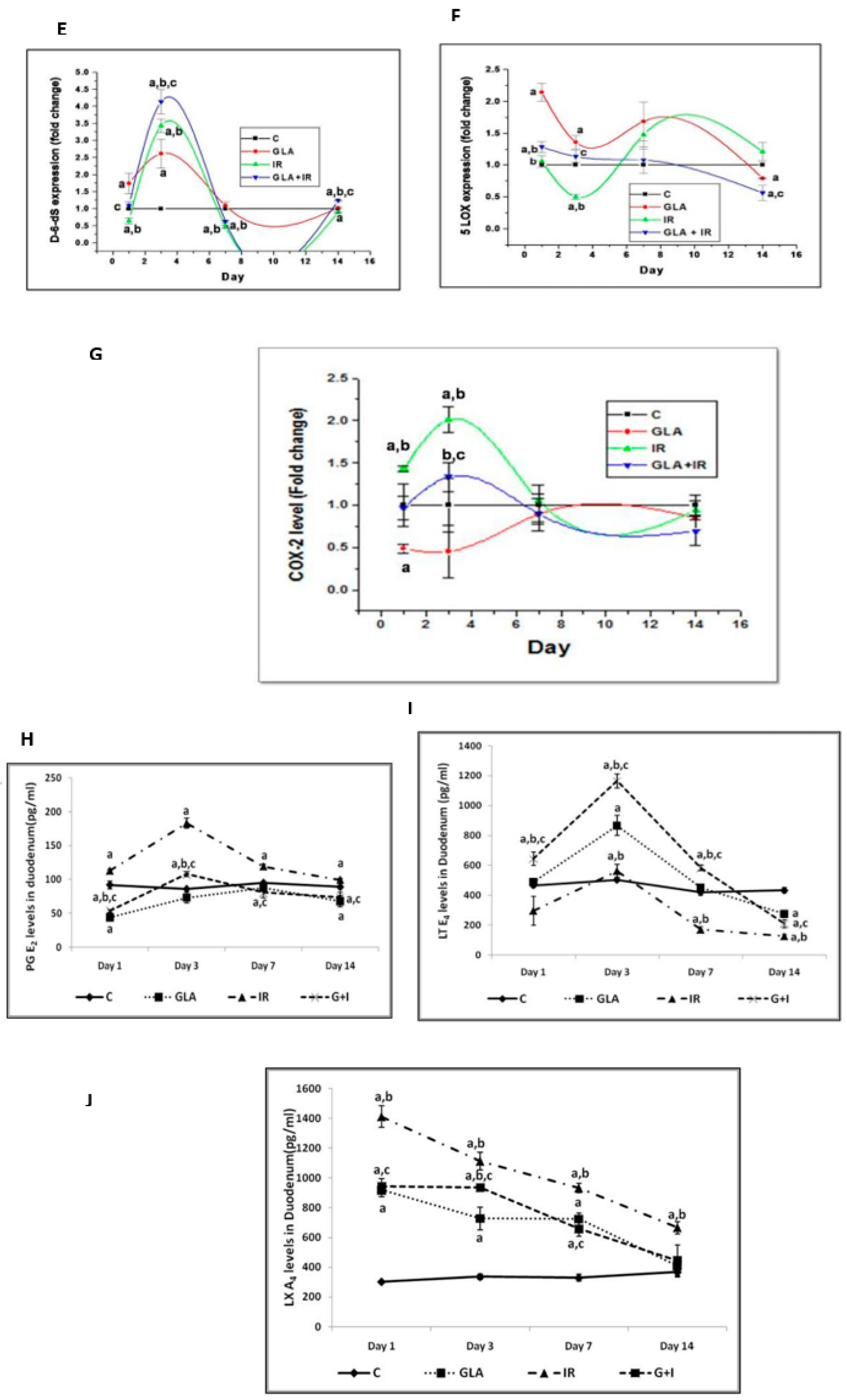
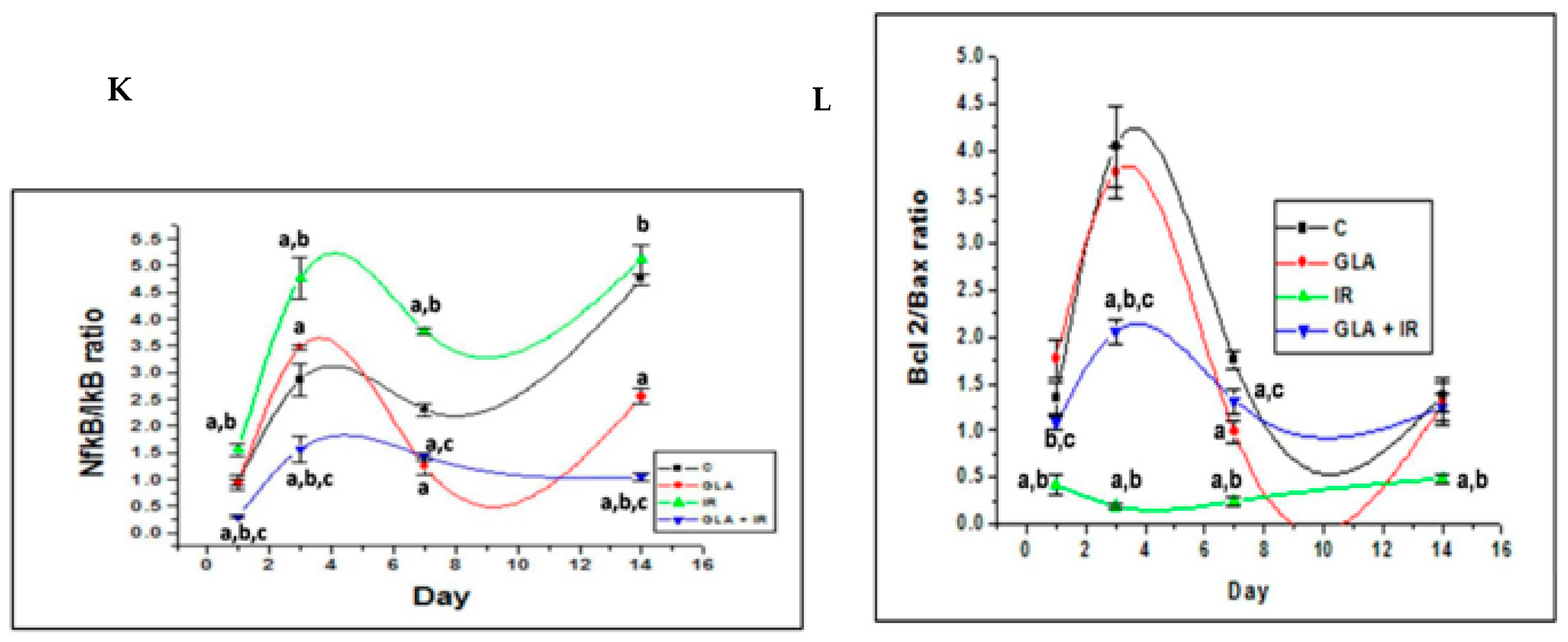
- Rengachar, P.; Bhatt, A.N.; Polavarapu, S.; Veeramani, S.; Krishnan, A.; Sadananda, M.; Das, U.N. Gamma-Linolenic Acid (GLA) Protects against Ionizing Radiation-Induced Damage: An In Vitro and In Vivo Study. Biomolecules 2022, 12, 797. [Google Scholar] [CrossRef]
- Abraham, R.T.; McKinney, M.M.; Forray, C.; Shipley, G.D.; Handwerger, B.S. Stimulation of arachidonic acid release and eicosanoid biosynthesis in an interleukin 2-dependent T cell line. J. Immunopharmacol. 1986, 8, 165–204. [Google Scholar] [CrossRef]
- Das, U.N. Can bioactive lipid arachidonic acid prevent and ameliorate COVID-19? Medicina 2020, 56, 418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlager, S.I.; Madden, L.D.; Meltzer, M.S.; Bara, S.; Mamula, M.J. Role of macrophage lipids in regulating tumoricidal activity. Cell. Immunol. 1983, 77, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Milella, M.; Gismondi, A.; Roncaioli, P.; Palmieri, G.; Morrone, S.; Piccoli, M.; Frati, L.; Cifone, M.G.; Santoni, A. Beta 1 integrin crosslinking inhibits CD16-induced phospholipase D and secretory phospholipase A2 activity and granule exocytosis in human NK cells: Role of phospholipase D in CD16-triggered degranulation. J. Immunol. 1999, 162, 2064–2072. [Google Scholar] [CrossRef]
- Baranov, V.; Nagaeva, O.; Hammarström, S.; Mincheva-Nilsson, L. Lipids are a constitutive component of cytolytic granules. Histochem. Cell Biol. 2000, 14, 167–171. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid in (for) COVID-19. Agro Food Ind. Hi-Tech 2012, 32, 52–55. [Google Scholar]
- Sheppe, A.E.F.; Kummari, E.; Walker, A.; Richards, A.; Hui, W.W.; Lee, J.H.; Mangum, L.; Borazjani, A.; Ross, M.K.; Edelmann, M.J. PGE2 Augments Inflammasome Activation and M1 Polarization in Macrophages Infected with Salmonella Typhimurium and Yersinia enterocolitica. Front. Microbiol. 2018, 9, 2447. [Google Scholar] [CrossRef]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Gambari, L.; Piacentini, A.; Filardo, G.; Fleury-Cappellesso, S.; Barbero, A.; Murphy, M.; Lisignoli, G. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: In vitro evaluation. Osteoarthr. Cartil. 