Intensive Intervention on Smoking Cessation in Patients Undergoing Elective Surgery: The Role of Family Physicians
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Ethical Considerations
2.3. Study Design
- F-0: Randomisation phase—four weeks before surgery;
- F-1: From three to seven days before surgery;
- F-2: Forty days after surgery;
- F-3: Four months after surgery;
- F-4: Six months after surgery;
- F-5: Follow-up was conducted 12 months after surgery.
2.4. Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Global Report on Trends in Prevalence of Tobacco Use 2000-2025; World Health Organization: Geneva, Switzerland, 2021; pp. 1–150. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 11 January 2024).
- Domic, A.; Tomasovic, I.; Bernaciak, P.; Voronov, G.; Igic, R.; Skrbic, R. Role of Physicians in Eliminating Risk of Cancer Caused by Combustible Tobacco Smoke. Med. Pregl. 2022, 75, 363–369. [Google Scholar] [CrossRef]
- Babb, S.; Malarcher, A.; Schauer, G.; Asman, K.; Jamal, A. Quitting Smoking Among Adults—United States, 2000–2015. Morb. Mortal. Wkly. Rep. 2017, 65, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Micic, L.; Vukojevic, B.; Pepic, A.; Preradovic, D.; Gligoric, D. Tobacco Consumption in Bosnia and Herzegovina, 2019; University of Banja Luka: Banja Luka, Bosnia and Herzegovina, 2020; Available online: https://www.tobacconomics.org/files/research/642/209-bih-report-v3.pdf (accessed on 11 January 2024).
- Widysanto, A.; Combest, F.; Dhakal, A.; Saadabadi, A. Nicotine Addiction. [Updated 8 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, January 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499915/ (accessed on 11 January 2024).
- Fan Chiang, Y.; Lee, Y.; Lam, F.; Liao, C.; Chang, C.; Lin, C. Smoking Increases the Risk of Postoperative Wound Complications: A Propensity Score-matched Cohort Study. Int. Wound J. 2023, 20, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Gavilan, E.; Fernández, E.; Minguell, J.; Trilla, E.; Zuriguel-Pérez, E.; Martínez, C. Efficacy of Presurgical Interventions to Promote Smoking Cessation: A Systematic Review. Anesth. Analg. 2023, 136, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; ElNemer, W.; Cartagena-Reyes, M.A.; Marrache, M.; Thompson, J.M.; Aiyer, A.A. The Cost-Effectiveness of Smoking Cessation Programs for Prevention of Wound Complications Following Total Ankle Arthroplasty: A Break-Even Analysis. Foot Ankle Orthop. 2024, 9, 24730114241239315. [Google Scholar] [CrossRef] [PubMed]
- Lindström, D.; Azodi, O.S.; Wladis, A.; Tønnesen, H.; Linder, S.; Nåsell, H.; Ponzer, S.; Adami, J. Effects of a Perioperative Smoking Cessation Intervention on Postoperative Complications: A Randomized Trial. Ann. Surg. 2008, 248, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Eyawo, O.; Lockhart, I.; Kelly, S.; Wu, P.; Ebbert, J.O. Smoking Cessation Reduces Postoperative Complications: A Systematic Review and Meta-Analysis. Am. J. Med. 2011, 124, 144–154.e8. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Tursan d’Espaignet, E.; Wiggers, J.; Claire, S.; Mellin-Olsen, J.; Grady, A. Tobacco and Postsurgical Outcomes: WHO Tobacco Knowledge Summaries; World Health Organization: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/330485/9789240000360-eng.pdf (accessed on 5 February 2024).
