Pleomorphic Liposarcoma Unraveled: Investigating Histopathological and Immunohistochemical Markers for Tailored Diagnosis and Therapeutic Innovations
Abstract
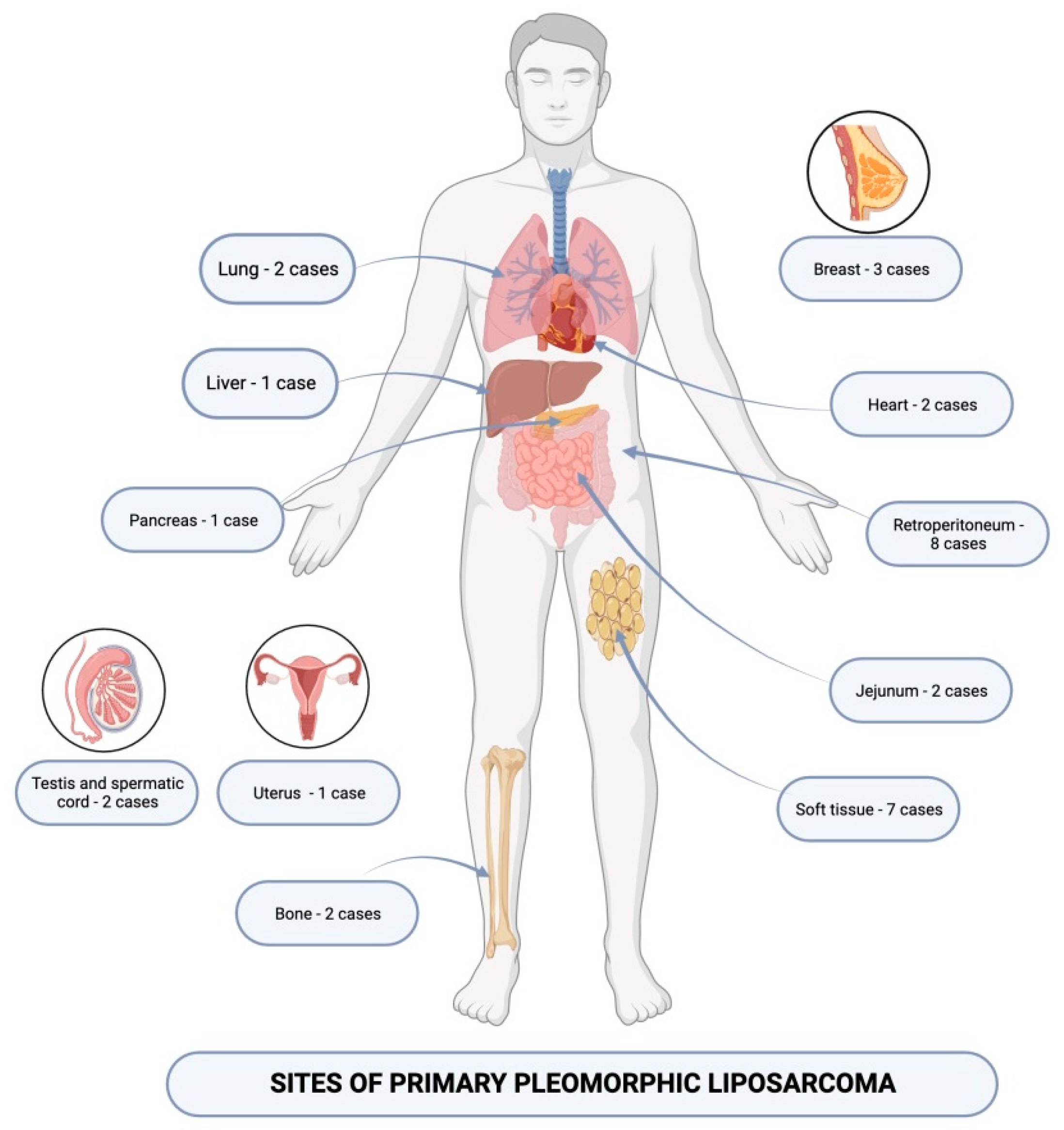
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Suarez-Kelly, L.P.; Baldi, G.G.; Gronchi, A. Pharmacotherapy for liposarcoma: Current state of the art and emerging systemic treatments. Expert Opin. Pharmacother. 2019, 20, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Machhada, A.; Emam, A.; Colavitti, G.; Maggiani, F.; Coelho, J.A.; Ayre, G.; Mahrous, A.M.; Khundkar, R.; Wright, T.C.; Wilson, P. Liposarcoma subtype recurrence and survival: A UK regional cohort study. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, F.E.; Wilding, C.; Jones, R.L.; Huang, P. Pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Expert Opin. Investig. Drugs 2019, 28, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Jones, R.L.; Noujaim, J.; Zaidi, S.; Miah, A.B.; Fisher, C. Dedifferentiated Liposarcoma: Updates on Morphology, Genetics, and Therapeutic Strategies. Adv. Anat. Pathol. 2016, 23, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Warnick, J.; Lahiri, R.; Karanjia, N.; Fisher, C.; Bagwan, I. Well Differentiated Liposarcoma Presenting as a Duodenal Polyp: A Case Report and Review of the Literature. Int. J. Surg. Pathol. 2023, 31, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Lucas, D.R. Dedifferentiated Liposarcoma with Myofibroblastic Differentiation. Arch. Pathol. Lab. Med. 2018, 142, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Mashima, E.; Sawada, Y.; Saito-Sasaki, N.; Yamamoto, K.; Ohmori, S.; Omoto, D.; Yoshioka, H.; Yoshioka, M.; Okada, E.; Aoki, T.; et al. A Retrospective Study of Superficial Type Atypical Lipomatous Tumor. Front. Med. 2020, 7, 609515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Briski, L.M.; Jorns, J.M. Primary Breast Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma and Dedifferentiated Liposarcoma. Arch. Pathol. Lab. Med. 2018, 142, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.T.J.; Thway, K.; Huang, P.H.; Jones, R.L. Clinical and Molecular Spectrum of Liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, J.; Wood, D.; Ingley, E.; Koks, S.; Wong, D. Update on genomic and molecular landscapes of well-differentiated liposarcoma and dedifferentiated liposarcoma. Mol. Biol. Rep. 2021, 48, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Abdul Razak, A.R.; Bauer, S.; Suarez, C.; Lin, C.C.; Quek, R.; Hütter-Krönke, M.L.; Cubedo, R.; Ferretti, S.; Guerreiro, N.; Jullion, A.; et al. Co-Targeting of MDM2 and CDK4/6 with Siremadlin and Ribociclib for the Treatment of Patients with Well-Differentiated or Dedifferentiated Liposarcoma: Results from a Proof-of-Concept, Phase Ib Study. Clin. Cancer Res. 2022, 28, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Crago, A.M.; Dickson, M.A. Liposarcoma: Multimodality Management and Future Targeted Therapies. Surg. Oncol. Clin. N. Am. 2016, 25, 761–773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narla, S.L.; Stephen, P.; Kurian, A.; Annapurneswari, S. Well-differentiated liposarcoma of the breast arising in a background of malignant phyllodes tumor in a pregnant woman: A rare case report and review of literature. Indian J. Pathol. Microbiol. 