2017, 25, 1161–1171. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huang, M.Y.; Lee, M.S.; Hsieh, C.C.; Kuo, H.F.; Kuo, C.H.; Hung, C.H. Effects of montelukast on M2-related cytokine and chemokine in M2 macrophages. J. Microbiol. Immunol. Infect. 2018, 51, 18–26. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Zhang, Y.; Desai, A.; Yang, S.Y.; Bae, K.B.; Antczak, M.I.; Fink, S.P.; Tiwari, S.; Willis, J.E.; Williams, N.S.; Dawson, D.M.; et al. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 2015, 348, 1223. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipids as Mediators of the beneficial action(s) of Mesenchymal Stem Cells in COVID-19. Aging Dis. 2020, 11, 746–755. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease. Prostaglandins Leukot Essen Fat. Acids 1995, 52, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Jin HLi, M.; Jeong, E.; Castro-Martinez, F.; Zuker, C.S. A body–brain circuit that regulates body inflammatory responses. Nature, 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Syntaxin interacts with arachidonic acid to prevent diabetes mellitus. Lipids Health Dis. 2022, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Kurata-Sato, I.; Mughrabi, I.T.; Rana, M.; Gerber, M.; Al-Abed, Y.; Sherry, B.; Zanos, S.; Diamond, B. Vagus nerve stimulation modulates distinct acetylcholine receptors on B cells and limits the germinal center response. Sci. Adv. 2024, 10, eadn3760. [Google Scholar] [CrossRef]
- Thornton, J.M.; Walker, J.M.; Sundarasivarao, P.Y.K.; Spur, B.W.; Rodriguez, A.; Yin, K. Lipoxin A4 promotes reduction and antibiotic efficacy against Pseudomonas aeruginosa biofilm. Prostaglandins Other Lipid Mediat. 2021, 152, 106505. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Ohira, T.; Arita, M.; Omori, K.; Recchiuti, A.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 2010, 285, 3451–3461. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Álvarez, J.; Fernández Real, J.M.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; Saenz de Pipaon, M.; Sanz, Y. Gut microbes and health. Gastroenterol. Hepatol. 2021, 44, 519–535, (In English; Spanish). [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, I.; WPelzer, B.; Khodamoradi, Y.; Vehreschild, M.J.G.T. The role of the human gut microbiota in colonization and infection with multidrug-resistant bacteria. Gut Microbes 2021, 13, 1911279. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, V.; Mani, I. Dysbiosis of human microbiome and infectious diseases. Prog. Mol. Biol. Transl. Sci. 2022, 192, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Xue, J.; Shao, R.; Mo, C.; Wang, F.; Chen, G. Postbiotics as potential new therapeutic agents for sepsis. Burn. Trauma 2023, 11, tkad022. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, H.; Jia, W.; Shou, Q.; Zhu, Y.; Mao, L.; Wang, W.; Wu, F.; Chen, X.; Wan, X.; et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 2021, 9, 185. [Google Scholar] [CrossRef]
- Zhuang, P.; Zhang, Y.; Shou, Q.; Li, H.; Zhu, Y.; He, L.; Chen, J.; Jiao, J. Eicosapentaenoic and Docosahexaenoic Acids Differentially Alter Gut Microbiome and Reverse High-Fat Diet-Induced Insulin Resistance. Mol. Nutr. Food Res. 2020, 64, e1900946. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, R.; Matsudo, A.; Sugimoto, K.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Fat Influences the Expression of Genes Related to Sterol Metabolism and the Composition of Cecal Microbiota and Its Metabolites in Rats. J. Oleo Sci. 2019, 68, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Suryaprabha, P.; Das, U.N.; Ramesh, G.; Kumar, K.V.; Kamalakar, V. Free radicals, lipid peroxidation and essential fatty acids in patients with septicemia. Prostaglandins Leukot Essen Fat. Acids 1991, 42, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Ye, D.; Zhao, M.; Wan, J. Resolvin D1 protects against sepsis-induced cardiac injury in mice. Biofactors 2020, 46, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Liao, R.; Li, Y.; Jiang, L.; Zhang, Z.; Geng, J.; Fu, P.; Su, B.; Zhao, Y. Resolvin D1 attenuates sepsis induced acute kidney injury targeting mitochondria and NF-κB signaling pathway. Heliyon 2022, 8, e12269. [Google Scholar] [CrossRef]
- Muzaffar, S.N.; Saran, S.; Siddiqui, S.S. Vitamin C therapy in septic shock. Crit. Care 2022, 26, 87. [Google Scholar] [CrossRef]
