Prevalence, Predictors, and Outcomes of Pulmonary Hypertension in Patients with Lupus Nephritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. PH Definition and Covariates
2.3.1. PH Definition and Pulmonary Artery Systolic Pressure (PASP) Equation
2.3.2. SLE Disease Activity
2.3.3. Estimated Glomerular Filtration Rate (eGFR)
2.3.4. Mean Arterial Pressure
2.3.5. Other Covariates
2.4. Endpoints
2.5. Analysis
2.6. Patient and Public Involvement
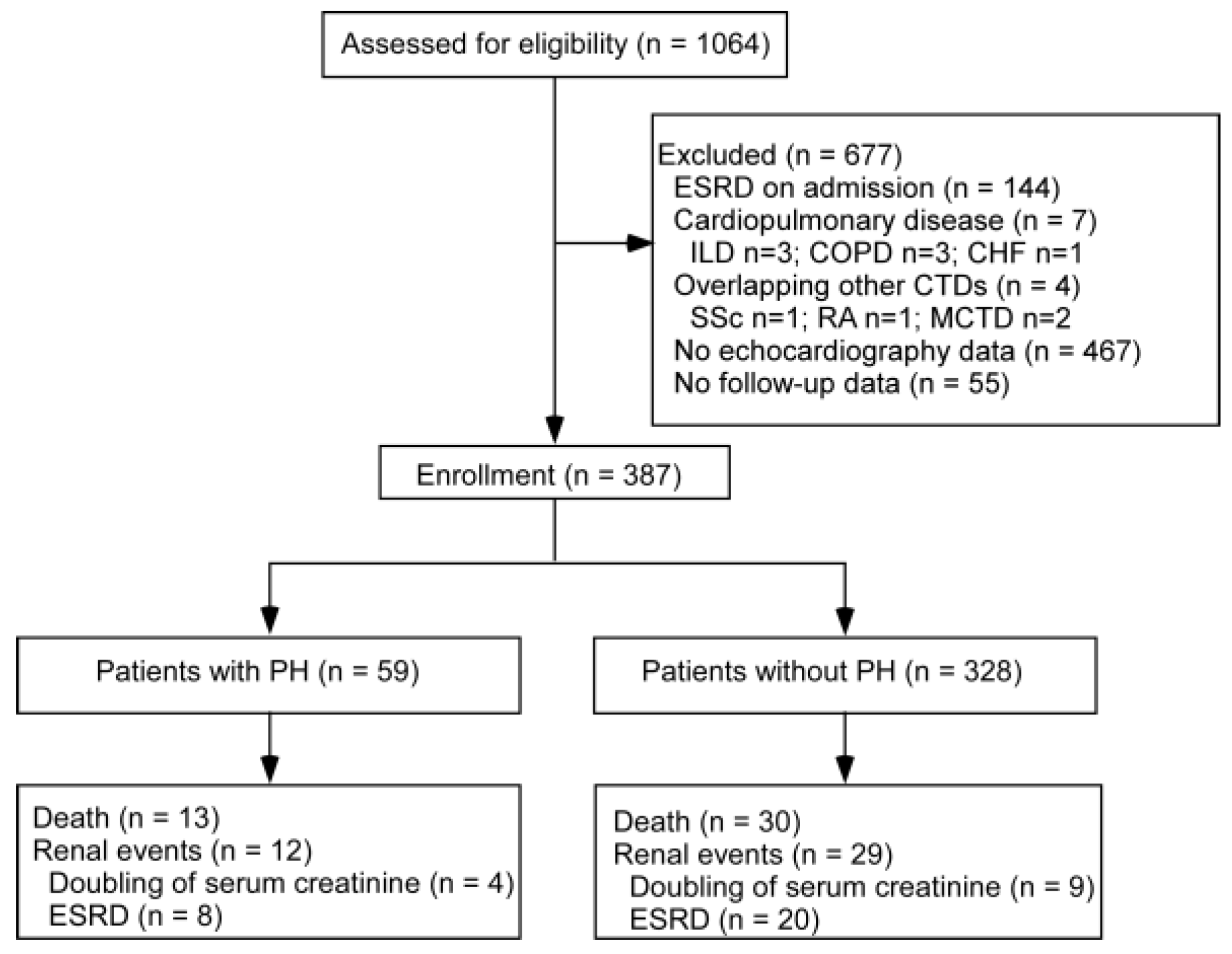
3. Results
3.1. Prevalence and Baseline Characteristics
3.2. Risk Factors for PH in Patients with Lupus Nephritis
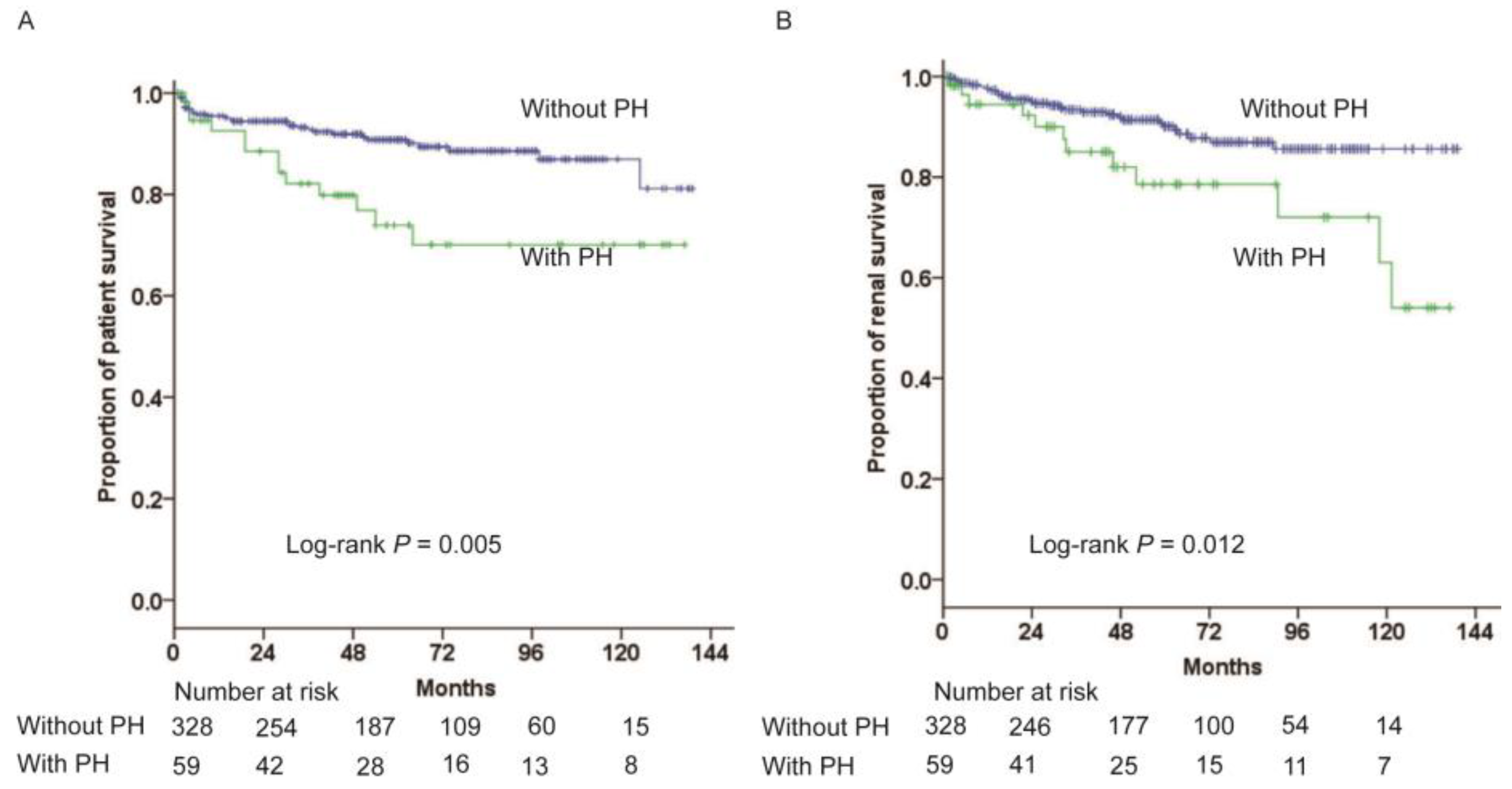
3.3. Association between PH and Clinical Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, K. Pulmonary Hypertension. JAMA 2021, 326, 1116. [Google Scholar] [CrossRef] [PubMed]
- Poch, D.; Mandel, J. Pulmonary Hypertension. Ann. Intern. Med. 2021, 174, ITC49–ITC64. [Google Scholar] [CrossRef] [PubMed]
- Thoreau, B.; Mouthon, L. Pulmonary arterial hypertension associated with connective tissue diseases (CTD-PAH): Recent and advanced data. Autoimmun. Rev. 2024, 23, 103506. [Google Scholar] [CrossRef] [PubMed]
- McGoon, M.D.; Miller, D.P. REVEAL: A contemporary US pulmonary arterial hypertension registry. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2012, 21, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.J.; Jiang, X.; Zhou, W.; Wang, Y.; Gao, L.; Wang, Y.; Li, G.T.; Hong, T.; Huo, Y.; Jing, Z.C.; et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur. Respir. J. 2014, 44, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Ko, C.-H.; Hsu, C.-Y.; Chen, H.-A. Epidemiology and mortality of connective tissue disease-associated pulmonary arterial hypertension: A national cohort study in taiwan. Semin. Arthritis Rheum. 2020, 50, 957–962. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Y.; Huang, C.; Yang, X.; Zhao, J.; Wang, Q.; Tian, Z.; Li, M.; Zeng, X. Survival and prognostic factors of systemic lupus erythematosus-associated pulmonary arterial hypertension: A PRISMA-compliant systematic review and meta-analysis. Autoimmun. Rev. 2016, 15, 250–257. [Google Scholar] [CrossRef]