- Musallam, K.M.; Rosendaal, F.R.; Zaatari, G.; Soweid, A.; Hoballah, J.J.; Sfeir, P.M.; Zeineldine, S.; Tamim, H.M.; Richards, T.; Spahn, D.R.; et al. Smoking and the Risk of Mortality and Vascular and Respiratory Events in Patients Undergoing Major Surgery. JAMA Surg. 2013, 148, 755. [Google Scholar] [CrossRef] [PubMed]
- Harrogate, S.; Barnes, J.; Thomas, K.; Isted, A.; Kunst, G.; Gupta, S.; Rudd, S.; Banerjee, T.; Hinchliffe, R.; Mouton, R. Peri-operative Tobacco Cessation Interventions: A Systematic Review and Meta-analysis. Anaesthesia 2023, 78, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.; Ferguson, S.G.; Nash, R. Barriers to Smoking Interventions in Community Healthcare Settings: A Scoping Review. Health Promot. Int. 2024, 39, daae036. [Google Scholar] [CrossRef] [PubMed]
- Kastaun, S.; Leve, V.; Hildebrandt, J.; Funke, C.; Klosterhalfen, S.; Lubisch, D.; Reddemann, O.; McRobbie, H.; Raupach, T.; West, R.; et al. Training General Practitioners in the ABC versus 5As Method of Delivering Stop-Smoking Advice: A Pragmatic, Two-Arm Cluster Randomised Controlled Trial. ERJ Open Res. 2021, 7, 00621–2020. [Google Scholar] [CrossRef] [PubMed]
- Aslan, D.; Gurbay, A.; Hayran, M.; Sengelen, M.; Pasli, D.; Huseyin, B. Carbon Monoxide in the Expired Air and Urinary Cotinine Levels of E-Cigarette Users. Turk. Thorac. J. 2019, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K. The Fagerström Test for Nicotine Dependence: A Revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Cirino, P.T.; Chin, C.E.; Sevcik, R.A.; Wolf, M.; Lovett, M.; Morris, R.D. Measuring Socioeconomic Status: Reliability and Preliminary Validity for Different Approaches. Assessment 2002, 9, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Berlin, N.L.; Cutter, C.; Battaglia, C. Will Preoperative Smoking Cessation Programs Generate Long-Term Cessation? A Systematic Review and Meta-Analysis. Am. J. Manag. Care 2015, 21, e623. [Google Scholar] [PubMed]
- Thomsen, T.; Tonnesen, H.; Okholm, M.; Kroman, N.; Maibom, A.; Sauerberg, M.-L.; Moller, A.M. Brief Smoking Cessation Intervention in Relation to Breast Cancer Surgery: A Randomized Controlled Trial. Nicotine Tob. Res. 2010, 12, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Sadr Azodi, O.; Lindström, D.; Adami, J.; Tønnesen, H.; Nåsell, H.; Gilljam, H.; Wladis, A. The Efficacy of a Smoking Cessation Programme in Patients Undergoing Elective Surgery—A Randomised Clinical Trial. Anaesthesia 2009, 64, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Villebro, N.M.; Pedersen, T.; Møller, A.M.; Tønnesen, H. Long-term Effects of a Preoperative Smoking Cessation Programme. Clin. Respir. J. 2008, 2, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, T.; Villebro, N.; Møller, A.M. Interventions for Preoperative Smoking Cessation. Cochrane Database Syst. Rev. 2014, 2014, CD002294. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Affentranger, A.; Cortonesi, B.; Jeker, U.; Gass, M.; Minervini, F.; Jung, G.; Christmann, C.; Brambs, C.; Puhan, M.A.; et al. Preoperative Smoking Cessation Program in Patients Undergoing Intermediate to High-Risk Surgery: A Randomized, Single-Blinded, Controlled, Superiority Trial. Trials 2022, 23, 717. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Landry, J.; Jones, P.M.; Buhrmann, O.; Morley-Forster, P. The Effectiveness of a Perioperative Smoking Cessation Program: A Randomized Clinical Trial. Anesth. Analg. 2013, 117, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Landry, J.; Jones, P.M.; Buhrmann, O.; Morley-Forster, P. Long-Term Quit Rates After a Perioperative Smoking Cessation Randomized Controlled Trial. Anesth. Analg. 2015, 120, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; de Voer, N.; Croes, E.; Brouwer, M. Outcomes of an Intensive Smoking Cessation Program Implemented in General Practices in the Netherlands. Tob. Prev. Cessat. 2022, 8, A29. [Google Scholar] [CrossRef]
- Lee, S.M. Perioperative Smoking Cessation Programs Should Be Standard-of-Care. Can. J. Anesth. Can. Anesth. 2019, 66, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Elmoghazy, E.; Mostafa, N.; Zaki, L.; Amin, W. Effect of Training and Smoking Status of Physicians on Smoking Cessation Practices in Egypt. Egypt. J. Chest Dis. Tuberc. 2018, 67, 323. [Google Scholar] [CrossRef]
- Buczkowski, K.; Marcinowicz, L.; Czachowski, S.; Piszczek, E.; Sowinska, A. “What Kind of General Practitioner Do I Need for Smoking Cessation?” Results from a Qualitative Study in Poland. BMC Fam. Pract. 2013, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Duaso, M.J.; McDermott, M.S.; Mujika, A.; Purssell, E.; While, A. Do Doctors’ Smoking Habits Influence Their Smoking Cessation Practices? A Systematic Review and Meta-analysis. Addiction 2014, 109, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Pipe, A.; Sorensen, M.; Reid, R. Physician Smoking Status, Attitudes toward Smoking, and Cessation Advice to Patients: An International Survey. Patient Educ. Couns. 2009, 74, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Al-Hagabani, M.; Khan, M.; Al-Hazmi, A.; Shaher, B.; El-Fahel, A. Smoking Behavior of Primary Care Physicians and Its Effect on Their Smoking Counseling Practice. J. Fam. Med. Prim. Care 2020, 9, 1053. [Google Scholar] [CrossRef]
- Todorović, I.; Cheng, F.; Stojisavljević, S.; Marinković, S.; Kremenović, S.; Savić, P.; Golić-Jelić, A.; Stojaković, N.; Stoisavljević-Šatara, S.; Igić, R.; et al. Prevalence of Cigarette Smoking and Influence of Associated Factors among Students of the University of Banja Luka: A Cross-Sectional Study. Medicina 2022, 58, 502. [Google Scholar] [CrossRef] [PubMed]
- Igić, R.; Bernaciak, P. Tobacco Smoking among Physicians and Medical Students. Scr. Med. 2022, 53, 77–81. [Google Scholar] [CrossRef]