- Fujii, T.; Salanti, G.; Belletti, A.; Bellomo, R.; Carr, A.; Furukawa, T.A.; Luethi, N.; Luo, Y.; Putzu, A.; Sartini, C.; et al. Effect of adjunctive vitamin C, glucocorticoids, and vitamin B1 on longer-term mortality in adults with sepsis or septic shock: A systematic review and a component network meta-analysis. Intensive Care Med. 2022, 48, 16–24. [Google Scholar] [CrossRef]
- Wald, E.L.; Badke, C.M.; Hintz, L.K.; Spewak, M.; Sanchez-Pinto, L.N. Vitamin therapy in sepsis. Pediatr. Res. 2022, 91, 328–336. [Google Scholar] [CrossRef]
- Hu, X.H.; Situ, H.L.; Chen, J.P.; Yu, R.H. Lipoxin A4 alleviates lung injury in sepsis rats through p38/MAPK signaling pathway. J. Biol. Regul. Homeost. Agents 2020, 34, 807–814. [Google Scholar] [CrossRef]
- Walker, J.; Dichter, E.; Lacorte, G.; Kerner, D.; Spur, B.; Rodriguez, A.; Yin, K. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock 2011, 36, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, D.; Tian, X.; Xiao, J.; Xu, C.; Du, L.; Li, J.; Zhou, S.; Chen, Y.; Zhuang, R.; et al. Protectin conjugates in tissue regeneration 1 alleviates sepsis-induced acute lung injury by inhibiting ferroptosis. J. Transl. Med. 2023, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wang, Z.; Ma, R.; Chen, Y.; Yan, Y.; Miao, S.; Jiao, J.; Cheng, X.; Kong, L.; Ye, D. Lipoxin A4 protects against lipopolysaccharide-induced sepsis by promoting innate response activator B cells generation. Int. Immunopharmacol. 2016, 39, 229–235. [Google Scholar] [CrossRef]
- Jundi, B.; Lee, D.H.; Jeon, H.; Duvall, M.G.; Nijmeh, J.; Abdulnour, R.E.; Pinilla-Vera, M.; Baron, R.M.; Han, J.; Voldman, J.; et al. Inflammation resolution circuits are uncoupled in acute sepsis and correlate with clinical severity. JCI Insight 2021, 6, e148866. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, Q.; Zhang, Y.; Xu, H.; Ye, Y.; Li, L.; Yang, Y.; Jin, S. Maresin conjugates in tissue regeneration-1 suppresses ferroptosis in septic acute kidney injury. Cell Biosci. 2021, 11, 221. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wang, L.; Jiang, L.; Qin, Z.; Zhao, Y.; Su, B. Maresin 1 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury via Inhibiting NOX4/ROS/NF-κB Pathway. Front. Pharmacol. 2021, 12, 782660. [Google Scholar] [CrossRef]