- Qian, J.; Li, M.; Zhang, X.; Wang, Q.; Zhao, J.; Tian, Z.; Wei, W.; Zuo, X.; Zhang, M.; Zhu, P.; et al. Long-term prognosis of patients with systemic lupus erythematosus-associated pulmonary arterial hypertension: CSTAR-PAH cohort study. Eur. Respir. J. 2019, 53, 1800081. [Google Scholar] [CrossRef]
- Yu, F.; Haas, M.; Glassock, R.; Zhao, M.-H. Redefining lupus nephritis: Clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 2017, 13, 483–495. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Roy, J.; Tao, K.; Brecklin, C.S.; Chen, J.; Deo, R.; Flack, J.M.; Ojo, A.O.; Plappert, T.J.; Raj, D.S.; et al. Prevalence, Predictors, and Outcomes of Pulmonary Hypertension in CKD. J. Am. Soc. Nephrol. JASN 2016, 27, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Hsu, V.M.; Moreyra, A.E.; Wilson, A.C.; Shinnar, M.; Shindler, D.M.; Wilson, J.E.; Desai, A.; Seibold, J.R. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: Comparison of noninvasive tests with results of right-heart catheterization. J. Rheumatol. 2008, 35, 458–465. [Google Scholar] [PubMed]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.X.; Coates-Bradshaw, L.D.; Willis, J.; Harkness, A.; Ring, L.; Grapsa, J.; Coghlan, G.; Kaye, N.; Oxborough, D.; Robinson, S.; et al. Echocardiographic assessment of pulmonary hypertension: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2018, 5, G11–G24. [Google Scholar] [CrossRef] [PubMed]
- Fayed, A.; Soliman, A.; El-Khasab, S.O.; Hammad, H.; Magdy, M.; Abu-Sheaishaa, M.A.; El Wahab, H.A.; Hegazy, M.T. Noninvasive Assessment of Endothelial Dysfunction in Egyptian Patients with Lupus Nephritis. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2021, 32, 671–679. [Google Scholar] [CrossRef]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Zuo, L.; Chen, J.H.; Luo, Q.; Yu, X.Q.; Li, Y.; Xu, J.S.; Huang, S.M.; Wang, L.N.; Huang, W.; et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. JASN 2006, 17, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Haycock, G.B.; Edelmann, C.M., Jr.; Spitzer, A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976, 58, 259–263. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef]