- Prijić, Ž.; Igić, R. Cigarette Smoking and Medical Students. J. BUON 2021, 26, 1709–1718. [Google Scholar] [PubMed]
- Buczkowski, K.; Marcinowicz, L.; Czachowski, S.; Piszczek, E. Motivations toward Smoking Cessation, Reasons for Relapse, and Modes of Quitting: Results from a Qualitative Study among Former and Current Smokers. Patient Prefer. Adherence 2014, 8, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.M.; Lindson, N.; Farley, A.; Leinberger-Jabari, A.; Sawyer, K.; Te Water Naudé, R.; Theodoulou, A.; King, N.; Burke, C.; Aveyard, P. Smoking Cessation for Improving Mental Health. Cochrane Database Syst. Rev. 2021, 3, CD013522. [Google Scholar] [CrossRef] [PubMed]
- Stojaković, N.; Jonjev, Ž.; Igić, R. Smoking Abstinence in Patients Scheduled for Elective Surgery. Scr. Med. 2013, 44, 97–99. [Google Scholar] [CrossRef]
- Bosch-Capblanch, X.; Abba, K.; Prictor, M.; Garner, P. Contracts between Patients and Healthcare Practitioners for Improving Patients’ Adherence to Treatment, Prevention and Health Promotion Activities. Cochrane Database Syst. Rev. 2007, 2007, CD004808. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sane, N.; Anand, S.; Marimutthu, P.; Benegal, V. Assessment of Cotinine in Urine and Saliva of Smokers, Passive Smokers, and Nonsmokers: Method Validation Using Liquid Chromatography and Mass Spectrometry. Indian J. Psychiatry 2019, 61, 270. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Bernert, J.T.; Foulds, J.; Hecht, S.S.; Jacob, P.; Jarvis, M.J.; Joseph, A.; Oncken, C.; Piper, M.E. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob. Res. 2020, 22, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Wan Puteh, S.E.; Mohd Ismail, N.; Md Isa, Z.; Ban, A.Y.-L. Exhaled Carbon Monoxide Level and Practices among Tobacco and Nicotine Adult Users in Klang Valley, Malaysia. Int. J. Environ. Res. Public Health 2023, 20, 4443. [Google Scholar] [CrossRef] [PubMed]
- Herbeć, A.; Perski, O.; Shahab, L.; West, R. Smokers’ Views on Personal Carbon Monoxide Monitors, Associated Apps, and Their Use: An Interview and Think-Aloud Study. Int. J. Environ. Res. Public Health 2018, 15, 288. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.O.; Gans, S.P.; Ripley-Moffitt, C.; Kotsen, C.; Bars, M. Use of Expired Air Carbon Monoxide Testing in Clinical Tobacco Treatment Settings. Chest 2018, 153, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.C.; Byron, M.J.; Baig, S.A.; Stepanov, I.; Brewer, N.T. How People Think about the Chemicals in Cigarette Smoke: A Systematic Review. J. Behav. Med. 2017, 40, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Brenner, L.; Moser, J.; Trudzinski, F.; Köllner, V.; Bals, R. The Effects of a Short-Term Physician Training on Smoking Cessation in a University Pulmonary Department. Ger. Med. Sci. 2020, 18, Doc06. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Livingstone-Banks, J.; Ordóñez-Mena, J.M.; Fanshawe, T.R.; Lindson, N.; Freeman, S.C.; Sutton, A.J.; Theodoulou, A.; Aveyard, P. Behavioural Interventions for Smoking Cessation: An Overview and Network Meta-Analysis. Cochrane Database Syst. Rev. 2021, 1, CD013229. [Google Scholar] [CrossRef] [PubMed]
