The Association of a Single Nucleotide Variant in COL5A1 to Early Onset Keratoconus and Pectus Excavatum—Convergence of Extracellular Matrix Pathologies
Abstract
:1. Introduction
1.1. Keratoconus
1.2. Pectus Excavatum
2. Case Description
3. Material and Methods
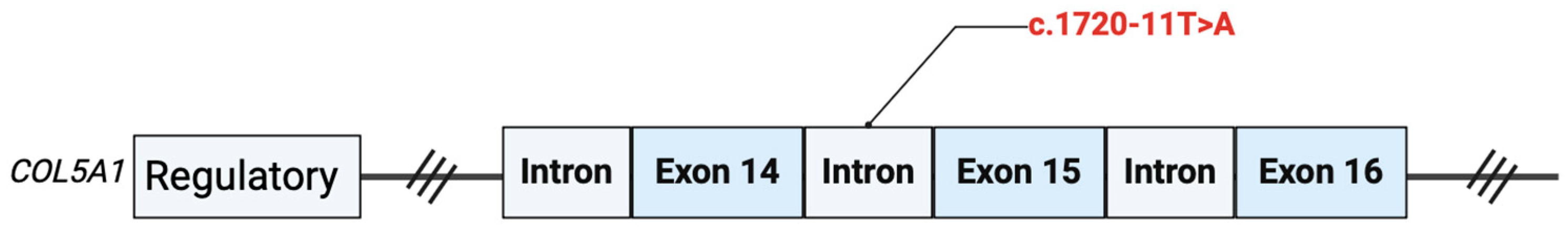
4. Genetic Results
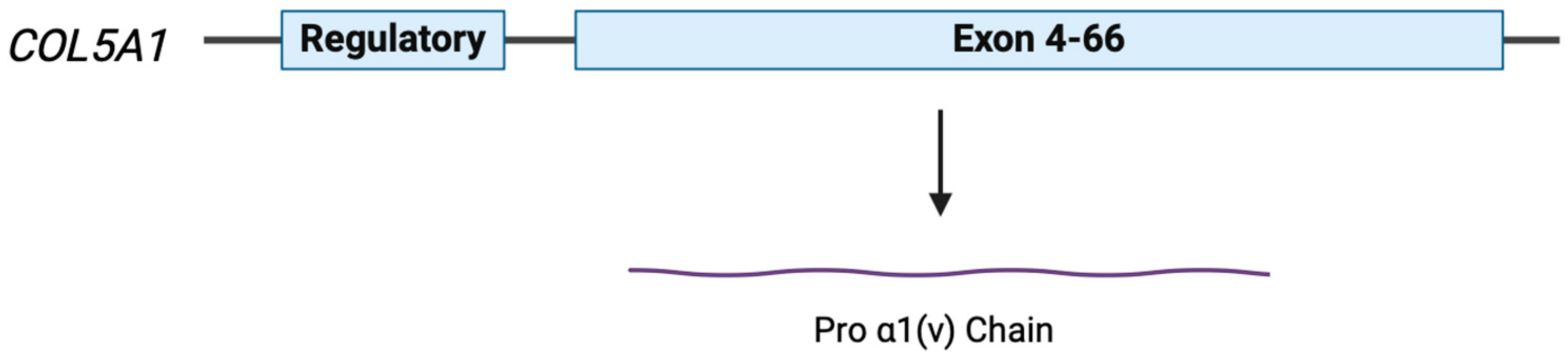
5. COL5A1
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.D.; Truong, A.; Pasricha, N.D.; Indaram, M. Keratoconus Diagnosis and Treatment: Recent Advances and Future Directions. Clin. Ophthalmol. 2023, 17, 2705–2718. [Google Scholar] [CrossRef] [PubMed]
- Espandar, L.; Meyer, J. Keratoconus: Overview and update on treatment. Middle East Afr. J. Ophthalmol. 2010, 17, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Rama, P. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul. Surf. 2020, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, Y.S.; Galvis, V.; Tello, A.; Rueda, D.; García, J.D. Genetics vs chronic corneal mechanical trauma in the etiology of keratoconus. Exp. Eye Res. 2021, 202, 108328. [Google Scholar] [CrossRef] [PubMed]
- Mazharian, A.; Panthier, C.; Courtin, R.; Jung, C.; Rampat, R.; Saad, A.; Gatinel, D. Incorrect sleeping position and eye rubbing in patients with unilateral or highly asymmetric keratoconus: A case-control study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Galvis, V.; Tello, A.; Barrera, R.; Niño, C.A. Inflammation in Keratoconus. Cornea 2015, 34, e22–e23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bykhovskaya, Y.; Canedo, A.L.C.; Haritunians, T.; Siscovick, D.; Aldave, A.J.; Szczotka-Flynn, L.; Iyengar, S.K.; Rotter, J.I.; Taylor, K.D.; et al. Genetic Association of COL5A1 Variants in Keratoconus Patients Suggests a Complex Connection between Corneal Thinning and Keratoconus. Investig. Opthalmol. Vis. Sci. 2013, 54, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Kundu, G.; Shetty, N.; Shetty, R.; Khamar, P.; D’souza, S.; Meda, T.R.; Nuijts, R.M.M.A.; Narasimhan, R.; Roy, A.S. Artificial intelligence–based stratification of demographic, ocular surface high-risk factors in progression of keratoconus. Indian J. Ophthalmol. 2023, 71, 1882–1888. [Google Scholar] [CrossRef]