- Abd ElHafeez, S.; D’Arrigo, G.; Leonardis, D.; Fusaro, M.; Tripepi, G.; Roumeliotis, S. Methods to Analyze Time-to-Event Data: The Cox Regression Analysis. Oxid. Med. Cell. Longev. 2021, 2021, 1302811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, W.; Zhu, P.; Zhang, X.; Liu, S.; Wu, L.; Ma, L.; Bi, L.; Zuo, X.; Sun, L.; et al. Chinese SLE Treatment and Research group (CSTAR) registry VII: Prevalence and clinical significance of serositis in Chinese patients with systemic lupus erythematosus. Lupus 2016, 25, 652–657. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Zhao, J.; Li, Z.; Ye, Z.; Li, C.; Li, X.; Zhu, P.; Wang, Z.; Zheng, Y.; et al. Chinese SLE Treatment and Research group (CSTAR) registry: II. Prevalence and risk factors of pulmonary arterial hypertension in Chinese patients with systemic lupus erythematosus. Lupus 2014, 23, 1085–1091. [Google Scholar] [CrossRef]
- Prabu, A.; Patel, K.; Yee, C.-S.; Nightingale, P.; Situnayake, R.D.; Thickett, D.R.; Townend, J.N.; Gordon, C. Prevalence and risk factors for pulmonary arterial hypertension in patients with lupus. Rheumatol. Oxf. Engl. 2009, 48, 1506–1511. [Google Scholar] [CrossRef]
- Álvarez Troncoso, J.; Soto Abánades, C.; Robles-Marhuenda, Á.; Alcolea Batres, S.; Fernández Velilla Peña, M.; Jiménez Valero, S.; Sorriguieta Torre, R.; Rios-Blanco, J.J. Prevalence, risk factors and echocardiographic predictors of pulmonary hypertension in systemic lupus erythematosus: Towards a screening protocol. RMD Open 2024, 10, e003674. [Google Scholar] [CrossRef]
- Reque, J.; Garcia-Prieto, A.; Linares, T.; Vega, A.; Abad, S.; Panizo, N.; Quiroga, B.; Collado Boira, E.J.; López-Gómez, J.M. Pulmonary Hypertension Is Associated with Mortality and Cardiovascular Events in Chronic Kidney Disease Patients. Am. J. Nephrol. 2017, 45, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Lennartz, S.; Leonardis, D.; D’Arrigo, G.; Tripepi, R.; Emrich, I.E.; Mallamaci, F.; Fliser, D.; Heine, G.; Zoccali, C. High estimated pulmonary artery systolic pressure predicts adverse cardiovascular outcomes in stage 2–4 chronic kidney disease. Kidney Int. 2015, 88, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Shah, S.J.; Ommerborn, M.J.; Clark, C.R.; Hall, M.E.; Mentz, R.J.; Qazi, S.; Robbins, J.M.; Skelton, T.N.; Chen, J.; et al. Pulmonary Hypertension Is Associated with a Higher Risk of Heart Failure Hospitalization and Mortality in Patients with Chronic Kidney Disease: The Jackson Heart Study. Circ. Heart Fail. 2017, 10, e003940. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-A.; Hsu, T.-C.; Yang, S.-C.; Weng, C.-T.; Wu, C.-H.; Sun, C.-Y.; Lin, C.-Y. Incidence and survival impact of pulmonary arterial hypertension among patients with systemic lupus erythematosus: A nationwide cohort study. Arthritis Res. Ther. 2019, 21, 82. [Google Scholar] [CrossRef]
- de Carvalho, J.F.; Bonfá, E.; Borba, E.F. Systemic lupus erythematosus and “lupus dyslipoproteinemia”. Autoimmun. Rev. 2008, 7, 246–250. [Google Scholar] [CrossRef]
- Robinson, G.; Pineda-Torra, I.; Ciurtin, C.; Jury, E.C. Lipid metabolism in autoimmune rheumatic disease: Implications for modern and conventional therapies. J. Clin. Investig. 2022, 132, e148552. [Google Scholar] [CrossRef] [PubMed]
- Nickel, N.P.; Galura, G.M.; Zuckerman, M.J.; Hakim, M.N.; Alkhateeb, H.; Mukherjee, D.; Austin, E.D.; Heresi, G.A. Liver abnormalities in pulmonary arterial hypertension. Pulm. Circ. 2021, 11, 20458940211054304. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.-M.; Meng, H.; Zhao, Q.-Z.; Zhen, Y.-Z.; Tian, L.; Wang, L.; Ji, L.-S.; Ma, G.-P.; Tian, Y.; et al. Relationship between lipids levels and right ventricular volume overload in congestive heart failure. J. Geriatr. Cardiol. JGC 2014, 11, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Hachulla, E.; Jais, X.; Cinquetti, G.; Clerson, P.; Rottat, L.; Launay, D.; Cottin, V.; Habib, G.; Prevot, G.; Chabanne, C.; et al. Pulmonary Arterial Hypertension Associated with Systemic Lupus Erythematosus: Results from the French Pulmonary Hypertension Registry. Chest 2018, 153, 143–151. [Google Scholar] [CrossRef]
- Lam, C.S.; Borlaug, B.A.; Kane, G.C.; Enders, F.T.; Rodeheffer, R.J.; Redfield, M.M. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009, 119, 2663–2670. [Google Scholar] [CrossRef]
