Effects of Daily Physical Activity on Exercise Capacity in Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Pulmonary Function Testing
2.2. Cardiopulmonary Exercise Test
2.3. Statistical Analysis
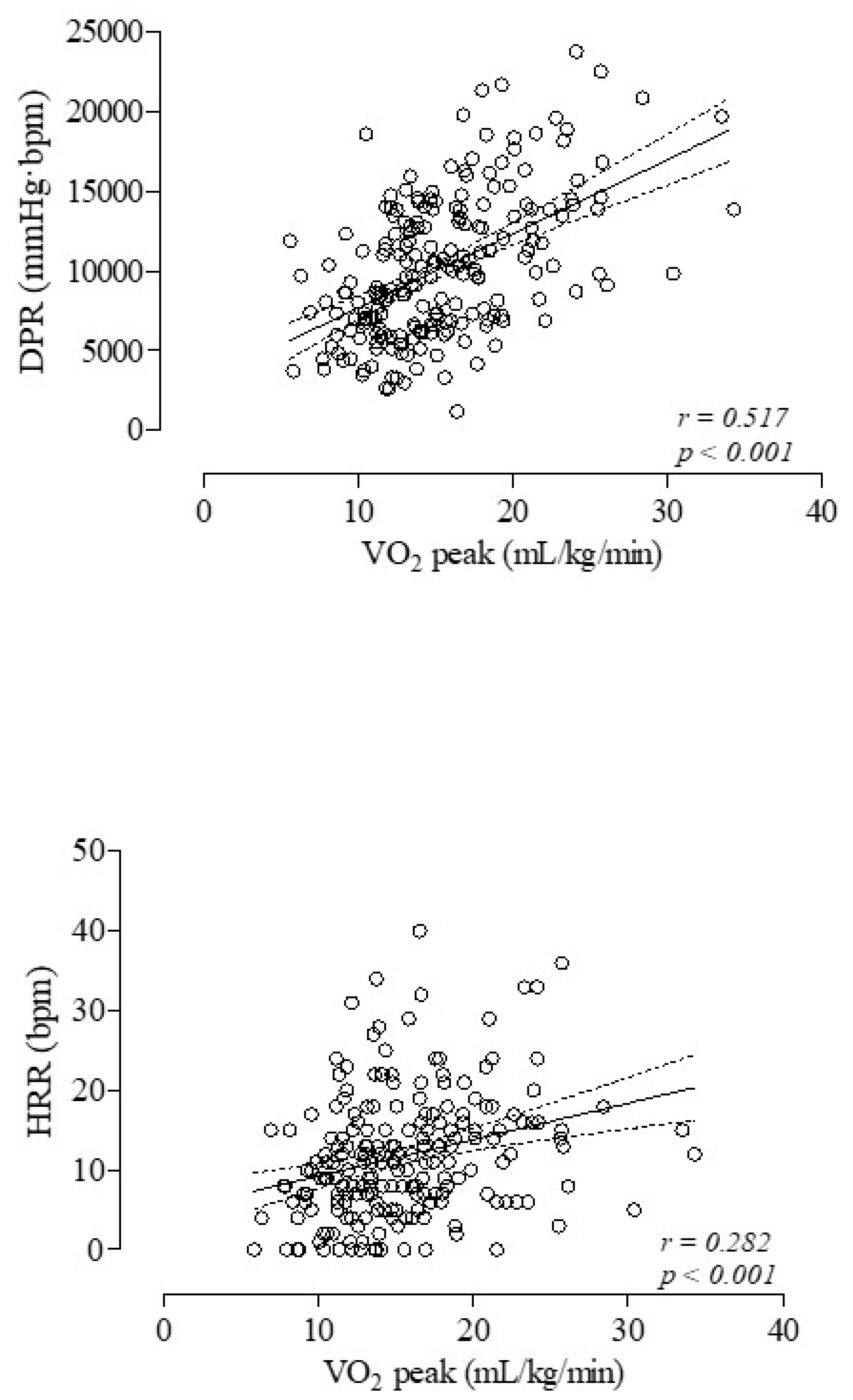
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Esteban, C.; Quintana, J.M.; Aburto, M.; Moraza, J.; Egurrola, M.; Pérez-Izquierdo, J.; Aizpiri, S.; Aguirre, U.; Capelastegui, A. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur. Respir. J. 2010, 36, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Kirsten, A.; Holz, O.; Müller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Waschki, B.; Boehme, C.; Claussen, M.; Meyer, T.; Magnussen, H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: A cross-sectional study. Am. J. Respir. Crit. Care Med. 2008, 177, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.E. Chapter 8: Exercise Capacity. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar] [PubMed]
- Pepin, V.; Saey, D.; Laviolette, L.; Maltais, F. Exercise capacity in chronic obstructive pulmonary disease: Mechanisms of limitation. COPD 2007, 4, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Carlacci, C.; Frizzelli, A.; Bondarenko, O.; Accogli, R.; Tzani, P.; Pisi, R.; Aiello, M.; Chetta, A. Daily Physical Activity and Maximal Exercise Capacity in COPD Patients, ID 129. In Proceedings of the XXIV National Congress of Italian Pulmonology—XLVII ITS-AIPO Congress PneumoLogica 2023—The Enlightenment of Pulmonology for Respiratory Health, Bari, Italy, 9–11 June 2023. [Google Scholar]
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Available online: http://goldcopd.org/ (accessed on 7 December 2023).
