Significance of Duodenal Prolactin Receptor Modulation by Calcium and Vitamin D in Sulpiride-Induced Hyperprolactinemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Laboratory Investigations
2.4. Tissue Preparation
2.5. RNA Isolation and cDNA Synthesis
2.6. Relative Quantification of Prolactin Receptor Gene Expression Using RealTime PCR (RT-PCR)
2.7. Analysis of Real-Time PCR Results
2.8. Statistical Analyses
2.8.1. Statistical Analyses of Laboratory Results
2.8.2. Statistical Analyses of Real-Time PCR Results
3. Results
3.1. Prolactin Levels
3.2. Vitamin D Concentration in Experimental Groups
3.3. Parathyroid Hormone Levels
3.4. Mineral Analysis
3.5. Bone Formation Markers
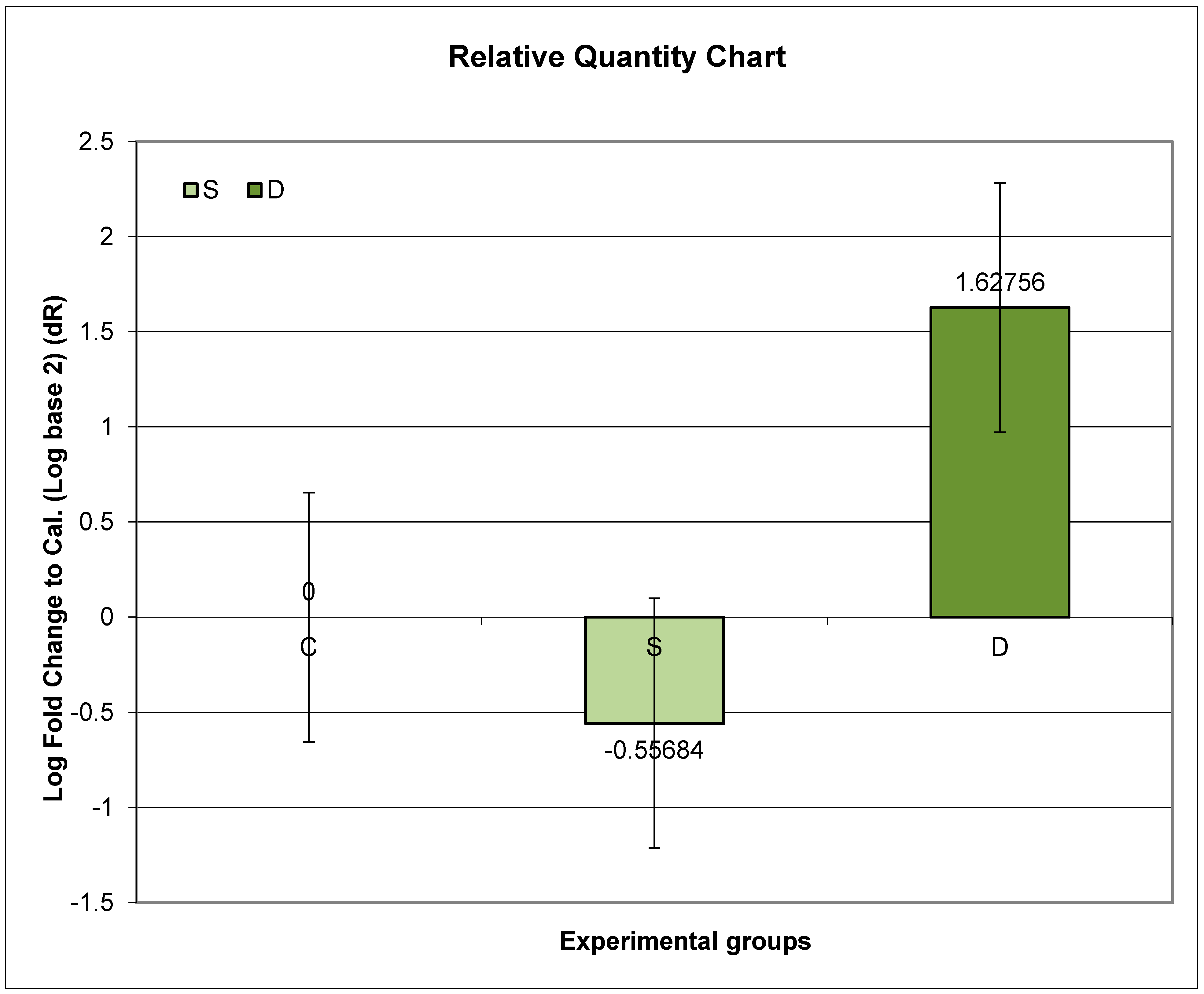
3.6. Expression of Prlr mRNA in the Small Intestine in Experimental Groups S and D
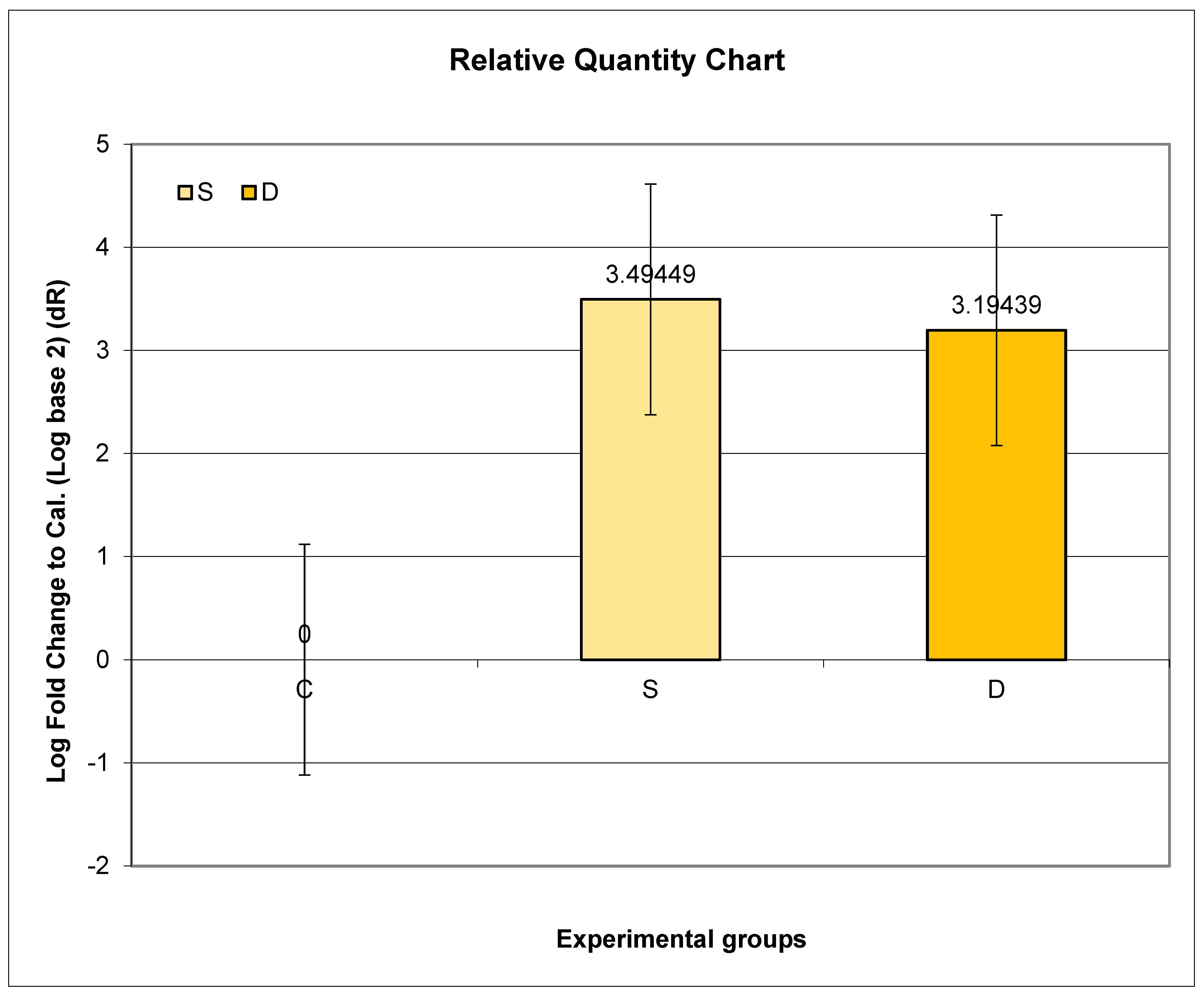
3.7. Expression of Prlr mRNA in Lumbar Spine in Experimental Groups S and D
3.8. Expression of Prlr mRNA in Renal Tissue in Experimental Groups S and D
4. Discussion
4.1. Increased Prolactin Concentration in Experimental Groups
4.2. Changes in Mineral Analysis during Sulpiride-Induced HP
4.2.1. Serum-Ionized Calcium and Phosphorous
4.2.2. Urinary Calcium and Phosphorus Excretion
4.3. Changes in Bone Turnover Markers during Sulpiride-Induced HP
4.4. Analysis of Prlr Expression in the Duodenum during Sulpiride-Induced HP with and without Vitamin D Supplementation
4.5. Analysis of Prlr Expression in the Vertebrae during Sulpiride-Induced HP with and without Vitamin D Supplementation
4.6. Analysis of Prlr Expression in the Kidney during Sulpiride-Induced HP with and without Vitamin D Supplementation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peirce, S.K.; Chen, W.Y. Quantification of prolactin receptor mRNA in multiple human tissues and cancer cell lines by real time RT-PCR. J. Endocrinol. 2001, 171, R1–R4. [Google Scholar] [CrossRef] [PubMed]
- Ouhtit, A.; Kelly, P.A.; Morel, G. Visualization of gene expression of short and long forms of prolactin receptor in rat digestive tissues. Am. J. Physiol. 1994, 266, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Bataille-Simoneau, N.; Gerland, K.; Chappard, D.; Basle, M.F.; Mercier, L. Expression of prolactin receptors in human osteosarcoma cells. Biochem. Biophys. Res. Commun. 1996, 229, 323–328. [Google Scholar] [CrossRef]