- Xu, Q.; Xiong, L.; Fan, L.; Xu, F.; Yang, Y.; Li, H.; Peng, X.; Cao, S.; Zheng, Z.; Yang, X.; et al. Association of Pulmonary Hypertension with Mortality in Incident Peritoneal Dialysis Patients. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2015, 35, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Hao, Y.; Fan, Y.; Huang, H.; Yang, X.; Xie, A.; Zhang, X.; Ji, L.; Geng, Y.; Zhang, Z. Mortality and prognostic factors in Chinese patients with systemic lupus erythematosus. Lupus 2018, 27, 1742–1752. [Google Scholar] [CrossRef]
- Edmonston, D.L.; Parikh, K.S.; Rajagopal, S.; Shaw, L.K.; Abraham, D.; Grabner, A.; Sparks, M.A.; Wolf, M. Pulmonary Hypertension Subtypes and Mortality in CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 75, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Walther, C.P.; Gregg, L.P.; Bansal, S.; Winkelmayer, W.C.; Nambi, V.; Niu, J. Mortality, Kidney Failure, and Hospitalization among Medicare Beneficiaries with CKD and Pulmonary Hypertension. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, 78, 700–708.e1. [Google Scholar] [CrossRef]
- Fei, Y.; Shi, X.; Gan, F.; Li, X.; Zhang, W.; Li, M.; Hou, Y.; Zhang, X.; Zhao, Y.; Zeng, X.; et al. Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin. Rheumatol. 2014, 33, 57–63. [Google Scholar] [CrossRef]
| Variables | Total (n = 387) | With PH (n = 59) | Without PH (n = 328) | p Value a |
|---|---|---|---|---|
| Age(y) | 30 (21, 40) | 34 (23, 39) | 30 (20, 40) | 0.190 |
| Female, n (%) | 322 (83.2) | 47 (79.7) | 275 (83.8) | 0.450 |
| Lupus duration on admission (months) | 6 (2, 30) | 13 (2, 49) | 6 (2, 25) | 0.128 |
| Lupus nephritis duration on admission (months) | 3 (1, 19) | 5 (1, 25) | 3 (1, 18) | 0.488 |
| Mean arterial pressure (mmHg) | 99 (88, 111) | 109 (101, 119) | 97 (87, 109) | <0.001 |
| Fever, n (%) | 105 (27.1) | 17 (28.8) | 88 (26.8) | 0.752 |
| Rash, n (%) | 156 (40.3) | 21 (35.6) | 135 (41.2) | 0.473 |
| Ulceration, n (%) | 17 (4.4) | 3 (5.1) | 14 (4.3) | 0.732 |
| Arthritis/Myositis, n (%) | 161 (41.6) | 25 (42.4) | 136 (41.5) | 0.887 |
| Pleuritis, n (%) | 128 (33.1) | 29 (49.2) | 99 (30.2) | 0.006 |
| Pericarditis, n (%) | 211 (54.5) | 35 (59.3) | 176 (53.7) | 0.479 |
| Vasculitis, n (%) | 5 (1.3) | 0 (0) | 5 (1.5) | 1.000 |