- Pipe, A.L.; Evans, W.; Papadakis, S. Smoking Cessation: Health System Challenges and Opportunities. Tob. Control. 2022, 31, 340–347. [Google Scholar] [CrossRef] [PubMed]
- United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Interventions for Smoking Cessation and Treatments for Nicotine Dependence. In Smoking Cessation: A Report of the Surgeon General; US Department of Health and Human Services: Washington, DC, USA, 2020. [Google Scholar]
- Scott-Sheldon, L.A.J.; Lantini, R.; Jennings, E.G.; Thind, H.; Rosen, R.K.; Salmoirago-Blotcher, E.; Bock, B.C. Text Messaging-Based Interventions for Smoking Cessation: A Systematic Review and Meta-Analysis. JMIR mHealth uHealth 2016, 4, e49. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y.; Dobson, R. Mobile Phone Text Messaging and App-Based Interventions for Smoking Cessation. Cochrane Database Syst. Rev. 2019, 10, CD006611. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Chepkin, S.C.; Ye, W.; Bullen, C.; Lancaster, T. Nicotine Replacement Therapy versus Control for Smoking Cessation. Cochrane Database Syst. Rev. 2018, 5, CD000146. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, S. The Use of Bupropion SR in Cigarette Smoking Cessation. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Puente, D.; Cabezas, C.; Rodriguez-Blanco, T.; Fernández-Alonso, C.; Cebrian, T.; Torrecilla, M.; Clemente, L.; Martín, C. The Role of Gender in a Smoking Cessation Intervention: A Cluster Randomized Clinical Trial. BMC Public Health 2011, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Chinwong, D.; Mookmanee, N.; Chongpornchai, J.; Chinwong, S. A Comparison of Gender Differences in Smoking Behaviors, Intention to Quit, and Nicotine Dependence among Thai University Students. J. Addict. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, R.; Bauld, L.; Amos, A.; Fidler, J.A.; Munafò, M. Socioeconomic Status and Smoking: A Review. Ann. N. Y. Acad. Sci. 2012, 1248, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.; Kendzor, D.; Roys, M.; Stewart, S.; Copeland, A. Financial Strain Mediates the Relationship Betweensocioeconomic Status and Smoking. Tob. Prev. Cessat. 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total N = 120 | IG N = 59 | CG N = 61 | p | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 62 (51.7) | 33 (55.9) | 29 (47.5) | 0.357 | |
| Female | 58 (48.3) | 26 (44.1) | 32 (52.5) | ||
| Age (years), mean (SD) | 48.2 (11.8) 21–65 | 46.9 (11.9) 21–65 | 49.4 (11.7) 25–65 | ||
| Marital status, n (%) | |||||
| Married | 93/119 (78.2) | 47/58 (81.0) | 46 (75.4) | 0.457 | |