- Charoenphandhu, N.; Tudpor, K.; Thongchote, K.; Saengamnart, W.; Puntheeranurak, S.; Krishnamra, N. High-calcium diet modulates effects of long term prolactin exposure on the cortical content in ovariectomized rats. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Clement-Lacroix, P.; Ormandy, C.; Lepescheux, L.; Ammann, P.; Damotte, D.; Goffin, V.; Bouchard, B.; Amling, M.; Gaillard-Kelly, M.; Binart, N.; et al. Osteoblasts are a new target for prolactin: Analysis of bone formation in prolactin receptor knockout mice. Endocrinology 1999, 140, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Charoenphandhu, N.; Krishnamara, N. Prolactin is an important regulator of intestinal calcium transport. Can. J. Physiol. Pharmacol. 2007, 85, 569–581. [Google Scholar] [CrossRef]
- Charoenphandhu, N.; Wongdee, K.; Krishnamara, N. Is prolactin the cardinal calciotropic maternal hormone? Trnds Endocrinol. Metab. 2010, 21, 395–401. [Google Scholar] [CrossRef]
- Kovacs, C.S. Calcium and bone metabolism in pregnancy and lactation. J. Clin. Endocrinol. Metab. 2001, 86, 2344–2348. [Google Scholar]
- Karlsson, C.; Obrant, K.J.; Karlsson, M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos. Int. 2001, 12, 828–834. [Google Scholar] [CrossRef]
- Streeten, E.A.; Ryan, K.A.; McBride, D.J.; Pollin, T.I.; Shuldiner, A.R.; Mitchell, B.D. The relationship between parity and bone mineral density in women characterized by a homogeneous lifestyle and high parity. J. Clin. Endocrinol. Metab. 2005, 90, 4536–4541. [Google Scholar] [CrossRef]
- Lenora, J.; Lekamwasam, S.; Karlsson, M.K. Effects of multiparity and prolonged breast-feeding on maternal bone mineral density: A community-based cross-sectional study. BMC Women’s Health 2009, 9, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.T.; Solimeo, S. Osteoporosis and depression: A historical perspective. Curr. Osteoporos. Rep. 2006, 4, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Papakostas, G.I.; Klibanski, A. Effects of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. J. Clin. Psychiatry 2004, 65, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Joe, S.H.; Lee, M.S.; Ko, Y.H.; Jung, I.K.; Kim, S.H. Effects of long-term combination treatment with valproate and atypical antipsychotics on bone mineral density and bone metabolism in premenopausal patients with bipolar disorder: A preliminary study. Psychiatry Investig. 2011, 8, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Radojkovic, D.; Pesic, M.; Radojkovic, M.; Dimic, D.; Vukelic Nikolic, M.; Jevtovic Stoimenov, T.; Radenkovic, S.; Velojic Golubovic, M.; Radjenovic Petkovic, T.; Antic, S. Expression of prolactin receptors in the duodenum, kidneys and skeletal system during physiological and sulpiride-induced hyperprolactinaemia. Endocrine 2018, 62, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Rady, A.; Elsheshai, A.; Elkholy, O.; Abouelwafa, H.; Eltawil, M. Long Term Use of Antipsychotics and Adverse Effects on Bone Density. Neuropsychiatry 2018, 8, 1559–1563. [Google Scholar] [CrossRef]
