Pharmacological Treatments and Therapeutic Targets in Muscle Dystrophies Generated by Alterations in Dystrophin-Associated Proteins
Abstract
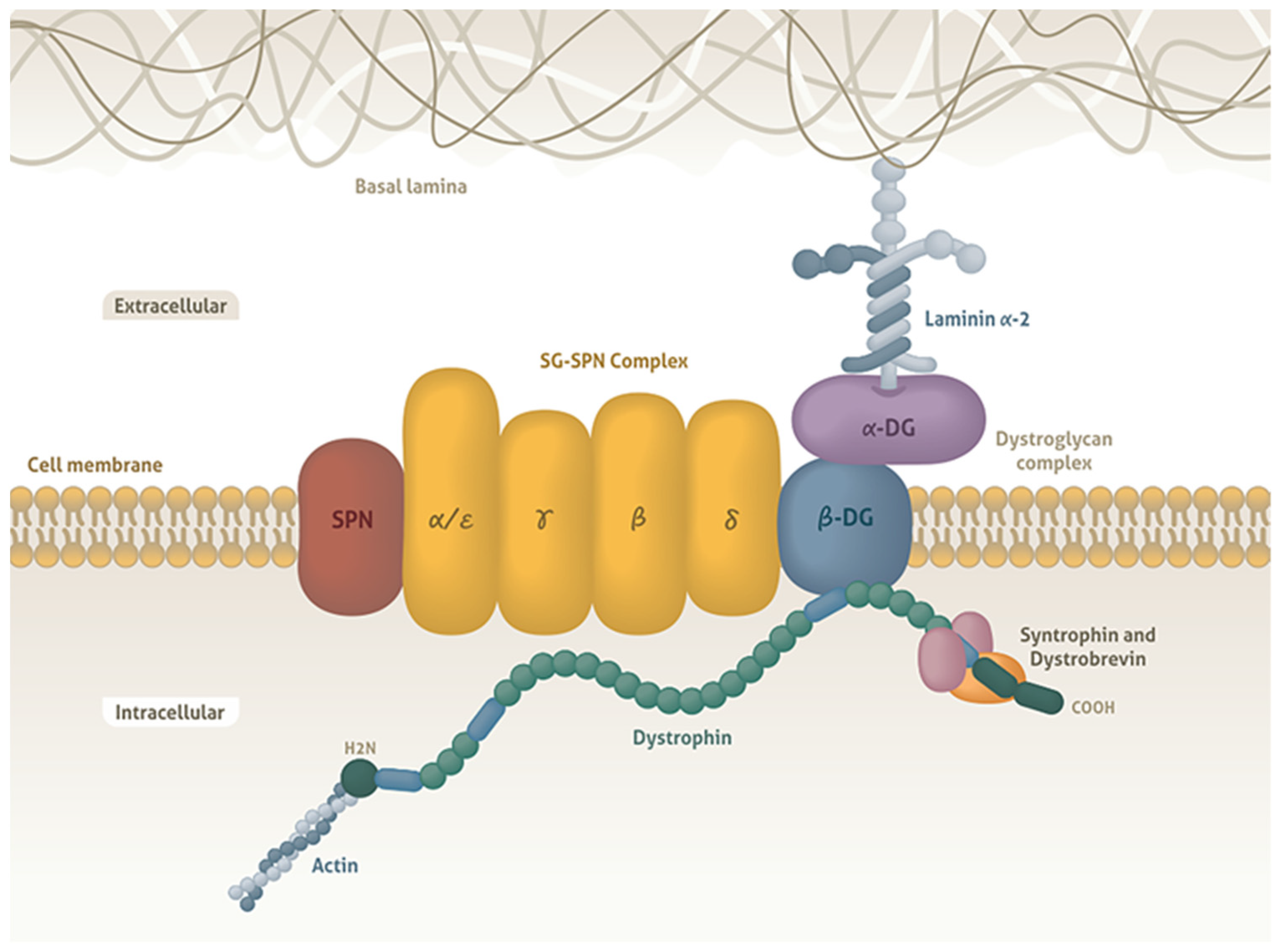
1. Introduction
2. Pharmacological Treatment Applied to Patients with MD
2.1. Corticosteroid Treatment in DMD
2.2. Corticosteroid Treatment in Dystrophies with Alterations in DAPs
3. Other Experimental Drugs Used in Models of DAP Alterations
3.1. Drug Repurposing
3.2. Novel Molecule Research
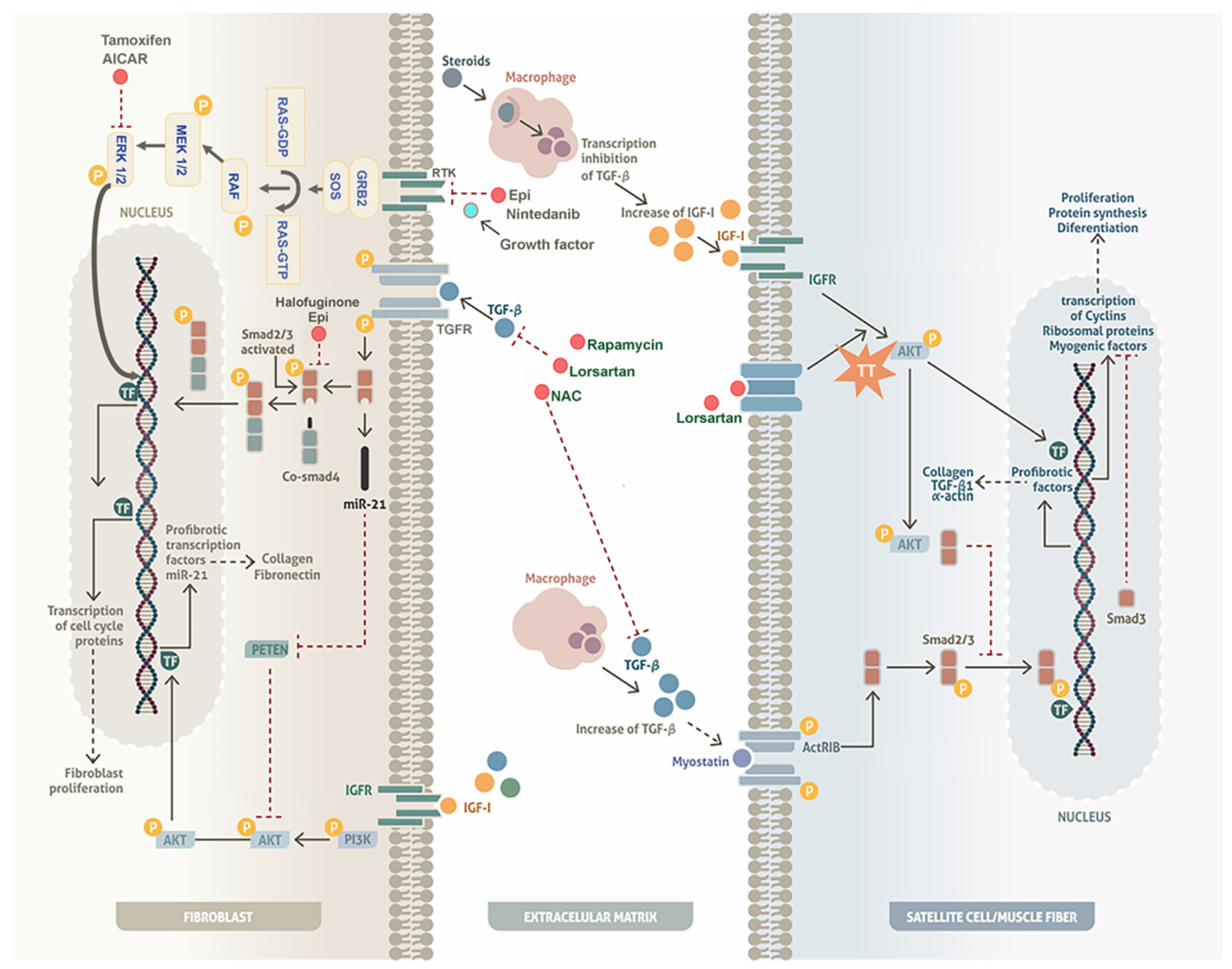
4. Mechanisms of Experimental Drugs to Regulate Fibrosis in Models with DAP Alterations
4.1. Inhibition of Fibrosis
4.1.1. The TGF-β/Smad Pathway
4.1.2. Extracellular Signal-Regulated Kinase (ERK) Pathway
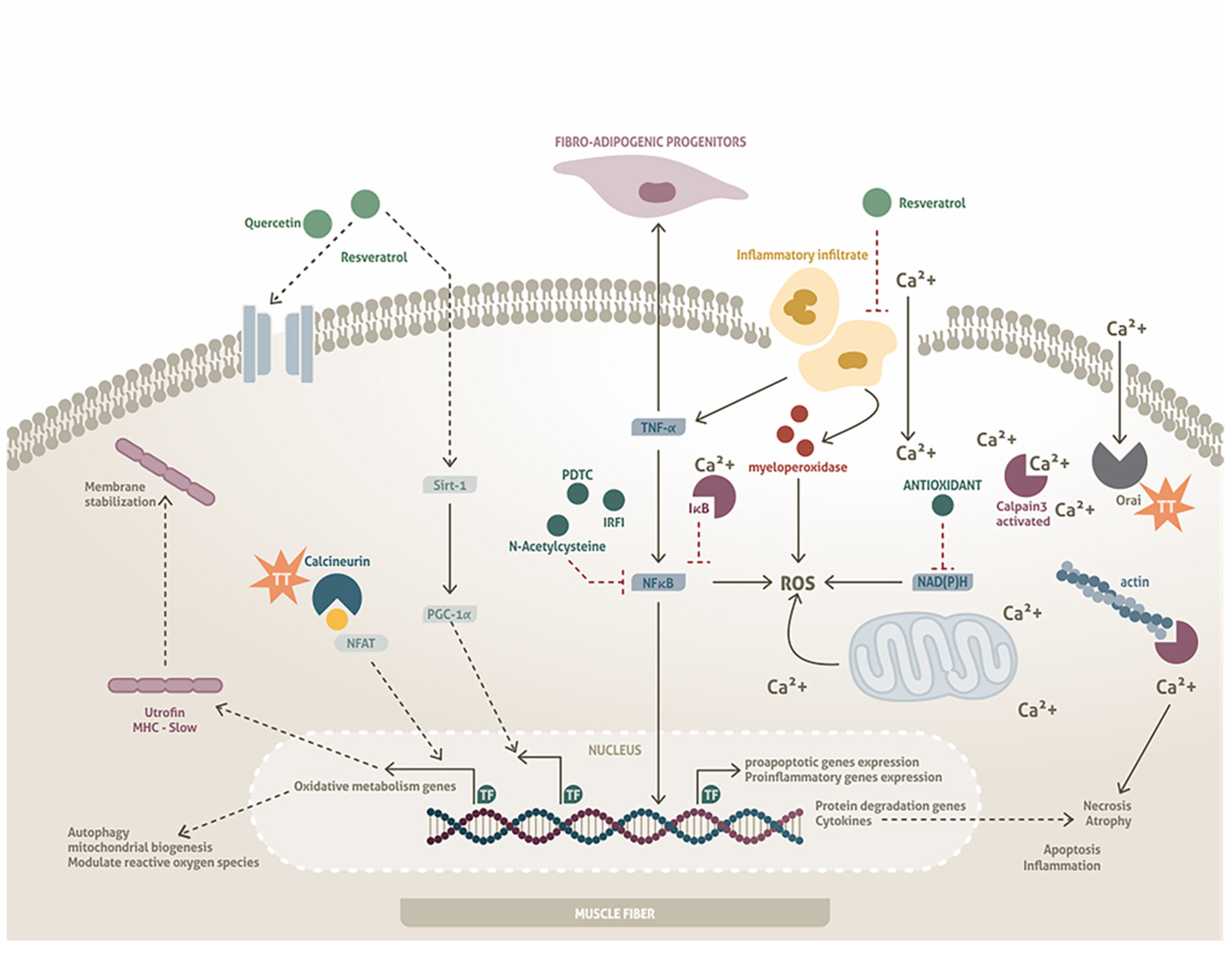
5. Antioxidants as Possible Pharmacological Molecules in MDs with Alterations in DAPs
6. Evaluation of Target Sites as New Therapeutic Strategies