| Single | 25/119 (21.0) | 11/58 (19.0) | 14 (23.0) | ||
| Widower/Widow | 1/119 (0.8) | 0/58 (0) | 1 (1.6) | ||
| Education, n (%) | |||||
| Elementary school | 10/119 (8.4) | 5/58 (8.6) | 5 (8.2) | ||
| Trade | 11/119 (9.2) | 7/58 (12.1) | 4 (6.6) | ||
| Secondary school | 59/119 (49.6) | 28/58 (48.3) | 31 (50.8) | ||
| School of higher education | 12/119 (10.1) | 5/58 (8.6) | 7 (11.5) | 0.905 | |
| College | 24/119 (20.2) | 12/58 (20.7) | 12 (19.7) | ||
| Postgraduate education | 3/119 (2.5) | 1/58 (1.7) | 2 (3.3) | ||
| SES, points | 34.2 (11.6) 11–63 | 33.8 (10.5) 17–62 | 34.6 (12.6) 11–63 | ||
| SES, n (%) | |||||
| Low (8–27) | 44/119 (37.0) | 21/58 (36.2) | 23 (37.7) | ||
| Medium (28–47) | 60/119 (50.4) | 30/58 (51.7) | 30 (49.2) | 0.959 | |
| High (48–66) | 15/119 (12.6) | 7/58 (12.1) | 8 (13.1) | ||
| Phases of Intervention | IG | CG | RR (95% CI) | p | ||
|---|---|---|---|---|---|---|
| n = 59 | n = 61 | |||||
| Smoking status 7 days before surgery | ||||||
| Abstinence FTND n (%) | ||||||
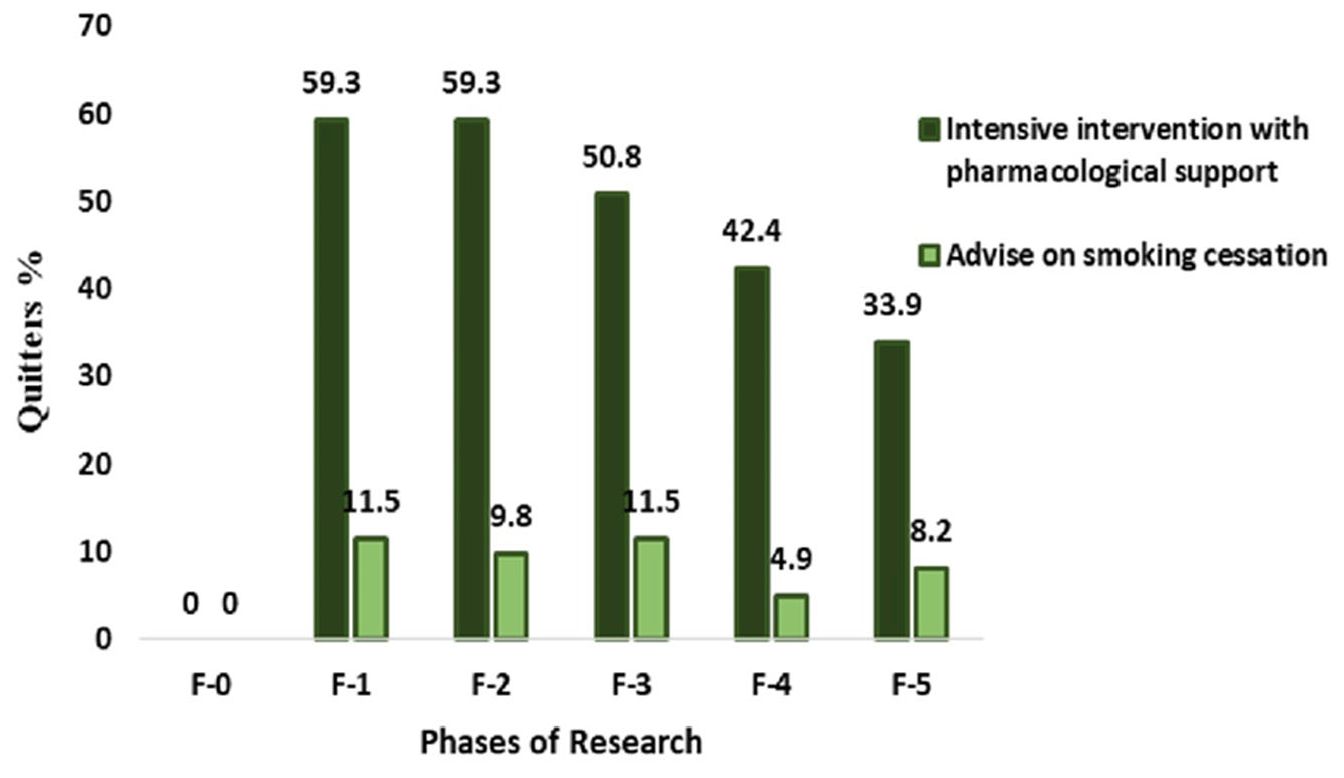
| Quitters | 35 (59.3) | 7 (11.5) | 5.17 (2.49–10.7) | 0.0001 | ||
| Abstinence biochemically validated * n (%) | ||||||
| Yes | 29 (49.2) | 5 (8.2) | 5.99 (2.48–14.44) | 0.0001 | ||
| Smoking status 40 days postoperatively | ||||||
| Abstinence FTND n (%) | ||||||
| Quitters | 35 (59.3) | 6 (9.8) | 6.03 (2.74–12.27) | 0.0001 | ||
| Abstinence biochemically validated * n (%) | ||||||
| Yes | 32 (54.2) | 4 (6.6) | 8.27 (3.11–21.94) | 0.0001 | ||
| Smoking status 4 months postoperatively | ||||||
| Abstinence FTND n (%) | ||||||
| Quitters | 30 (50.8) | 7 (11.5) | 4.43 (2.11–9.29) | 0.0001 | ||
| Abstinence biochemically validated * | ||||||
| Yes | 29 (49.2) | 5 (8.2) | 6.89 (2.90–16.37) | 0.0001 | ||
| Smoking status 6 months postoperatively | ||||||
| Abstinence FTND n (%) | ||||||
| Quitters | 25 (42.4) | 3 (4.9) | 8.61 (2.74–27.01) | 0.0002 | ||
| Abstinence biochemically validated * | ||||||
| Yes | 24 (40.7) | 4 (6.6) | 6.20 (2.29–16.79) | 0.0003 | ||
| Follow-up after 1 year | ||||||
| Abstinence FTND n (%) | ||||||
| Quitters | 20 (33.9) | 5 (8.2) | 4.13 (1.66–10.29) | 0.0023 | ||
| Abstinence biochemically validated * | ||||||
| Yes | 18 (30.5) | 3 (4.9) | 6.20 (1.92–19.96) | 0.0022 | ||
| pCQ(6) abstinence according FTND | <0.001 | 0.002 | ||||
| pCQ(5) abstinence according to FTND | 0.037 | 0.212 | ||||
| pCQ(6) * abstinence biochemically validated | <0.001 | 0.06 | ||||
| pCQ(5) * abstinence biochemically validated | 0.287 | 0.896 | ||||
| Study Phase | F-0 | F-1 | F-2 | F-3 | F-4 | F-5 (Follow-Up) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | IG | CG | IG | CG | IG | CG | IG | CG | IG | CG | IG | CG | ||
| FTND—points | ||||||||||||||
| p-value | p = 0.484 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | pFIG(5) < 0.001 pFCG(5) < 0.001 pFIG(4) = 0.068 pFCG(4) = 0.064 | ||||||||