- Haddad, P.M.; Wieck, A. Antipsychotic-induced hyperprolactinemia: Mechanisms, clinical features and management. Drugs 2004, 64, 2291–2314. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Nemere, I. Endocrine regulation of calcium transport in epithelia. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Besnard, I.; Auclair, V.; Callery, G.; Gabriel-Bordenave, C.; Roberge, C. Hyperprolactinémies induites par les antipsychotiques: Physiopathologie, clinique et surveillance [Antipsychotic-drug-induced hyperprolactinemia: Physiopathology, clinical features and guidance]. Encephale 2014, 40, 86–94. [Google Scholar] [CrossRef]
- Torre, D.L.; Falorni, A. Pharmacological causes of hyperprolactinemia. Ther. Clin. Risk Manag. 2007, 3, 929–951. [Google Scholar]
- Tonini, M.; Cipollina, L.; Poluzzi, E.; Cremas, F.; Corazzas, G.R.; De Ponti, F. Review article: Clinical implications of enteric and central D2 receptor blocade by antidopaminergic gastrointestinal prokinetics. Aliment. Pharmacol. Ther. 2004, 19, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, W.; Jeong, S.; Kim, D.; Yoo, J.W.; Jung, Y. Therapeutic switching of sulpiride, an anti-psychotic and prokinetic drug, to an anti-colitic drug using colon-specific drug delivery. Drug Deliv. Transl. Res. 2019, 9, 334–343. [Google Scholar] [CrossRef]
- Baastrup, P.C.; Christiansen, C.; Transbol, I. Calcium metabolism in schizophrenic patients on long-term neuroleptic therapy. Neuropsychobiology 1980, 6, 56–59. [Google Scholar] [CrossRef]
- Nechifor, M.; Vaideanu, C.; Palamaru, I.; Borza, C.; Mindreci, I. The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia. J. Am. Coll. Nutr. 2004, 23, 549S–551S. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.; Mutlu, N.; Akbas, N.B. Treatment of a major depression episode suppresses markers of bone turnover in premenopausal women. J. Psychiatr. Res. 2011, 45, 1316–1320. [Google Scholar] [CrossRef]
- Wyszogrodzka-Kucharska, A.; Rabe-Jablonska, J. Calcium balance regulation in schizophrenic patients treated with second generation antipsychotics. Psychiatr. Pol. 2005, 39, 1157–1171. [Google Scholar]
- Zornic, N.; Radojevic, D.J.; Jankovic, S.; Djuric, D.; Varjacic, M.; Simic, V.D.; Milovanovic, D.R. Monitoring of drug-associated electrolyte disturbances in a hospital. Pharmacoepidemiol. Drug Saf. 2009, 18, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, D.R.; Janjic, V.; Zornic, N.; Djukic Dejanovic, S.; Jankovic, S.M. Risperidone-associated hypocalcemia. Am. J. Psychiatry 2010, 167, 1533–1534. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, D.R.; Pirkovic, M.S.; Simonovic, S.Z.; Matovic, M.; Dejanovic, S.D.; Jankovic, S.M.; Ravanic, D.; Petronijevic, M.; Ristic, D.I.; Mladenovic, V.; et al. Parameters of Calcium Metabolism Fluctuated during Initiation or Changing of Antipsychotic Drugs. Psychiatry Investig. 2016, 13, 89–101. [Google Scholar] [CrossRef]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 23–30. [Google Scholar] [CrossRef]
