CBP Expression Contributes to Neuropathic Pain via CREB and MeCP2 Regulation in the Spared Nerve Injury Rat Model
Abstract
:1. Introduction
2. Methods
2.1. Experimental Animals
2.2. SNI Model Creation
2.3. siRNA Preparation
- CBP siRNA
- MeCP2 siRNA
- Non-targeting (NT) siRNA
2.4. Intrathecal Injection
2.5. Measurement of Pain Response
2.6. IHC Method
2.7. Statistical Analyses
3. Results
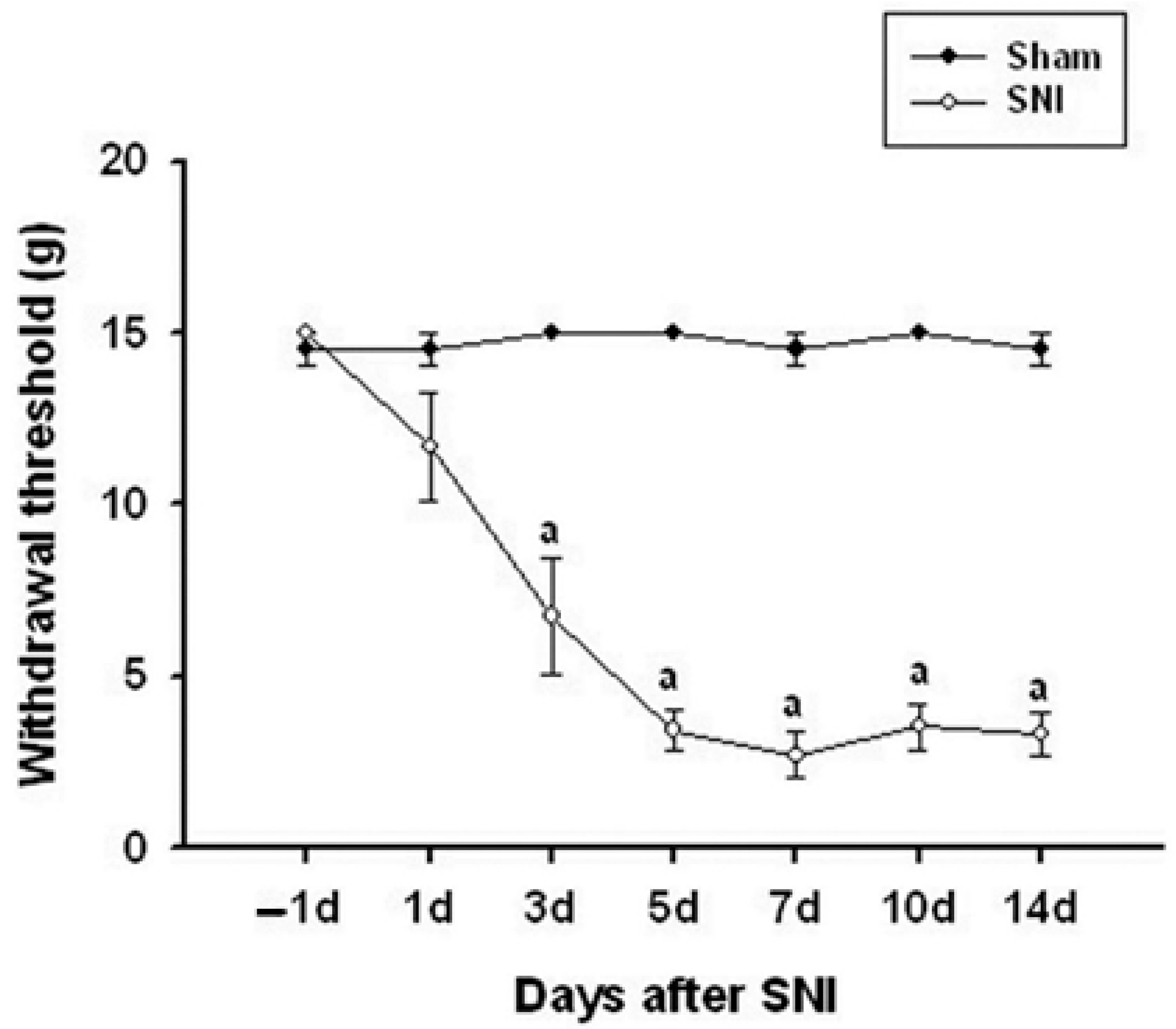
3.1. Pain Changes in the SNI Model
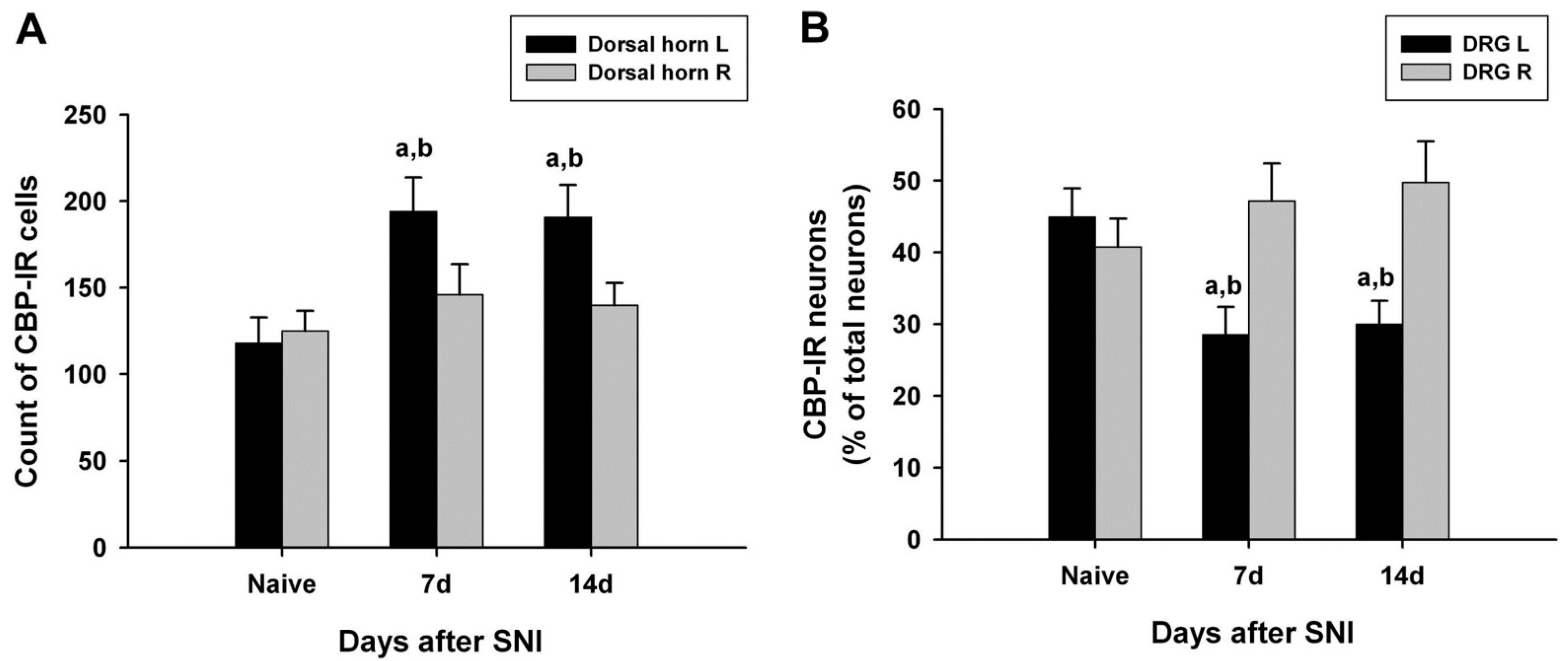
3.2. Changes in CBP Expression in the SC
3.3. Changes in CBP Expression in the DRG
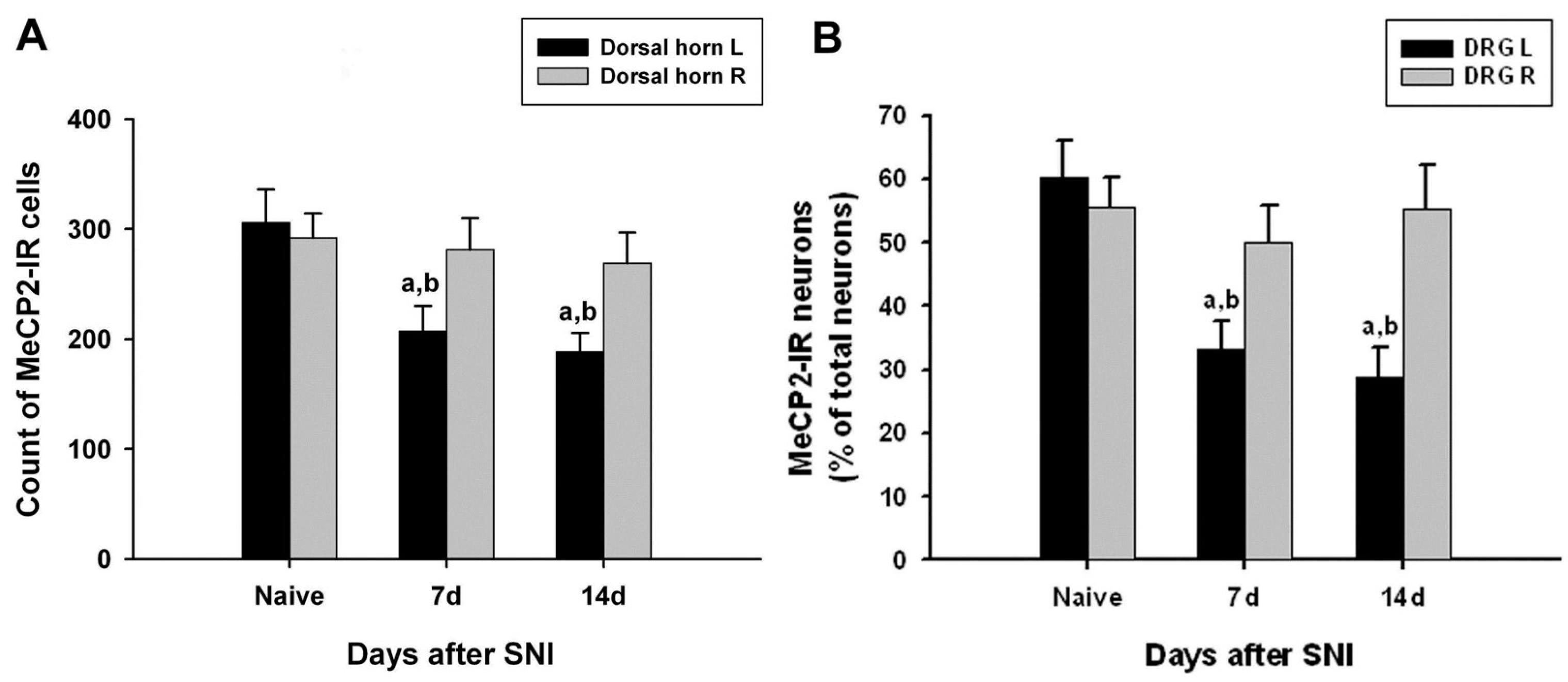
3.4. Changes in MeCP2 Expression in the SC
3.5. Changes in MeCP2 Expression in the DRG
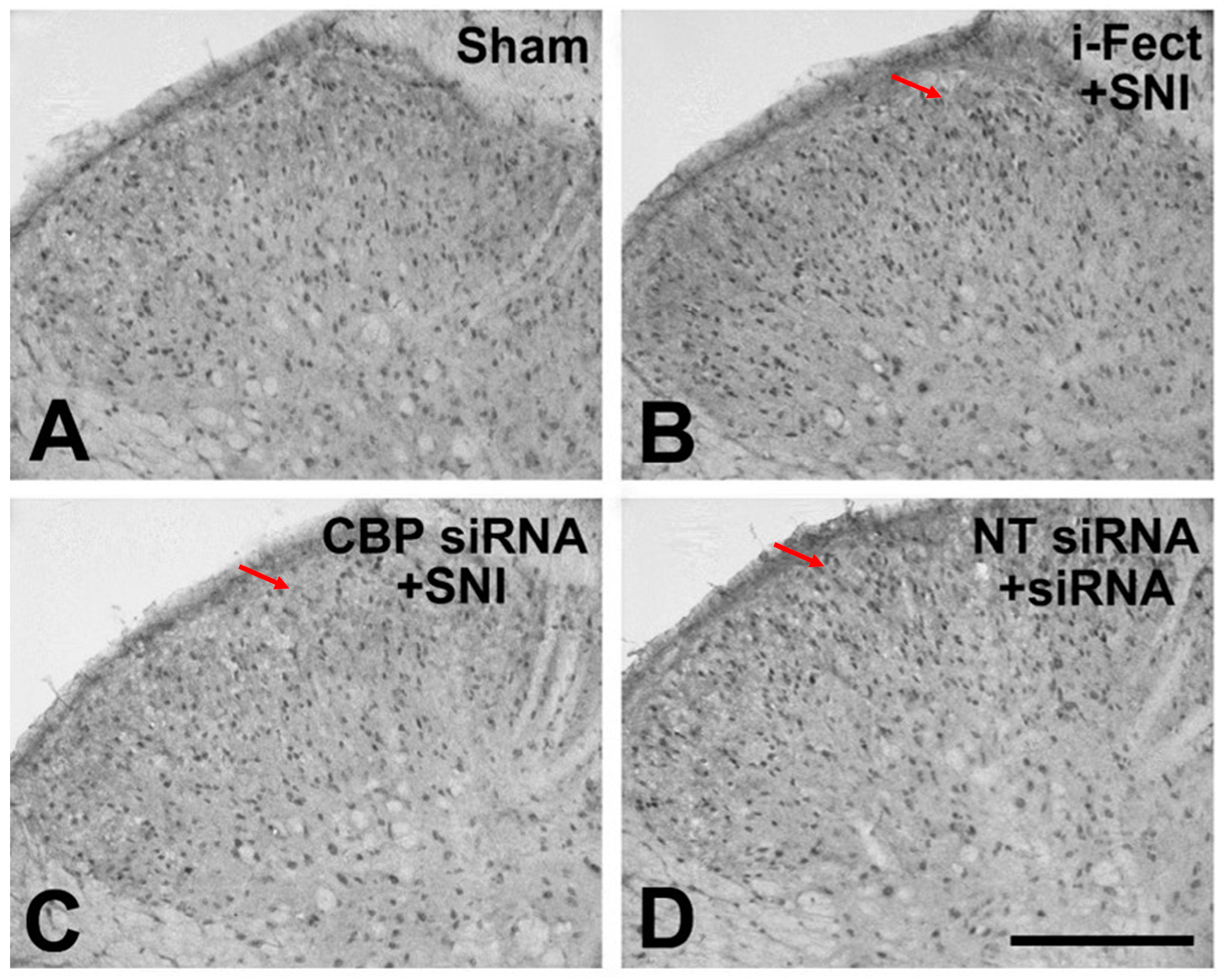
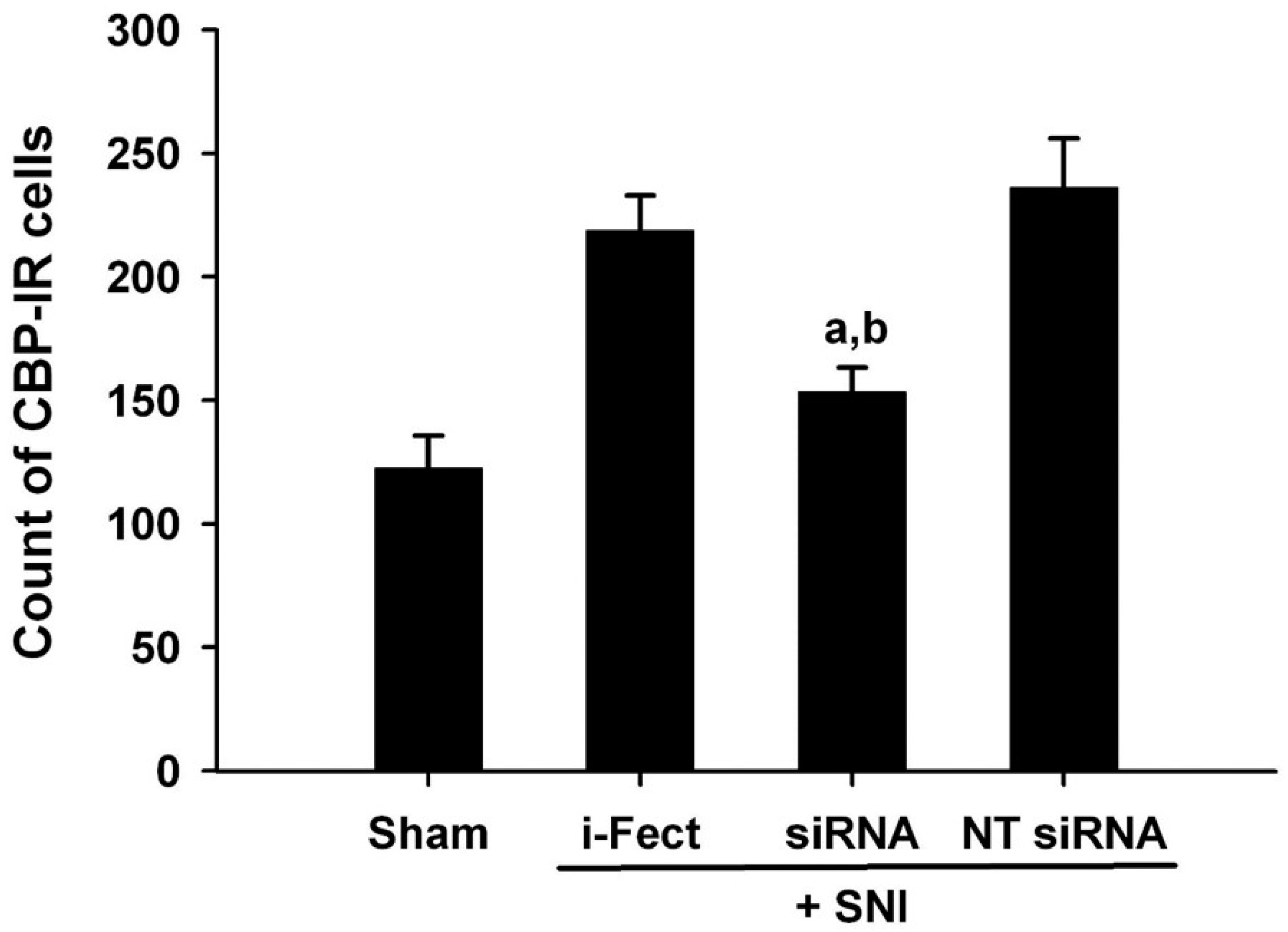
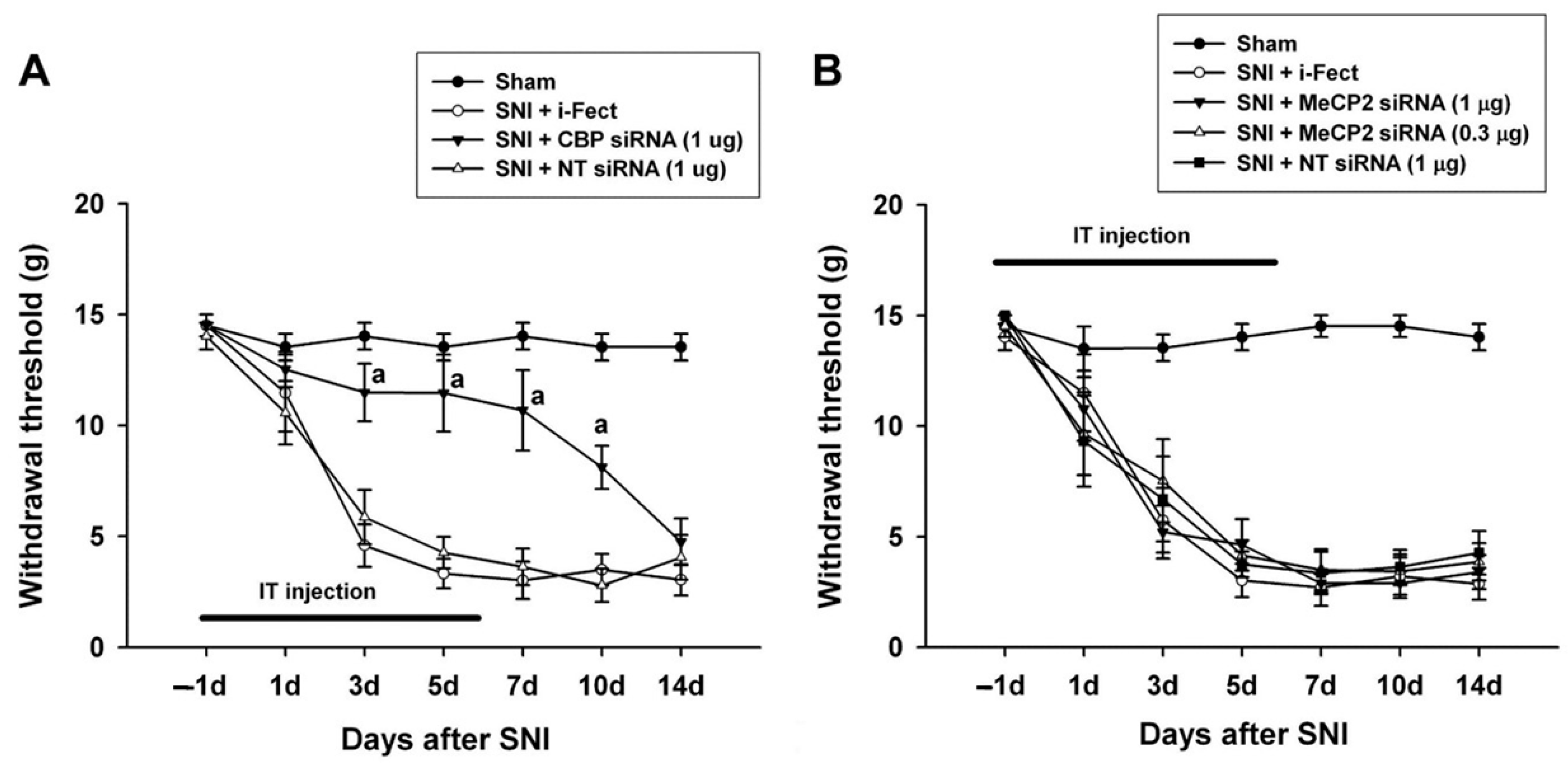
3.6. Changes in Pain Response after CBP siRNA Injection