- Gu, J.; Luo, L.; Wang, Q.; Yan, S.; Lin, J.; Li, D.; Cao, B.; Mei, H.; Ying, B.; Bin, L.; et al. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab. Investig. 2018, 98, 715–733. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Ma, Z.; Ma, M.; Wang, D.; Xie, G.; Yin, Y.; Zhang, P.; Tao, K. Maresin 1 Mitigates Inflammatory Response and Protects Mice from Sepsis. Mediat. Inflamm. 2016, 2016, 3798465. [Google Scholar] [CrossRef] [PubMed]
- Miller-Little, W.A.; Chen, X.; Salazar, V.; Liu, C.; Bulek, K.; Zhou, J.Y.; Li, X.; Stüve, O.; Stappenbeck, T.; Dubyak, G.; et al. A TH17-intrinsic IL-1β-STAT5 axis drives steroid resistance in autoimmune neuroinflammation. Sci. Immunol. 2024, 9, eabq1558. [Google Scholar] [CrossRef] [PubMed]
- Furse, R.K.; Rossetti, R.G.; Seiler, C.M.; Zurier, R.B. Oral administration of gammalinolenic acid, an unsaturated fatty acid with anti-inflammatory properties, modulates interleukin-1beta production by human monocytes. J. Clin. Immunol. 2002, 22, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D.; Tecklenburg, S.L.; Montgomery, G.S.; Lindley, M.R. Eicosapentaenoic acid is more eff7ective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin. Nutr. 2009, 28, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ait-Said, F.; Elalamy, I.; Werts, C.; Gomard, M.T.; Jacquemin, C.; Couetil, J.P.; Hatmi, M. Inhibition by eicosapentaenoic acid of IL-1beta-induced PGHS-2 expression in human microvascular endothelial cells: Involvement of lipoxygenase-derived metabolites and p38 MAPK pathway. Biochim. Biophys. Acta 2003, 1631, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wu, S.H.; Zhang, L.; Chen, X.Q. Roles of lipoxin A4 receptor activation and anti-interleukin-1β antibody on the toll-like receptor 2/mycloid differentiation factor 88/nuclear factor-κB pathway in airway inflammation induced by ovalbumin. Mol. Med. Rep. 2015, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Lamont, G.J.; Lamont, R.J.; Uriarte, S.M.; Wang, H.; Scott, D.A. Resolvin D1, resolvin D2 and maresin 1 activate the GSK3β anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. 2016, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.G.; Colas, R.A.; Newton, J.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Appleton, L.; Wheway, K.; Watkins, B.; et al. 15-Epi-LXA4 and MaR1 counter inflammation in stromal cells from patients with Achilles tendinopathy and rupture. FASEB J. 2019, 33, 8043–8054. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 872–882. [Google Scholar] [CrossRef]