- Taheri, M.; Tavasoli, S.; Shokrzadeh, F.; Amiri, F.B.; Basiri, A. Effect of vitamin D supplementation on 24-hour urine calcium in patients with calcium urolithiasis and vitamin D deficiency. Int. Braz. J. Urol. 2019, 45, 340–346. [Google Scholar] [CrossRef]
- Jang, H.R.; Lee, J.W.; Kim, S.; Heo, N.J.; Lee, J.H.; Kim, H.S.; Jung, J.Y.; Oh, Y.K.; Na, K.Y.; Han, J.S.; et al. High dose vitamin D3 attenuates the hypocalciuric effect of thiazide in hypercalciuric rats. J. Korean Med. Sci. 2010, 25, 1305–1312. [Google Scholar] [CrossRef]
- Herrán, A.; Amado, J.A.; García-Unzueta, M.T.; Vázquez-Barquero, J.L.; Perera, L.; González-Macías, J. Increased bone remodeling in first-episode major depressive disorder. PsychosomMed. 2000, 62, 779–782. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and Matrix Gla Protein: Vitamin K-dependent Proteins in Bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Takeda, T.; Sato, Y. Role of Vitamin K2 in the Treatment of Postmenopausal Osteoporosis. Curr. Drug Saf. 2006, 1, 87–97. [Google Scholar] [CrossRef]
- Jadhav, N.; Ajgaonkar, S.; Saha, P.; Gurav, P.; Pandey, A.; Basudkar, V.; Gada, Y.; Panda, S.; Jadhav, S.; Mehta, D.; et al. Molecular Pathways and Roles for Vitamin K2-7 as a Health-Beneficial Nutraceutical: Challenges and Opportunities. Front. Pharmacol. 2022, 13, 896920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gram, J.; Junker, P.; Nielsen, H.K.; Bollerslev, J. Dose-response effect of short-term calcitriol treatment on bone and mineral metabolism in normal males. Bone 1996, 18, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Radojković, D.; Pesić, M.; Ristić, T. Bone turnover markers in medicamentous and physiological hyperprolactinemia in female rats. Vojnosanit. Pregl. 2014, 71, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chung, M.Y.; Chung, H.K.; Choi, J.H.; Kim, T.Y.; So, H.S. Bone density in chronic schizophrenia with long-term antipsychotic treatment: Preliminary study. Psychiatry. Investig. 2010, 7, 278–284. [Google Scholar] [CrossRef]
- Sirtori, P.; Sosio, C.; Resmini, G.; Rubinacci, A. Effect of short course of 1,25-dihydroxyvitamin D3 on biochemical markers of bone remodelling in postmenopausal women. Pharmacol. Res. 1996, 33, 353–359. [Google Scholar] [CrossRef]