3.7. Changes in Pain Response after MeCP2 siRNA Injection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A landscape takes shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Nestler, E.J.; Allis, C.D.; Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 2008, 60, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Langley, B.; Lubin, F.D.; Renthal, W.; Wood, M.A.; Yasui, D.H.; Kumar, A.; Nestler, E.J.; Akbarian, S.; Beckel-Mitchener, A.C. Epigenetics in the nervous system. J. Neurosci. 2008, 28, 11753–11759. [Google Scholar] [CrossRef] [PubMed]
- Kishi, N.; Macklis, J.D. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol. Cell. Neurosci. 2004, 27, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.M.; Wood, M.A. Beyond transcription factors: The role of chromatin modifying enzymes in regulating transcription required for memory. Learn. Mem. 2008, 15, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Turnell, A.S.; Mymryk, J.S. Roles for the coactivators CBP and p300 and the APC/C E3 ubiquitin ligase in E1A-dependent cell transformation. Br. J. Cancer 2006, 95, 555–560. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Huang, C.S.; Li, Q.; Guo, Q.L.; Wang, Y.; He, X.; Liao, J. Temporal distribution of p300/CBP immunoreactivity in the adult rat spinal dorsal horn following chronic constriction injury (CCI). Cell. Mol. Neurobiol. 2013, 33, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Meehan, R.R.; Henzel, W.J.; Maurer-Fogy, I.; Jeppesen, P.; Klein, F.; Bird, A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 1992, 69, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Akbarian, S.; Chen, R.Z.; Gribnau, J.; Rasmussen, T.P.; Fong, H.; Jaenisch, R.; Jones, E.G. Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol. Dis. 2001, 8, 784–791. [Google Scholar] [CrossRef]
- Géranton, S.M.; Morenilla-Palao, C.; Hunt, S.P. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J. Neurosci. 2007, 27, 6163–6173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Guo, Q.L.; Yan, J.Q.; Zhu, X.Y.; Huang, C.S.; Zou, W.Y. Intrathecal 5-azacytidine inhibits global