- Hartmann, L.M.; Langhans, D.S.; Eggarter, V.; Freisenich, T.J.; Hillenmayer, A.; König, S.F.; Vounotrypidis, E.; Wolf, A.; Wertheimer, C.M. Keratoconus Progression Determined at the First Visit: A Deep Learning Approach with Fusion of Imaging and Numerical Clinical Data. Transl. Vis. Sci. Technol. 2024, 13, 7. [Google Scholar] [CrossRef]
- Vohra, S.; Dudeja, L.; Chauhan, T. Sequence of events leading to diagnosis of keratoconus and its impact on quality of life. Indian J. Ophthalmol. 2021, 69, 3478. [Google Scholar] [CrossRef] [PubMed]
- Santhiago, M.R. Chapter 14—Corneal Topography in Keratoconus. ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323759786000145 (accessed on 1 June 2024).
- Gomes, J.A.P.; Tan, D.; Rapuano, C.J.; Belin, M.W.; Ambrósio, R., Jr.; Guell, J.L.; Malecaze, F.; Nishida, K.; Sangwan, V.S.; Group of Panelists for the Global Delphi Panel Panel of Keratoconus and Ectatic Disease. Global Consensus on Keratoconus and Ectatic Diseases. Cornea 2015, 34, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Grisevic, S.; Gilevska, F.; Biscevic, A.; Pjano, M.; Bohac, M.; Pidro, A. Keratoconus Progression Classification One Year after Performed Crosslinking Method Based on ABCD Keratoconus Grading System. Acta Inform. Medica 2020, 28, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Lombardo, M.; Grasso, E.; Gensini, G.F.; Ambrosio, G. The influence of pectus excavatum on cardiac kinetics and function in otherwise healthy individuals: A systematic review. Int. J. Cardiol. 2023, 381, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, M. Increased incidence of mitral valve prolapse in children with pectus chest wall deformity. Pediatr. Int. 2023, 65, e15582. [Google Scholar] [CrossRef] [PubMed]
- Katrancioglu, O.; Ozgel, M.; Inceoglu, F.; Katrancioglu, N.; Sahin, E. Is there a relationship between Haller Index and cardiopulmonary function in children with pectus excavatum? Turk. J. Thorac. Cardiovasc. Surg. 2023, 31, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Carter, Y.M. Pectus Excavatum. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430918/ (accessed on 1 June 2024).