- Hitz, M.F.; Jensen, J.E.; Eskildsen, P.C. Bone mineral density and bone markers in patients with recent low-energy fracture: Effect of 1 y of treatment with calcium and vitamin D. Am. J. Clin. Nut 2007, 86, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Peppone, L.J.; Hebl, S.; Purnell, J.Q.; Reid, M.E.; Rosier, R.N.; Mustian, K.M.; Palesh, O.G.; Huston, A.J.; Ling, M.N.; Morrow, G.R. The efficacy of calcitriol therapy in the management of bone loss and fractures: A qualitative review. Osteoporos. Int. 2010, 21, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; Nilius, B.; Bindels, R.J. Calcium absorption across epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Evolution of our understanding of vitamin D. Nutr. Rev. 2008, 66 (Suppl. 2), S73–S87. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S. Vitamin D Gene Regulation. In Principles of Bone Biology, 3rd ed.; Elsevier: San Diego, CA, USA, 2008; pp. 779–794. [Google Scholar]
- Amnattanakul, S.; Charoenphandhu, N.; Limlomwongse, L.; Krishnamara, N. Endogenous prolactin modulated calcium absorption in the jejunum of suckling rats. Can. J. Physiol. Pharmacol. 2005, 83, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Charoenphandhu, N.; Limlomwongse, L.; Krishnamara, N. Prolactin directly enhanced Na+/K+- and Ca2+-ATPase activities in the duodenum of female rats. Can. J. Physiol. Pharmacol. 2006, 84, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Piyabhan, P.; Krishnamara, N.; Limlomwongse, L. Changes in the regulation of calcium metabolism and bone calcium content during growth in the absence of endogenous prolactin and during hyperprolactinemia: Longitudinal study in male and female Wistar rats. Can. J. Physiol. Pharmacol. 2000, 78, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Tanrattana, C.; Charoenphandhu, N.; Limlomwongse, L.; Krishnamara, N. Prolactin directly stimulated the solvent drag-induced calcium transport in the duodenum of female rats. Biochim. Biophys. Acta 2004, 1665, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Charoenphandhu, N.; Wongdee, K.; Teerapornpuntakit, J.; Thongchote, K.; Krishnamara, N. Transcriptome responses of duodenal epithelial cells to prolactin in pituitary grafted rats. Mol. Cell Endocrinol. 2008, 296, 41–52. [Google Scholar] [CrossRef]
- Tudpor, K.; Charoenphandhu, N.; Saengamnart, W.; Krishnamra, N. Long-term prolactin exposure differentially stimulated the transcellular and solvent drag-induced calcium transport in the duodenum of ovariectomized rats. Exp. Biol. Med. 2005, 230, 836–844. [Google Scholar] [CrossRef]
- Charoenphandhu, N.; Nakkrasae, L.I.; Kraidith, K.; Teerapornpuntakit, J.; Thongchote, K.; Thongon, N.; Krishnamara, N. Two-step stimulation for intestinal Ca2+ absorption during lactation by long-term prolactin exposure and suckling induced prolactin surge. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E609–E619. [Google Scholar] [CrossRef] [PubMed]