- Duffy, M.M.; Pindjakova, J.; Hanley, S.A.; McCarthy, C.; Weidhofer, G.A.; Sweeney, E.M.; English, K.; Shaw, G.; Murphy, J.M.; Barry, F.P.; et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur. J. Immunol. 2011, 41, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, H.; Zhang, X.; Wen, D.; Yu, F.; Yang, S.; Jia, X.; Cong, B.; Ma, C. Prostaglandin I2-IP signalling regulates human Th17 and Treg cell differentiation. Prostaglandins Leukot Essent Fat. Acids 2013, 89, 335–344. [Google Scholar] [CrossRef]
- Shoda, H.; Yanai, R.; Yoshimura, T.; Nagai, T.; Kimura, K.; Sobrin, L.; Connor, K.M.; Sakoda, Y.; Tamada, K.; Ikeda, T.; et al. Dietary Omega-3 Fatty Acids Suppress Experimental Autoimmune Uveitis in Association with Inhibition of Th1 and Th17 Cell Function. PLoS ONE 2015, 10, e0138241. [Google Scholar] [CrossRef]
- Oner, F.; Alvarez, C.; Yaghmoor, W.; Stephens, D.; Hasturk, H.; Firatli, E.; Kantarci, A. Resolvin E1 Regulates Th17 Function and T Cell Activation. Front. Immunol. 2021, 12, 637983. [Google Scholar] [CrossRef]
| Fatty Acid | Control | HTN | CHD | T2DM | DN |
|---|---|---|---|---|---|
| 18:2 n-6 (LA) | 18.6 ± 3.1 | 14.5 ± 3.1 * | 17.8 ± 5.0 | 13.9 ± 5.3 | 15.1 ± 3.1 |
| 18:3 n-6 (GLA) | 0.14 ± 0.1 | 0.4 ± 0.3 * | 0.1 ± 0.1 * | 0.2 ± 0.3 | 0.1 ± 0.2 |
| 20:3 n-6 (DGLA) | 3.4 ± 1.0 | 3.1 ± 0.9 | 2.7 ± 1.1 | 1.7 ± 1.0 * | 2.0 ± 0.8 * |
| 20:4 n-6 (AA) | 9.4 ± 1.8 | 7.8 ± 2.0 * | 7.0 ± 2.1 * | 4.6 ± 1.8 * | 6.6 ± 2.6 * |
| 22:5 n-6 | 0.7 ± 0.4 | 0.4 ± 0.4 * | 1.0 ± 0.9 | 2.1 ± 0.6 * | 1.3 ± 0.5 * |
| 20:4 n-6/18-2 n-6 | 0.51 | 0.54 | 0.39 | 0.33 | 0.43 |
| 18:3 n-3 (ALA) | 0.2 ± 0.1 | 0.4 ± 0.2 * | 0.3 ± 0.5 | 0.1 ± 0.2 * | 0.1 ± 0.1 |
| 20:5 n-3 (EPA) | 0.4 ± 0.4 | 0.6 ± 0.6 | 0.1 ± 0.2 * | 0.3 ± 0.3 | 0.2 ± 0.3 |
| 22:5 n-3 | 0.5 ± 0.2 | 0.4 ± 0.5 | 0.3 ± 0.3 * | 1.6 ± 1.3 | 1.7 ± 1.1 |
| 22:6 n-3 (DHA) | 1.4 ± 0.5 | 1.2 ± 0.6 | 0.8 ± 0.4 * | 0.5 ± 0.4 * | 0.5 ± 0.3 * |
| 20:5 n-3/18:3 n-3 | 1.8 | 1.39 | 0.41 | 3.2 | 4.0 |