- American Thoracic Society. Surveillance for respiratory hazards in the occupational setting. Am. Rev. Respir. Dis. 1982, 126, 952–956. [Google Scholar]
- Available online: https://depts.washington.edu/hprc/programs-tools/tools-guides/rapa/ (accessed on 7 December 2023).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. 1993, 6 (Suppl. 16), 5–40. [Google Scholar] [CrossRef]
- Ross, R.M. ATS/ACCP Statement on cardiopulmonary exercise testing. Methodology: Exercising test protocol. Am. J. Respir. Crit. Care Med. 2003, 167, 224. [Google Scholar] [CrossRef]
- Ross, R.M. ATS/ACCP Statement on cardiopulmonary exercise testing. Methodology: Interpretation. Am. J. Respir. Crit. Care Med. 2003, 167, 245. [Google Scholar] [CrossRef]
- Ross, R.M. ATS/ACCP Statement on cardiopulmonary exercise testing. Criteria for terminating the exercise test. Am. J. Respir. Crit. Care Med. 2003, 167, 227. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Casaburi, R.; Whipp, B.J. (Eds.) Normal Values. In Principles of Exercise Testing & Interpretation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994; pp. 143–162. [Google Scholar]
- Ross, R.M. ATS/ACCP Statement on cardiopulmonary exercise testing. Ventilatory reserve. Am. J. Respir. Crit. Care Med. 2003, 167, 235. [Google Scholar] [CrossRef] [PubMed]
- Stubbing, D.G.; Pengelly, L.D.; Morse, J.L.; Jones, N.L. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. J. Appl. Physiol. 1980, 49, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Le, V.V.; Mitiku, T.; Sungar, G.; Myers, J.; Froelicher, V. The blood pressure response to dynamic exercise testing: A systematic review. Prog. Cardiovasc. Dis. 2008, 51, 135–160. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Frizzelli, A.; Di Spigno, F.; Moderato, L.; Halasz, G.; Aiello, M.; Tzani, P.; Manari, G.; Calzetta, L.; Pisi, R.; Pelà, G.; et al. An Impairment in Resting and Exertional Breathing Pattern May Occur in Long-COVID Patients with Normal Spirometry and Unexplained Dyspnoea. J. Clin. Med. 2022, 11, 7388. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Zwerink, M.; van der Palen, J.; van der Valk, P.; Brusse-Keizer, M.; Effing, T. Relationship between daily physical activity and exercise capacity in patients with COPD. Respir. Med. 2013, 107, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.; Dolmage, T.E.; Woon, L.; Coutts, D.; Goldstein, R.; Brooks, D. Defining the relationship between average daily energy expenditure and field-based walking tests and aerobic reserve in COPD. Chest 2012, 141, 406–412. [Google Scholar] [CrossRef]
- Task Force of the Italian Working Group on Cardiac Rehabilitation Prevention; Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology; Piepoli, M.F.; Corrà, U.; Agostoni, P.G.; Belardinelli, R.; Cohen-Solal, A.; Hambrecht, R.; Vanhees, L. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: Recommendations for performance and interpretation. Part I: Definition of cardiopulmonary exercise testing parameters for appropriate use in chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 150–164. [Google Scholar]