| Mean (SD) | 5.58 (2.31) | 5.26 (2.24) | 1.29 (2.22) | 4.10 (2.56) | 1.00 (2.01) | 3.95 (2.48) | 1.10 (2.00) | 3.86 (2.43) | 1.27 (1.87) | 4.07 (2.21) | 2.0 (2.9) | 5.4 (2.1) | ||
| >4 n% | 48 (81.4) | 48 (78.6) | 7 (11.9) | 36 (59.0) | 5 (8.5) | 31 (50.8) | 5 (8.5) | 32 (52.5) | 5 (8.5) | 33 (54.1) | 22 (37.3) | 43 (70.5) | pFIG(6) < 0.001 pFCG(6) < 0.808 pFIG(5) = 0.527 pFCG(5) = 0.426 | |
| <4 n;% | 11 (18.6) | 13 (21.3) | 52 (88.1) | 23 (37.7) | 49 (83.1) | 25 (40.9) | 48 (81.4) | 24 (39.4) | 47 (79.6) | 23 (37.7) | 22 (37.3) | 5 (8.2) | ||
| Nicotine dependence n (%) | ||||||||||||||
| p-value | p = 0.984 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| High (8+ points) | 11 (18.6) | 10 (16.4) | 2 (3.4) | 5 (8.5) | 1 (1.9) | 5 (8.9) | 1 (1.9) | 4 (7.1) | 1 (1.9) | 3 (5.4) | 2 (3.4) | 2 (3.27) | pFIG(6) < 0.001 pFCG(6) < 0.001 pFIG(5) = 0.007 pFCG(5) = 0.123 | |
| Medium (5–7) | 28 (47.5) | 29 (47.5) | 3 (5.2) | 21 (35.6) | 3 (5.7) | 17 (30.4) | 3 (5.8) | 18 (32.1) | 3 (5.8) | 20 (35.7) | 14 (23.7) | 33 (54.1) | ||
| Moderate (3–4) | 15 (25.4) | 16 (26.2) | 5 (8.6) | 18 (30.5) | 3 (5.7) | 19 (33.9) | 3 (5.8) | 19 (33.9) | 4 (7.7) | 24 (42.9) | 8 (13.5) | 8 (13.1) | ||
| Low (1–2) | 5 (8.5) | 6 (9.8) | 13 (22.4) | 8 (13.6) | 11 (20.8) | 9 (16.1) | 15 (28.8) | 8 (14.3) | 19 (36.5) | 6 (10.7) | 2 (3.4) | 2 (3.3) | ||
| None (0) | 0 (0) | 0 (0) | 35 (60.3) | 7 (11.9) | 35 (66.0) | 6 (10.7) | 30 (57.7) | 7 (12.5) | 25 (48.1) | 3 (5.4) | 20 (33.9) | 4 (6.5) | ||
| Number of cigarettes smoked per day | ||||||||||||||
| Mean (SD) | 17.8 (7.2) | 17.0 (8.3) | 5.6 (8.2) | 13.3 (8.3) | 4.4 (7.5) | 13.0 (8.1) | 5.1 (7.5) | 13.0 (8.4) | 5.6 (6.6) | 14.2 (7.6) | 8.8 (8.7) | 15.3 (6.4) | ||
| p | 0.574 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domić, A.; Pilipović-Broćeta, N.; Grabež, M.; Divac, N.; Igić, R.; Škrbić, R. Intensive Intervention on Smoking Cessation in Patients Undergoing Elective Surgery: The Role of Family Physicians. Medicina 2024, 60, 965. https://doi.org/10.3390/medicina60060965
Domić A, Pilipović-Broćeta N, Grabež M, Divac N, Igić R, Škrbić R. Intensive Intervention on Smoking Cessation in Patients Undergoing Elective Surgery: The Role of Family Physicians. Medicina. 2024; 60(6):965. https://doi.org/10.3390/medicina60060965
Chicago/Turabian StyleDomić, Anto, Nataša Pilipović-Broćeta, Milkica Grabež, Nevena Divac, Rajko Igić, and Ranko Škrbić. 2024. "Intensive Intervention on Smoking Cessation in Patients Undergoing Elective Surgery: The Role of Family Physicians" Medicina 60, no. 6: 965. https://doi.org/10.3390/medicina60060965
APA StyleDomić, A., Pilipović-Broćeta, N., Grabež, M., Divac, N., Igić, R., & Škrbić, R. (2024). Intensive Intervention on Smoking Cessation in Patients Undergoing Elective Surgery: The Role of Family Physicians. Medicina, 60(6), 965. https://doi.org/10.3390/medicina60060965