- Kraidith, K.; Jantarajit, W.; Teerapornpuntakit, J.; Nakkrasae, L.I.; Krishnamara, N.; Charoenphandhu, N. Direct stimulation of the transcellular and paracellular calcium transport in the cecum by prolactin. Pflugers Arch. 2009, 458, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Charoenphandhu, N.; Tudpor, K.; Pulsook, N.; Krishnamara, N. Chronic metabolic acidosis stimulated transcellular and solvent drag-induced calcium transport in the duodenum of female rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G446–G455. [Google Scholar] [CrossRef] [PubMed]
- Charoenphandhu, N.; Limlomwongse, L.; Krishnamara, N. Prolactin directly stimulates transcellular active calcium transport in the duodenum of female rats. Can. J. Physiol. Pharmacol. 2001, 79, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Van Cromphaut, S.J.; Rummens, K.; Stockmans, I.; van Herck, E.; Dijcks, F.A.; Ederveen, A.G.; Carmeliet, P.; Verhaeghe, J.; Bouillon, R.; Carmeliet, G. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J. Bone Miner. Res. 2003, 18, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Meyer, M.B.; Pike, J.W.; Christakos, S. Evidence for role of prolactin in calcium homeostasis: Regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption and 25-hydroxyvitamin D3 1α hydroxylase gene by prolactin. Endocrinology 2010, 15, 2974–2984. [Google Scholar] [CrossRef] [PubMed]
- Seriwatanachai, D.; Thongchote, K.; Charoenphandhu, N.; Pandaranandaka, J.; Tudpor, K.; Teerapornpuntakit, J.; Suthiphongchai, T.; Krishnamra, N. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone 2008, 42, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Coss, D.; Yang, L.; Kuo, C.B.; Xu, X.; Luben, R.A.; Walker, A.M. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Glowacki, J.; Kim, S.W.; Hahne, J.; Geng, S.; Mueller, S.M.; Shen, L.; Bleiberg, I.; LeBoff, M.S. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D 3in human marrow stromal cells. J. Bone Miner. Res. 2012, 27, 1992–2000. [Google Scholar] [CrossRef]
- van Driel, M.; Koedam, M.; Buurman, C.J.; Hewison, M.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.; van Leeuwen, J.P. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006, 20, 2417–2419. [Google Scholar] [CrossRef]