2018, 61, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, J.; Wei, S.; Wang, D.; Brandwein, M. Well-Differentiated Laryngeal/Hypopharyngeal Liposarcoma in the MDM2 Era Report of Three Cases and Literature Review. Head Neck Pathol. 2017, 11, 146–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eyermann, C.; Raguin, T.; Hemar, P.; Debry, C. Well-differentiated, pedunculated liposarcoma of the hypopharynx. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.C.; Yuen, H.K.L.; Cheuk, W. Primary Well-Differentiated Liposarcoma of the Orbit. Int. J. Surg. Pathol. 2021, 29, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Liu, Q.Y.; Gou, X.N.; Zhao, Y.W.; Cheng, Q.; Kong, L.F. [Well-differentiated/dedifferentiated liposarcoma associated with myxoid-like morphology: A clinicopathological and molecular genetic characteristics analysis of 34 cases]. Zhonghua Bing Li Xue Za Zhi 2024, 53, 168–173. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Stock, N. Tumeurs adipeuses [Adipocytic tumors]. Ann. Pathol. 2015, 35, 41–53. (In French) [Google Scholar] [CrossRef] [PubMed]
- Kammerer-Jacquet, S.F.; Thierry, S.; Cabillic, F.; Lannes, M.; Burtin, F.; Henno, S.; Dugay, F.; Bouzillé, G.; Rioux-Leclercq, N.; Belaud-Rotureau, M.A.; et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: Utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum. Pathol. 2017, 59, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ciongariu, A.M.; Dumitru, A.V.; Cîrstoiu, C.; Crețu, B.; Sajin, M.; Țăpoi, D.A.; Ciobănoiu, A.D.; Bejenariu, A.; Marin, A.; Costache, M. The Conundrum of Dedifferentiation in a Liposarcoma at a Peculiar Location: A Case Report and Literature Review. Medicina 2023, 59, 967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traweek, R.S.; Cope, B.M.; Roland, C.L.; Keung, E.Z.; Nassif, E.F.; Erstad, D.J. Targeting the MDM2-p53 pathway in dedifferentiated liposarcoma. Front. Oncol. 2022, 12, 1006959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bill, K.L.; Casadei, L.; Prudner, B.C.; Iwenofu, H.; Strohecker, A.M.; Pollock, R.E. Liposarcoma: Molecular targets and therapeutic implications. Cell. Mol. Life Sci. 2016, 73, 3711–3718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dubois-Silva, A.; Barbagelata-Lopez, C. Retroperitoneal dedifferentiated liposarcoma. Intern. Emerg. Med. 2019, 14, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, S.P.; Gabriel, E.; Mann, G.N. Multiply recurrent retroperitoneal liposarcoma. J. Surg. Oncol. 2018, 117, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, S.E. Dedifferentiated Liposarcoma: A Comprehensive Historical Review with Proposed Evidence-based Guidelines Regarding a Diagnosis in Need of Further Clarification. Adv. Anat. Pathol. 2021, 28, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Kito, M.; Yoshimura, Y.; Isobe, K.; Aoki, K.; Suzuki, S.; Tanaka, A.; Okamoto, M.; Sano, K.; Kato, H. Clinical outcome of dedifferentiated liposarcoma in the extremities: A retrospective case series of 7 patients. J. Orthop. Sci. 2016, 21, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.H.; Liu, W.F.; Ding, Y.; Li, L.; Zhang, M.; Sun, X.Q.; Huang, X.Y. [Dedifferentiated liposarcoma of extremities: A clinicopathologic analysis]. Zhonghua Bing Li Xue Za Zhi 2018, 47, 511–516. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Sengul, D.; Sengul, I.; Ustun, H. Dedifferentiated Liposarcoma of the Left Thigh: A Rare Case. Med. Arch. 2019, 73, 121–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Child, J.R.; Young, C.R.; Amini, B. Liposarcoma of the thigh with mixed calcification and ossification. Radiol. Case Rep. 2016, 11, 217–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakata, E.; Kunisada, T.; Hasei, J.; Nakahara, R.; Yanai, H.; Toji, T.; Inoue Ct, H.; Ozaki, T. What Are the Results of Resection of Localized Dedifferentiated Liposarcomas in the Extremities? Clin. Orthop. Relat. Res. 2020, 478, 2550–2561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scapa, J.V.; Cloutier, J.M.; Raghavan, S.S.; Peters-Schulze, G.; Varma, S.; Charville, G.W. DDIT3 Immunohistochemistry Is a Useful Tool for the Diagnosis of Myxoid Liposarcoma. Am. J. Surg. Pathol. 2021, 45, 230–239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mujtaba, B.; Wang, F.; Taher, A.; Aslam, R.; Madewell, J.E.; Nassar, S. Myxoid Liposarcoma with Skeletal Metastases: Pathophysiology and Imaging Characteristics. Curr. Probl. Diagn. Radiol. 2021, 50, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hindi, N.; Haas, R.L. Management of Synovial Sarcoma and Myxoid Liposarcoma. Surg. Oncol. Clin. N. Am. 2022, 31, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.P. Myxoid Liposarcoma: How to Stage and Follow. Curr. Treat. Options Oncol. 2023, 24, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.E.; Colborne, S.; Hughes, C.S.; Morin, G.B.; Nielsen, T.O. The FUS-DDIT3 Interactome in Myxoid Liposarcoma. Neoplasia 2019, 21, 740–751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dürr, H.R.; Rauh, J.; Baur-Melnyk, A.; Knösel, T.; Lindner, L.; Roeder, F.; Jansson, V.; Klein, A. Myxoid liposarcoma: Local relapse and metastatic pattern in 43 patients. BMC Cancer 2018, 18, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Redroban, L.; Montalvo, N. Vulvar Myxoid Liposarcoma, an Extremely Rare Diagnosis: A Case Report and Review of Literature. Int. J. Gynecol. Pathol. 2019, 38, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Zullow, H.J.; Sankar, A.; Ingram, D.R.; Samé Guerra, D.D.; D’Avino, A.R.; Collings, C.K.; Lazcano, R.; Wang, W.L.; Liang, Y.; Qi, J.; et al. The FUS::DDIT3 fusion oncoprotein inhibits BAF complex targeting and activity in myxoid liposarcoma. Mol. Cell 2022, 82, 1737–1750.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dolatabadi, S.; Jonasson, E.; Andersson, L.; Luna Santamaría, M.; Lindén, M.; Österlund, T.; Åman, P.; Ståhlberg, A. FUS-DDIT3 Fusion Oncoprotein Expression Affects JAK-STAT Signaling in Myxoid Liposarcoma. Front. Oncol. 2022, 12, 816894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mantilla, J.G.; Ricciotti, R.W.; Chen, E.Y.; Liu, Y.J.; Hoch, B.L. Amplification of DNA damage-inducible transcript 3 (DDIT3) is associated with myxoid liposarcoma-like morphology and homologous lipoblastic differentiation in dedifferentiated liposarcoma. Mod. Pathol. 2019, 32, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Hasegawa, T. Practical use and utility of fluorescence in situ hybridization in the pathological diagnosis of soft tissue and bone tumors. J. Orthop. Sci. 2017, 22, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Lansu, J.; Bovée, J.V.M.G.; Braam, P.; van Boven, H.; Flucke, U.; Bonenkamp, J.J.; Miah, A.B.; Zaidi, S.H.; Thway, K.; Bruland, Ø.S.; et al. Dose Reduction of Preoperative Radiotherapy in Myxoid Liposarcoma: A Nonrandomized Controlled Trial. JAMA Oncol. 