- Ortiz, J.A.R.; Abrego, B.V. Surgical correction of recurrent pectus excavatum of an adult patient, case report, and review of literature. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewski, D.; Notrica, D.; McMahon, L.; Steidley, D.E.; Deschamps, C. Current Management of Pectus Excavatum: A Review and Update of Therapy and Treatment Recommendations. J. Am. Board Fam. Med. 2010, 23, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Wenstrup, R.; De Paepe, A. Classic Ehlers-Danlos Syndrome. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1244/ (accessed on 1 June 2024).
- TAAD Syndrome Genetic Testing|TAADNext|Ambry Genetics. Available online: https://www.ambrygen.com/providers/genetic-testing/12/cardiology/taadnext (accessed on 23 March 2023).
- gnomAD. Available online: https://gnomad.broadinstitute.org/variant/9-134753839-T-A?dataset=gnomad_r4 (accessed on 23 March 2024).
- VCV000212940.11—ClinVar—NCBI. Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/212940/ (accessed on 23 March 2024).
- Image Created with Biorender.com. Available online: https://www.biorender.com (accessed on 26 May 2024).
- Mak, K.M.; Yi, C.; Png, M.; Lee, D.J. Type V Collagen in Health, Disease, and Fibrosis. Anat. Rec. 2016, 299, 613–629. [Google Scholar] [CrossRef]
- Love, A.N.; Palmer, B. Presentation and Management of a Novel Ehlers-Danlos COL5A1 Variant with Birt-Hogg-Dube Syndrome: A Case Study. Cureus 2023, 15, e35866. [Google Scholar] [CrossRef]
- Loukovitis, E.; Sfakianakis, K.; Syrmakesi, P.; Tsotridou, E.; Orfanidou, M.; Bakaloudi, D.R.; Stoila, M.; Kozei, A.; Koronis, S.; Zachariadis, Z.; et al. Genetic Aspects of Keratoconus: A Literature Review Exploring Potential Genetic Contributions and Possible Genetic Relationships with Comorbidities. Ophthalmol. Ther. 2018, 7, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, X.; Zhu, W.; Huang, Y.; Mou, L.; Liu, M.; Li, X.; Li, F.; Li, X.; Zhang, Y.; et al. Evidence for GAL3ST4 mutation as the potential cause of pectus excavatum. Cell Res. 2012, 22, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- Symoens, S.; Malfait, F.; Vlummens, P.; Hermanns-Lê, T.; Syx, D.; De Paepe, A. A Novel Splice Variant in the N-propeptide of COL5A1 Causes an EDS Phenotype with Severe Kyphoscoliosis and Eye Involvement. PLoS ONE 2011, 6, e20121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, G.; Moore, P.; Sathyamoorthy, M. The Association of a Single Nucleotide Variant in COL5A1 to Early Onset Keratoconus and Pectus Excavatum—Convergence of Extracellular Matrix Pathologies. Medicina 2024, 60, 974. https://doi.org/10.3390/medicina60060974
Bryant G, Moore P, Sathyamoorthy M. The Association of a Single Nucleotide Variant in COL5A1 to Early Onset Keratoconus and Pectus Excavatum—Convergence of Extracellular Matrix Pathologies. Medicina. 2024; 60(6):974. https://doi.org/10.3390/medicina60060974
Chicago/Turabian StyleBryant, Griffin, Peyton Moore, and Mohanakrishnan Sathyamoorthy. 2024. "The Association of a Single Nucleotide Variant in COL5A1 to Early Onset Keratoconus and Pectus Excavatum—Convergence of Extracellular Matrix Pathologies" Medicina 60, no. 6: 974. https://doi.org/10.3390/medicina60060974
APA StyleBryant, G., Moore, P., & Sathyamoorthy, M. (2024). The Association of a Single Nucleotide Variant in COL5A1 to Early Onset Keratoconus and Pectus Excavatum—Convergence of Extracellular Matrix Pathologies. Medicina, 60(6), 974. https://doi.org/10.3390/medicina60060974