| Leukocytopenia, n (%) | 90 (23.3) | 16 (27.1) | 74 (22.6) | 0.503 |
| Thrombocytopenia, n (%) | 34 (8.8) | 5 (8.5) | 29 (8.8) | 1.000 |
| Hemoglobin (g/L) | 98 (81, 115) | 87 (75, 103) | 99 (82, 116) | 0.001 |
| 24 h proteinuria (g) | 2.6 (1.0, 5.3) | 2.7 (1.0, 5.5) | 2.5 (1.0, 5.3) | 0.895 |
| Blood urea nitrogen (mmol/L) | 8.2 (5.3, 13.2) | 11.0 (6.0, 16.9) | 7.9 (5.1, 12.7) | 0.006 |
| Serum creatinine (umol/L) | 90 (64, 144) | 106 (68, 228) | 86 (62, 136) | 0.007 |
| eGFR (mL/min/1.73 m2) | 71.3 (42.1, 109.4) | 58.2 (25.8, 93.6) | 74.0 (44.5, 111.7) | 0.004 |
| Serum uric acid (μmol/L) | 419 (326, 543) | 480 (333, 583) | 413 (326, 533) | 0.138 |
| Serum albumin (g/L) | 26 (21, 31) | 26 (21, 32) | 26 (21, 31) | 0.835 |
| Total cholesterol (mmol/L) | 5.6 (4.3, 7.0) | 5.0 (4.0, 6.5) | 5.7 (4.4, 7.2) | 0.069 |
| Triglycerides (mmol/L) | 2.2 (1.5, 3.0) | 1.8 (1.2, 2.5) | 2.2 (1.5, 3.1) | 0.028 |
| HDL-C (mmol/L) | 1.0 (0.7, 1.3) | 0.9 (0.7, 1.1) | 0.99 (0.72, 1.34) | 0.169 |
| LDL-C (mmol/L) | 3.3 (2.6, 4.5) | 3.1 (2.5, 4.1) | 3.4 (2.6, 4.5) | 0.178 |
| C3 (g/L) | 0.41 (0.26, 0.61) | 0.36 (0.25, 0.59) | 0.42 (0.27, 0.62) | 0.233 |
| Patients with low C3 n (%) | 334 (86.3) | 56 (94.9) | 278 (84.8) | 0.039 |
| C4 (g/L) | 0.08 (0.06, 0.15) | 0.07 (0.06, 0.15) | 0.08 (0.06, 0.15) | 0.302 |
| Patients with low C4 n (%) | 306 (79.1) | 45 (76.3) | 261 (79.6) | 0.602 |
| C-reactive protein (mg/L) | 1.95 (0.84, 8.03) | 5.3 (1.2, 17.85) | 1.5 (0.8, 6.0) | 0.002 |
| Erythrocyte sedimentation rate (mm/h) | 33 (15, 53) | 37 (10, 53) | 33 (16, 54) | 0.776 |
| ANA, n (%) | 380/386 (98.4) | 59/59 (100.0) | 321/327 (98.2) | 0.597 |
| Anti-dsDNA, n (%) | 331/386 (85.8) | 53/59 (89.8) | 278/327 (85.0) | 0.420 |
| Anti-Sm, n (%) | 103/371 (27.8) | 13/57 (22.8) | 90/314 (28.7) | 0.423 |
| Anti-RNP, n (%) | 116/371 (31.3) | 19/57 (33.3) | 97/314 (30.9) | 0.757 |
| Anti-SSA/Ro, n (%) | 217/371 (58.5) | 35/57 (61.4) | 182/314 (58.0) | 0.664 |
| Anti-SSB/La, n (%) | 78/371 (21.0) | 12/57 (21.1) | 66/314 (21.0) | 1.000 |
| Anticardiolipin-IgM, n (%) | 53/328 (16.2) | 8/48 (16.7) | 45/280 (16.1) | 1.000 |
| Anticardiolipin-IgG, n (%) | 78/328 (23.8) | 13/48 (27.1) | 65/280 (23.2) | 0.583 |