- Tzani, P.; Aiello, M.; Elia, D.; Boracchia, L.; Marangio, E.; Olivieri, D.; Clini, E.; Chetta, A. Dynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patients. Respir. Res. 2011, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, M.; Maltais, F.; Poirier, P.; Lacasse, Y.; Marquis, K.; Jobin, J.; LeBlanc, P. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.A.; Curb, J.D.; Petrovitch, H.; Rodriguez, B.L.; Yano, K.; Ross, G.W.; White, L.R.; Abbott, R.D. Effects of walking on coronary heart disease in elderly men: The Honolulu Heart Program. Circulation 1999, 100, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Rexrode, K.M.; Cook, N.R.; Manson, J.E.; Buring, J.E. Physical Activity and Coronary Heart Disease in Women: Is “No Pain, No Gain” Passé? JAMA 2001, 285, 1447–1454. [Google Scholar] [CrossRef] [PubMed]

| Parameter | All Patients N. 214 (100%) | Active Patients N. 83 (38.8%) | Inactive Patients N. 131 (61.2%) |
|---|---|---|---|
| Age (yr) | 68.2 ± 8.6 | 68.0 ± 8.7 | 68.2 ± 8.0 |
| M/F (N.) (F%) | 152/62 (28.9) | 62/21 (25.3) | 90/41 (31.3) |
| BMI (Kg/m2) | 27.4 ± 5.2 | 27.2 ± 5.1 | 27.5 ± 5.3 |
| mMRC (0–4) | 1 (1–2) | 1 (1–2) | 2 (1–2) ** |
| TLC (L) | 6.810 ± 1.346 | 6.808 ± 1.362 | 6.812 ± 1.339 |
| TLC (% predicted) | 115.2 ± 19.6 | 114.0 ± 19.4 | 116.1 ± 19.7 |
| FVC (L) | 2.842 ± 0.756 | 2.883 ± 0.704 | 2.816 ± 0.790 |
| FVC (% predicted) | 85.7 ± 17.6 | 86.3 ± 17.2 | 85.3 ± 17.9 |
| FEV1 (L) | 1.428 ± 0.592 | 1.504 ± 0.589 | 1.380 ± 0.590 |
| FEV1 (% predicted) | 56.2 ± 19.3 | 58.4 ± 18.4 | 54.7 ± 19.7 |
| FEV1/FVC (%) | 47.3 ± 12.5 | 48.3 ± 11.3 | 46.6 ± 13.1 |
| IC (L) | 2.02 ± 0.64 | 2.13 ± 0.58 | 1.95 ± 0.67 |
| IC/TLC (%) | 31.7 ± 9.5 | 33.1 ± 9.6 | 30.1 ± 9.4 |
| DLCO (% predicted) | 63.5 ± 23.3 | 65.5 ± 23.0 | 62.2 ± 23.5 |
| Parameter | All Patients N. 214 (100%) | Active Patients N. 83 (39%) | Inactive Patients N. 131 (61%) |
|---|---|---|---|
| Workload (watts) | 75.8 ± 35.5 | 86.8 ± 38.9 | 68.9 ± 31.4 ** |
| V’O2 peak (mL/kg/min) | 15.4 ± 4.9 | 16.9 ± 5.6 | 14.5 ± 4.3 ** |
| V’O2 peak (% pred) | 64.6 ± 21.5 | 70.9 ± 25 | 60.6 ± 17.7 ** |
| V’O2 @AT (mL/kg/min) | 11.5 ± 3.9 | 12.5 ± 4.5 | 10.9 ± 3.4 * |
| V’O2/HR peak (mL/bpm) | 10.0 ± 3.4 | 10.8 ± 3.6 | 9.5 ± 3.1 ** |
| V’O2/HR peak (% pred) | 83.7 ± 26.0 | 89.9 ± 29.5 | 79.8 ± 22.8 ** |
| DPR (mmHg·bpm) | 10,196 ± 4454 | 11,203 ± 4986 | 9557 ± 3970 ** |
| HRR (bpm) | 12.6 ± 12.2 | 13.0 ± 5.1 | 9.9 ± 7.0 * |
| BR (%) | 74.0 ± 18.0 | 74.1 ± 17.7 | 75.1 ± 21.8 |
| VE/V’CO2 slope | 33.2 ± 8.8 | 33.4 ± 10.1 | 34.3 ± 8.1 |
| VE/V’CO2 intercept | 3.7 ± 2.6 | 3.3 ± 2.5 | 3.9 ± 2.7 |
| ∆PETCO2 (mmHg) | 5.4 ± 4 | 5.7 ± 5 | 5.2 ± 4 |
| ∆SpO2 (%) | −1.8 ± 2.8 | −1.7 ± 2.9 | −1.9 ± 2.7 |
| ∆IC (L) | −0.251 ± 0.424 | −0.260 ± 0.437 | −0.245 ± 0.416 |
| VASdys/WL (mm/watts) | 1.22 ± 0.7 | 1.05 ± 0.6 | 1.32 ± 0.8 ** |
| VASfat/WL (mm/watts) | 1.13 ± 0.7 | 0.96 ± 0.6 | 1.23 ± 0.7 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, M.; Frizzelli, A.; Pisi, R.; Accogli, R.; Marchese, A.; Carlacci, F.; Bondarenko, O.; Tzani, P.; Chetta, A. Effects of Daily Physical Activity on Exercise Capacity in Chronic Obstructive Pulmonary Disease. Medicina 2024, 60, 1026. https://doi.org/10.3390/medicina60071026
Aiello M, Frizzelli A, Pisi R, Accogli R, Marchese A, Carlacci F, Bondarenko O, Tzani P, Chetta A. Effects of Daily Physical Activity on Exercise Capacity in Chronic Obstructive Pulmonary Disease. Medicina. 2024; 60(7):1026. https://doi.org/10.3390/medicina60071026
Chicago/Turabian StyleAiello, Marina, Annalisa Frizzelli, Roberta Pisi, Rocco Accogli, Alessandra Marchese, Francesca Carlacci, Olha Bondarenko, Panagiota Tzani, and Alfredo Chetta. 2024. "Effects of Daily Physical Activity on Exercise Capacity in Chronic Obstructive Pulmonary Disease" Medicina 60, no. 7: 1026. https://doi.org/10.3390/medicina60071026