2021, 7, e205865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schöffski, P. Established and Experimental Systemic Treatment Options for Advanced Liposarcoma. Oncol. Res. Treat. 2022, 45, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Hadjimichael, A.C.; Bekos, A.; Tsukamoto, S.; Nitta, Y.; Righi, A.; Errani, C.; Mavrogenis, A.F. Pleomorphic Liposarcoma Revisited. Orthopedics 2023, 46, e72–e80. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.J.; Jo, V.Y. Pleomorphic liposarcoma: Updates and current differential diagnosis. Semin. Diagn. Pathol. 2019, 36, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Creytens, D.; Folpe, A.L.; Koelsche, C.; Mentzel, T.; Ferdinande, L.; van Gorp, J.M.; Van der Linden, M.; Raman, L.; Menten, B.; Fritchie, K.; et al. Myxoid pleomorphic liposarcoma-a clinicopathologic, immunohistochemical, molecular genetic and epigenetic study of 12 cases, suggesting a possible relationship with conventional pleomorphic liposarcoma. Mod. Pathol. 2021, 34, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Al Kindi, A.H.; Al Kindi, F.A.; Al Riyami, M.; Khalil, E. Giant Mediastinal Myxoid Pleomorphic Liposarcoma. Sultan Qaboos Univ. Med. J. 2023, 23, 271–273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pink, D.; West, A.; Andreou, D. Systemische Therapieoptionen bei lokal fortgeschrittenen und metastasierten myxoiden, dedifferenzierten und pleomorphen Liposarkomen [Systemic Therapy Options for Locally Advanced and Metastatic Myxoid, Dedifferentiated and Pleomorphic Liposarcoma]. Zentralblatt Chir. 2020, 145, 160–167. (In Germany) [Google Scholar] [CrossRef] [PubMed]
- Wakely, P.E., Jr.; Wangsiricharoen, S.; Ali, S.Z. Pleomorphic liposarcoma: A clinicopathologic study of 20 FNA cases. Cancer Cytopathol. 2022, 130, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Tyler, R.; Wanigasooriya, K.; Taniere, P.; Almond, M.; Ford, S.; Desai, A.; Beggs, A. A review of retroperitoneal liposarcoma genomics. Cancer Treat. Rev. 2020, 86, 102013. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, J.S.; Dickson, M.A.; Schwartz, G.K.; Le Cesne, A.; Varga, A.; Bahleda, R.; Wagner, A.J.; Choy, E.; de Jonge, M.J.; et al. TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma. Nat. Commun. 2016, 7, 12609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Luo, R.; Xiong, Z.; Xu, J.; Fang, D. Pleomorphic liposarcoma: An analysis of 6 case reports and literature review. Medicine 2018, 97, e9986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, J.; Kadakia, S.; Agaimy, A.; Ogadzanov, A.; Khorsandi, A.; Chai, R.L. Pleomorphic liposarcoma of the head and neck: Presentation of two cases and literature review. Am. J. Otolaryngol. 2017, 38, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Halevi, P.D.; Ramirez-de-Noriega, F.; Fellig, Y.; Gomori, J.M.; Cohen, J.E.; Itshayek, E. Primary pleomorphic liposarcoma of the thoracic epidural space: Case report. Spine J. 2015, 15, e71–e75. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L. Subclassification of pleomorphic sarcomas: How and why should we care? Ann. Diagn. Pathol. 2018, 37, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, M.; Jnawali, A.; Yang, M. Rare Pleomorphic Liposarcoma Presented as Jejunal Obstruction. Case Rep. Pathol. 