DNA methylation and methyl-CpG-binding protein 2 expression and alleviates neuropathic pain in rats following chronic constriction injury. Brain Res. 2011, 1418, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Géranton, S.M.; Fratto, V.; Tochiki, K.K.; Hunt, S.P. Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol. Pain. 2008, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Richner, M.; Bjerrum, O.J.; Nykjaer, A.; Vaegter, C.B. The spared nerve injury (SNI) model of induced mechanical allodynia in Mice. J. Vis. Exp. 2011, 54, 3092. [Google Scholar]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S.; Zammataro, M.; Morales, M.E.; Busceti, C.L.; Drago, F.; Gereau, R.W.; Copani, A., 4th; Nicoletti, F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol. Pharmacol. 2009, 75, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Wei, D.; Zou, S.; Ren, K.; Dubner, R. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol. Pain. 2010, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Zhou, Y.; Xiao, Y.; Tao, J.; Gu, J.; Jiang, X.; Xu, G.Y. Promoter demethylation of cystathionine-beta-synthetase gene contributes to inflammatory pain in rats. Pain 2013, 154, 34–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Y.Q.; Zou, F.; Bie, B.; Pan, Z.Z. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat. Med. 2011, 17, 1448–1455. [Google Scholar] [CrossRef]
- Uchida, H.; Ma, L.; Ueda, H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J. Neurosci. 2010, 30, 4806–4814. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Fukazawa, Y.; Tohya, K.; Kimura, M.; Kishioka, S. Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. J. Pharmacol. Exp. Ther. 2012, 340, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Ikegami, D.; Yamashita, A.; Shimizu, T.; Narita, M.; Niikura, K.; Furuya, M.; Kobayashi, Y.; Miyashita, K.; Okutsu, D.; et al. Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain 2013, 136 Pt 3, 828–843. [Google Scholar] [CrossRef] [PubMed]