| SLEDAI score | 16 (12, 20) | 16 (13, 20) | 16 (12, 20) | 0.346 |
| PASP (mmHg) | 33 (29, 40) | 44 (41, 52) | 31 (28, 34) | NA |
| Pathological pattern, n (%) | 0.196 | |||
| Class I, n (%) | 1/286 (0.3) | 0/38 (0.0) | 1/248 (0.4) | |
| Class II, n (%) | 18/286 (6.3) | 3/38 (7.9) | 15/248 (6.0) | |
| Class III, n (%) | 31/286 (10.8) | 2/38 (5.3) | 29/248 (11.7) | |
| Class IV, n (%) | 151/286 (52.8) | 27/38 (71.1) | 124/248 (50.0) | |
| Class V/III + V/IV + V n (%) | 82/286 (28.7) | 6/38 (15.8) | 76/248 (30.6) | |
| Class VI, n (%) | 3/286 (1.0) | 0/38 (0.0) | 3/248 (1.2) | |
| Activity index | 7 (5, 9) | 7 (5, 10) | 7 (5, 9) | 0.478 |
| Chronic index | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 0.554 |
| Variables | Odds Ratio (95% Confidence Interval) | p Value |
|---|---|---|
| Mean arterial pressure (per 1 mmHg increase) | 1.05 (1.03–1.08) | <0.001 |
| Hemoglobin (per 1 g/L increase) | 0.98 (0.97–1.00) | 0.014 |
| Triglycerides (per 1 mmol/L increase) | 0.71 (0.52–0.98) | 0.038 |
| C-reactive protein (per 1 mg/L increase) | 1.02 (1.00–1.04) | 0.088 |
| Variables | All-Cause Mortality | Renal Events | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Unadjusted | 2.46 (1.25–4.85) | 0.010 | 2.58 (1.30–5.12) | 0.007 |
| Model 1 a | 2.18 (1.10–4.32) | 0.025 | 2.49 (1.25–4.95) | 0.010 |
| Model 2 b | 2.01 (1.01–4.00) | 0.047 | 2.07 (1.04–4.12) | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Guo, J.; Huang, X.; He, W.; Yu, X.; Xia, X.; Chen, W. Prevalence, Predictors, and Outcomes of Pulmonary Hypertension in Patients with Lupus Nephritis. Medicina 2024, 60, 988. https://doi.org/10.3390/medicina60060988
Chen S, Guo J, Huang X, He W, Yu X, Xia X, Chen W. Prevalence, Predictors, and Outcomes of Pulmonary Hypertension in Patients with Lupus Nephritis. Medicina. 2024; 60(6):988. https://doi.org/10.3390/medicina60060988
Chicago/Turabian StyleChen, Sixiu, Junhan Guo, Xiamin Huang, Wei He, Xueqing Yu, Xi Xia, and Wei Chen. 2024. "Prevalence, Predictors, and Outcomes of Pulmonary Hypertension in Patients with Lupus Nephritis" Medicina 60, no. 6: 988. https://doi.org/10.3390/medicina60060988