2023, 2023, 8040232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spałek, M.J.; Czarnecka, A.M.; Rutkowski, P. The Management of Radiation-Induced Sarcomas: A Cohort Analysis from a Sarcoma Tertiary Center. J. Clin. Med. 2021, 10, 694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gami, S.; Tiwari, S.B.; Gautam, K.; Sharma, S.; Shrivastav, S.; Sapkota, R. A rare case of myxoid pleomorphic liposarcoma in an infant: A report. Int. J. Surg. Case Rep. 2021, 87, 106365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Z.L.; Yong, M.H.; Tiu, L.A.; Dolendo, M.; Mok, Y. Myxoid pleomorphic liposarcoma of the orbit: Intratumoural genetic similarities and heterogeneity. Pathology 2023, 56, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Zare, S.Y.; Leivo, M.; Fadare, O. Recurrent Pleomorphic Myxoid Liposarcoma in a Patient with Li-Fraumeni Syndrome. Int. J. Surg. Pathol. 2020, 28, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Francom, C.R.; Leoniak, S.M.; Lovell, M.A.; Herrmann, B.W. Head and neck pleomorphic myxoid liposarcoma in a child with Li-Fraumeni syndrome. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.J.; Thorson, C.M.; Alvarez, E.; Tan, S.; Spunt, S.L.; Chao, S.D. Pleomorphic myxoid liposarcoma in an adolescent with Li-Fraumeni syndrome. Pediatr. Surg. Int. 2017, 33, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.D.; Schneider, K.W.; Suttman, A.; Garrington, T.; Jellins, T.; Tholen, K.; Francom, C.R.; Herrmann, B.W. Pediatric Head and Neck Tumors Associated with Li-Fraumeni Syndrome. Ann. Otol. Rhinol. Laryngol. 2021, 131, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, C.; Li, L. Simultaneous pulmonary and inferior vena cava tumor thromboembolism secondary to retroperitoneal pleomorphic liposarcoma. Hell. J. Nucl. Med. 2018, 21, 74–76. [Google Scholar] [CrossRef] [PubMed]
- El Haq, F.; Pramod, S.V.; Safriadi, F.; Hernowo, B.S. Pleomorphic retroperitoneal liposarcoma with kidney infiltration mimicking renal trauma. Urol. Case Rep. 2021, 38, 101647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciliberti, V.; Cretella, P.; Zeppa, P.; Caputo, A. Pleomorphic liposarcoma with liver metastasis diagnosed by combined fine-needle aspiration cytology and core-needle biopsy. Diagn. Cytopathol. 2022, 50, E28–E31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Liu, W.; Wang, B.; Zhu, N.; Guo, X.; Wang, Z.; Zhuang, R.; You, Y.; Zhang, Y.; Tong, H.; et al. Case report: Pathological complete response to perioperative treatment of radiotherapy combined with angiogenesis inhibitor in a patient with pleomorphic liposarcoma. Front. Oncol. 2023, 13, 925233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebey, B.N.; Naouar, S.; Faidi, B.; Lahouar, R.; Ben Khalifa, B.; El Kamel, R. Pleomorphic spermatic cord liposarcoma: A case report and review of management. Int. J. Surg. Case Rep. 2021, 81, 105725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jadhav, K.; Venkateswaran, R.; Landge, R.; Kadam, A.; Rashid, R. A Para-Scrotal Pleomorphic Liposarcoma Mimicking as Another Scrotum (Pseudo-Scrotum): A Case Report. Cureus 2023, 15, e46569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, B.; Xin, Z.; Li, Z.; Zhang, X. Giant pulmonary pleomorphic liposarcoma: A case report and literature review. Asian J. Surg. 2023, 46, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.; Khosla, D.; Kumar, D.; Chatterjee, D.; Madan, R.; Singh, H.; Singh, H.; Kapoor, R. Rare Case of Primary Pulmonary Pleomorphic Liposarcoma Treated with Multimodal Therapy. Ochsner J. 2021, 21, 431–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burt, J.R.; Limback, J.; Molina, M.; Suarez, J.; Mekhail, T.; Fanaian, N.; Aquino, G.; Kabakus, I.; Weyant, A.; Scherer, K. Fat-Finding Mission: Primary Pleomorphic Liposarcoma of the Heart and Pericardium. JACC Case Rep. 2020, 2, 1520–1526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, N.Y.; Najam, M.; Lyle, M.A.; Maleszewski, J.J.; Collins, J.D.; Klarich, K.W. Dramatic Presentation of Cardiac Pleomorphic Liposarcoma. Circ. Cardiovasc. Imaging 2021, 14, e012620. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Jung, H.K.; Nam, K.H. Recurrent Primary Pleomorphic Liposarcoma of the Breast: A Case Report with Imaging Findings. J. Breast Cancer 2020, 23, 567–573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, B.K.; Pol, M.M.; GA, T.; Barwad, A.W. Pleomorphic liposarcoma of the male breast: Lessons from a rare malignancy during COVID-19 pandemic. BMJ Case Rep. 2021, 14, e244056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yue, C.; Wang, A.; Hu, W.M.; Lu, H.M. Primary pleomorphic liposarcoma of the pancreas: A case report. Asian J. Surg. 2023, 46, 1902–1903. [Google Scholar] [CrossRef] [PubMed]
- Meijide Santos, G.; Poch Arenas, M.; Grau Polo, C.J.; Rodríguez Aguilar, R. Liposarcoma pleomórfico originado en una localización inusual. Presentación de caso [Pleomorphic liposarcoma arising in an unusual site. A case report]. Rev. Esp. Patol. 2022, 55, 249–253. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Valenciaga, A.; Iwenofu, O.H.; Tinoco, G. Larotrectinib in a Patient with Advanced Pleomorphic Liposarcoma of the Uterus. J. Natl. Compr. Cancer Netw. 2021, 19, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Tiemeier, G.L.; Brown, J.M.; Pratap, S.E.; McCarthy, C.; Kastrenopoulou, A.; Bradley, K.; Wilson, S.; Orosz, Z.; Gibbons, C.L.M.H.; Oppermann, U.; et al. Pleomorphic liposarcoma of bone: A rare primary malignant bone tumour. Clin. Sarcoma Res. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farah, S.; Haythem, M.; Ameni, A.; Samia, H.; Slim, H.; Mahmoud, S. Primary pleomorphic liposarcoma of bone: A case report with literature review. Int. J. Surg. Case Rep. 2023, 109, 108584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abe, M.; Hoshi, N.; Hoshi, S.; Hirabayashi, K.; Kikuta, K.; Hirozane, T.; Nakagawa, R.; Mizuno, T.; Nakamura, H.; Inoue, K.; et al. A Case of GATA3 Positive Pleomorphic Liposarcoma, Epithelioid Variant: A Diagnostic Pitfall. Case Rep. Pathol. 2023, 2023, 9443027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piplani, G.; Bhat, S.P.; Teerthanath, S.; Vishwanath, K.; Lobo, L.; Sajitha, K. Pleomorphic Liposarcoma of Finger-a Rare Entity. Indian J. Surg. Oncol. 2019, 10, 699–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nautiyal, H.; Egiz, A.; Farajzadeh Asl, S.; Fazail, A.H.; Nautiyal, S. Epithelioid Variant of Pleomorphic Liposarcoma: A Rare Challenging Diagnosis Causing Severe Medial Thigh Pain. Cureus 2021, 13, e19531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Histopathology | Immunophenotype | Treatment | |
|---|---|---|---|