- van de Peppel, J.; van Leeuwen, J.P. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Woeckel, V.J.; Alves, R.D.; Swagemakers, S.M.; Eijken, M.; Chiba, H.; van der Eerden, B.C.; van Leeuwen, J.P. 1Alpha,25-(OH)2D3 acts in the early phase of osteoblast differentiation to enhance mineralization via accelerated production of mature matrix vesicles. J. Cell Physiol. 2010, 225, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.A.; Proudman, J.A.; Bahr, J.M. Radioligand receptor assay for prolactin using chicken and turkey kidney membranes. Comp. Biochem. Physiol. 1991, 100B, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Hiraoka, Y.; Ogawa, M.; Takeuchi, Y.; Aiso, S. The prolactin gene is expressed in the mouse kidney. Kidney. Int. 1999, 55, 833–840. [Google Scholar] [CrossRef]
- Crambert, S.; Sjoberg, A.; Eklof, A.C.; Ibarra, F.; Holtback, U. Prolactin and dopamin 1-like receptor interaction in renal proximal tubular cells. Am. J. Physiol. Renal Physiol. 2010, 299, 49–54. [Google Scholar] [CrossRef]
- Ibarra, F.; Crambert, S.; Eklof, A.C.; Hansell, P.; Holtback, U. Prolactin, a natriuretic hormone, interacting with renal dopamine system. Kidney Int. 2005, 68, 1700–1707. [Google Scholar] [CrossRef]

| QuantiTect Primer Assay (Qiagen, Germany) | Detected Transcript | |
|---|---|---|
| β2 microglobulin | Rn_B2m_1_SG, QT00176295 | NM_012512 |
| Prolactin receptor gene | Rn_Prlr_vb.1_SG, QT01169518 | NM_001034111 |
| S | D | C | |
|---|---|---|---|
| PRL (pg/mL) X ± SD | 148.92 ± 20.46 a | 148.38 ± 27.25 b | 112.01 ± 11.92 |
| s-Ca ++ (mmol/L) X ± SD | 1.21 ± 0.03 | 1.17 ± 0.03 | 1.15 ± 0.02 |
| s-P (mmol/L) X ± SD | 1.70 ± 0.13 | 1.92 ± 0.16 | 1.89 ± 1.89 |
| u-Ca (mmol/24 h) X ± SD | 2.88 ± 0.60 | 8.63 ± 2.08 c,d | 3.37 ± 0.87 |
| u-P (mmol/24 h) X ± SD | 53.93 ± 14.05 | 67.83 ± 16.78 | 55.03 ± 20.37 |
| OC (ng/mL) X ± SD | 13.55 ± 3.42 | 9.90 ± 1.98 e,f | 16.18 ± 2.00 |
| P1NP (pg/mL) X ± SD | 291.70 ± 71.03 | 283.70 ± 86.52 | 314.86 ± 50.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radojkovic, D.B.; Pesic, M.; Radojkovic, M.; Vukelic Nikolic, M.; Jevtovic Stoimenov, T.; Radenkovic, S.; Ciric, V.; Basic, D.; Radjenovic Petkovic, T. Significance of Duodenal Prolactin Receptor Modulation by Calcium and Vitamin D in Sulpiride-Induced Hyperprolactinemia. Medicina 2024, 60, 942. https://doi.org/10.3390/medicina60060942
Radojkovic DB, Pesic M, Radojkovic M, Vukelic Nikolic M, Jevtovic Stoimenov T, Radenkovic S, Ciric V, Basic D, Radjenovic Petkovic T. Significance of Duodenal Prolactin Receptor Modulation by Calcium and Vitamin D in Sulpiride-Induced Hyperprolactinemia. Medicina. 2024; 60(6):942. https://doi.org/10.3390/medicina60060942
Chicago/Turabian StyleRadojkovic, Danijela Branislav, Milica Pesic, Milan Radojkovic, Marija Vukelic Nikolic, Tatjana Jevtovic Stoimenov, Sasa Radenkovic, Vojislav Ciric, Dijana Basic, and Tatjana Radjenovic Petkovic. 2024. "Significance of Duodenal Prolactin Receptor Modulation by Calcium and Vitamin D in Sulpiride-Induced Hyperprolactinemia" Medicina 60, no. 6: 942. https://doi.org/10.3390/medicina60060942