- Davis-Taber, R.A.; Scott, V.E.S. Transcriptional profiling of dorsal root ganglia in a neuropathic pain model using microarray and laser capture microdissection. Drug Dev. Res. 2006, 67, 308–330. [Google Scholar] [CrossRef]
- Lacroix-Fralish, M.L.; Tawfik, V.L.; Tanga, F.Y.; Spratt, K.F.; DeLeo, J.A. Differential spinal cord gene expression in rodent models of radicular and neuropathic pain. Anesthesiology 2006, 104, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Huang, C.S.; Li, Q.; Chang, R.M.; Song, Z.B.; Zou, W.Y.; Guo, Q.L. p300 exerts an epigenetic role in chronic neuropathic pain through its acetyltransferase activity in rats following chronic constriction injury (CCI). Mol. Pain. 2012, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; Huang, W.; Sidders, B.; Bithell, A.; Crow, M.; Grist, J.; Sharma, S.; Ziemek, D.; Rice, A.S.C.; Buckley, N.J.; et al. HDAC inhibitors attenuate the development of hypersensitivity in models of neuropathic pain. Pain 2013, 154, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Manners, M.T.; Ertel, A.; Tian, Y.; Ajit, S.K. Genome-wide redistribution of MeCP2 in dorsal root ganglia after peripheral nerve injury. Epigenetics Chromatin 2016, 9, 23. [Google Scholar] [CrossRef]
- Penas, C.; Navarro, X. Epigenetic modifications associated to neuroinflammation and neuropathic pain after neural trauma. Front. Cell. Neurosci. 2018, 12, 158. [Google Scholar] [CrossRef]
| Size of Neuron | Naive | SNI 7 Day | SNI 14 Day | ||||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| CBP | Small | 29.2 ± 2.5 | 25.7 ± 2.6 | 18.7 ± 3.0 | 32.5 ± 4.0 | 22.5 ± 2.1 | 35.1 ± 5.4 |
| Medium | 8.4 ± 1.2 | 8.90 ± 1.3 | 4.9 ± 0.8 | 7.3 ± 1.2 | 3.6 ± 0.8 | 7.8 ± 0.9 | |
| Large | 7.4 ± 0.8 | 6.15 ± 1.8 | 4.9 ± 1.3 | 7.4 ± 1.4 | 4.0 ± 1.4 | 6.8 ± 1.5 | |
| Total | 44.9 ± 4.0 | 40.7 ± 4.0 | 28.5 ± 3.9 | 47.2 ± 5.2 | 30.0 ± 3.2 | 49.8 ± 5.8 | |
| MeCP2 | Small | 41.6 ± 4.1 | 38.1 ± 4.5 | 24.4 ± 3.5 | 33.4 ± 3.7 | 21.7 ± 3.4 | 38.6 ± 5.4 |