| Well-Differentiated Liposarcoma | Lipogenic component with mature adipocytes of variable size Atypical spindle stromal cells | MDM2+, CDK+ DDIT3− S100+ CD34− p16+ p53 wildtype | Surgical resection with negative margins |
| Dedifferentiated Liposarcoma | Lipogenic component with an abrupt transition toward a non-lipogenic tumor area Necrosis [+/−] | MDM2+, CDK4+ DDIT3− S100+ CD34− p16+ p53 wildtype or mutant | Surgical resection Neoadjuvant radiotherapy Chemotherapy/targeted therapy |
| Myxoid Liposarcoma | Lipogenic areas Loose basophilic/myxoid stroma Necrosis [+/−] Atypical lipoblasts [+/−] Round cell component [+/−] | MDM2−, CDK4− DDIT3+ S100+ CD34− p16− p53 wildtype | Surgical resection Neoadjuvant or adjuvant radiotherapy Chemotherapy/targeted therapies |
| Pleomorphic Liposarcoma | High-grade undifferentiated sarcoma Pleomorphic lipoblasts Necrosis [+/−] | MDM2−, CDK4− DDIT3− S100+ CD34+ p16+ p53 mutant [hyperexpression] | Surgical resection or amputation Post-operative radiotherapy Chemotherapy |
| Myxoid Pleomorphic Liposarcoma | Myxoid liposarcoma-like areas Pleomorphic liposarcoma-like areas | MDM2−, CDK4− CD34+ p16+ p53 mutant [hyperexpression] Rb loss | Surgical resection with negative margins Benefits of radiotherapy and chemotherapy not well-established |
| S100 | MDM2 | CD 34 | SMA | P53 | P16 | |
|---|---|---|---|---|---|---|
| Abdominal cavity | ||||||
| Wang et al. [6] | + | + | - | |||
| Soft tissue | ||||||
| Abe M. [82] | + | - | - | |||
| Zhang C. [68] | + | |||||
| Piplani [83] | + | - | ||||
| Nautiyal [84] | + | |||||
| Pulmonary | ||||||
| Li B. [71] | + | + | - | Hyper-expression/Mutant | + | |
| Dey T. [72] | + | - | Hyper-expression/Mutant | |||
| Digestive tract | ||||||
| Yue [77] | + | + | - | + | Hyper-expression/Mutant | + |
| Meijide Santos G. [78] | + | - | - | |||
| Al Attar M. [56] | - | - | - | - | Hyper-expression/Mutant | |
| Bone | ||||||
| Farah S. [81] | + | - | - | - | ||
| Tiemeier [80] | - | - | ||||
| Cardiac | ||||||
| Tan NY. [74] | + | Hyper-expression/Mutant | ||||
| Burt JR. [73] | + | Hyper-expression/Mutant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciongariu, A.-M.; Țăpoi, D.-A.; Dumitru, A.-V.; Bejenariu, A.; Marin, A.; Costache, M. Pleomorphic Liposarcoma Unraveled: Investigating Histopathological and Immunohistochemical Markers for Tailored Diagnosis and Therapeutic Innovations. Medicina 2024, 60, 950. https://doi.org/10.3390/medicina60060950
Ciongariu A-M, Țăpoi D-A, Dumitru A-V, Bejenariu A, Marin A, Costache M. Pleomorphic Liposarcoma Unraveled: Investigating Histopathological and Immunohistochemical Markers for Tailored Diagnosis and Therapeutic Innovations. Medicina. 2024; 60(6):950. https://doi.org/10.3390/medicina60060950
Chicago/Turabian StyleCiongariu, Ana-Maria, Dana-Antonia Țăpoi, Adrian-Vasile Dumitru, Adrian Bejenariu, Andrei Marin, and Mariana Costache. 2024. "Pleomorphic Liposarcoma Unraveled: Investigating Histopathological and Immunohistochemical Markers for Tailored Diagnosis and Therapeutic Innovations" Medicina 60, no. 6: 950. https://doi.org/10.3390/medicina60060950
APA StyleCiongariu, A.-M., Țăpoi, D.-A., Dumitru, A.-V., Bejenariu, A., Marin, A., & Costache, M. (2024). Pleomorphic Liposarcoma Unraveled: Investigating Histopathological and Immunohistochemical Markers for Tailored Diagnosis and Therapeutic Innovations. Medicina, 60(6), 950. https://doi.org/10.3390/medicina60060950