| Fatty Acid | Control (n = 10) | Pneumonia (n = 12) | Septicemia (n = 14) | RA (n = 12) | SLE (n = 5) |
|---|---|---|---|---|---|
| 16:0 | 24.8 ± 3.4 | 32.5 ± 3.6 | 26.95 ± 4.1 | 30.2 ± 3.0 | 32.0 ± 3.75 |
| 18:0 | 23.3 ± 4.1 | 21.4 ± 7.1 | 24.58 ± 6.0 | 19.0 ± 6.1 | 14.6 ± 5.82 |
| 18:1 n-9 | 13.1 ± 2.3 | 15.6 ± 3.2 | 16.5 ± 3.3 * | 14.8 ± 2.1 | 16.0 ± 2.76 |
| 18:2 n-6 | 17.7 ± 3.1 | 14.2 ± 0.3 * | 16.3 ± 2.4 | 17.5 ± 2.7 | 20.8 ± 2.2 |
| 18:3 n-6 | 0.13 ± 0.09 | 0.13 ± 0.08 | 0.04 ± 0.05 * | 0.02 ± 0.04 ** | 0.01 ± 0.01 ** |
| 20:3 n-6 | 3.2 ± 0.79 | 1.5 ± 0.4 * | 0.46 ± 0.54 * | 2.5 ± 0.58 | 2.12 ± 0.52 |
| 20:4 n-6 | 8.8 ± 2.0 | 5.1 ± 0.4 * | 5.8 ± 1.6 * | 9.5 ± 2.2 | 8.93 ± 2.0 |
| 22:4 n-6 | 0.42 ± 0.23 | 0.8 ± 0.9 | 0.34 ± 0.28 | 0.26 ± 0.37 ** | 0.18 ± 0.18 ** |
| 22:5 n-6 | 0.73 ± 0.55 | 0.45 ± 0.63 | 1.5 ± 1.02 * | 0.6 ± 0.7 | 0.8 ± 1.0 |
| 18:3 n-6/18:2 n-6 | 0.007 | 0.0092 | 0.002 | 0.001 | 0.004 |
| 20:4 n-6/18:2 n-6 | 0.35 | 0.36 | 0.5 | 0.54 | 0.44 |
| 20:4 n-6/20:3 n-6 | 4.01 | 3.4 | 2.75 | 3.8 | 4.2 |
| 18:3 n-3 | 0.27 ± 0.12 | 0.09 ± 0.04 * | 0.16 ± 0.11 * | 0.12 ± 0.16 * | 0.1 ± 0.1 * |
| 20:5 n-3 | 0.25 ± 0.26 | 0.23 ± 0.24 | 0.01 ± 0.01 * | 0.05 ± 0.14 ** | 0.04 ± 0.04 ** |
| 22:5 n-3 | 0.54 ± 0.16 | 0.44 ± 0.53 | 0.29 ± 0.12 * | 0.69 ± 0.05 | 0.21 ± 0.35 * |
| 22:6 n-3 | 1.43 ± 0.43 | 0.54 ± 0.43 * | 1.2 ± 1.14 | 0.62 ± 0.56 * | 0.88 ± 0.75 * |
| 20:5 n-3/18:3 n-3 | 0.92 | 1.55 | 0.06 | 0.41 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, U.N. The Dysregulation of Essential Fatty Acid (EFA) Metabolism May Be a Factor in the Pathogenesis of Sepsis. Medicina 2024, 60, 934. https://doi.org/10.3390/medicina60060934
Das UN. The Dysregulation of Essential Fatty Acid (EFA) Metabolism May Be a Factor in the Pathogenesis of Sepsis. Medicina. 2024; 60(6):934. https://doi.org/10.3390/medicina60060934
Chicago/Turabian StyleDas, Undurti N. 2024. "The Dysregulation of Essential Fatty Acid (EFA) Metabolism May Be a Factor in the Pathogenesis of Sepsis" Medicina 60, no. 6: 934. https://doi.org/10.3390/medicina60060934
APA StyleDas, U. N. (2024). The Dysregulation of Essential Fatty Acid (EFA) Metabolism May Be a Factor in the Pathogenesis of Sepsis. Medicina, 60(6), 934. https://doi.org/10.3390/medicina60060934