| Medium | 10.5 ± 1.3 | 10.5 ± 1.3 | 4.9 ± 0.8 | 9.7 ± 1.3 | 3.3 ± 0.8 | 7.9 ± 0.9 | |
| Large | 8.2 ± 1.3 | 7.0 ± 0.7 | 4.0 ± 0.7 | 7.1 ± 1.3 | 3.7 ± 0.9 | 8.8 ± 1.3 | |
| Total | 60.4 ± 5.8 | 55.5 ± 4.8 | 33.3 ± 4.4 | 50.1 ± 5.6 | 28.7 ± 4.7 | 55.2 ± 7.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Park, K.-B.; Kim, M.S.; Jeon, Y.D. CBP Expression Contributes to Neuropathic Pain via CREB and MeCP2 Regulation in the Spared Nerve Injury Rat Model. Medicina 2024, 60, 989. https://doi.org/10.3390/medicina60060989
Lee C-C, Park K-B, Kim MS, Jeon YD. CBP Expression Contributes to Neuropathic Pain via CREB and MeCP2 Regulation in the Spared Nerve Injury Rat Model. Medicina. 2024; 60(6):989. https://doi.org/10.3390/medicina60060989
Chicago/Turabian StyleLee, Chae-Chil, Ki-Bong Park, Min Seok Kim, and Young Dae Jeon. 2024. "CBP Expression Contributes to Neuropathic Pain via CREB and MeCP2 Regulation in the Spared Nerve Injury Rat Model" Medicina 60, no. 6: 989. https://doi.org/10.3390/medicina60060989