6.1. Calcium Regulation
6.2. Regulation of Hypertrophy/Proliferation/Differentiation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Smad 2/3 | Mothers against decapentaplegic homolog 2/3 |
| Smad 4 | Mothers against decapentaplegic homolog 4 |
| miR-21 | microRNA-21 |
| PTEN | Phosphatase and tensin homolog |
| AKT | Serine/threonine kinase |
| IGF-1 | Insulin-like growth factor 1 |
| mTOR | Mammalian target of rapamycin |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| FOXO | Forkhead box O |
| HIF-1 | Hypoxia-inducible factor 1-alpha |
| NFAT | Nuclear factor of activated T-cells |
| p70S6K | Ribosomal protein S6 kinase beta-1 |
References
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Emery, A.E. The muscular dystrophies. Lancet 2002, 359, 687–695. [Google Scholar] [CrossRef]
- Salari, N.; Fatahi, B.; Valipour, E.; Kazeminia, M.; Fatahian, R.; Kiaei, A.; Shohaimi, S.; Mohammadi, M. Global prevalence of Duchenne and Becker muscular dystrophy: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 96. [Google Scholar] [CrossRef]
- Murphy, A.P.; Straub, V. The Classification, Natural History and Treatment of the Limb Girdle Muscular Dystrophies. J. Neuromuscul. Dis. 2015, 2, S7–S19. [Google Scholar] [CrossRef]
- Escobar-Cedillo, R.E.; López-Hernández, L.; Miranda-Duarte, A.; Curiel-Leal, M.D.; Suarez-Ocón, A.; Sánchez-Chapul, L.; Berenice Luna-Angulo, A.; Ávila-Ramírez, G.; López-Hernández, J.A.; Gómez-Díaz, B. Genetic analysis of muscular dystrophies: Our experience in Mexico. Folia Neuropathol. 2021, 59, 276–283. [Google Scholar] [CrossRef]
- Gómez-Díaz, B.; Rosas-Vargas, H.; Roque-Ramírez, B.; Meza-Espinoza, P.; Ruano-Calderón, L.A.; Fernández-Valverde, F.; Escalante-Bautista, D.; Escobar-Cedillo, R.E.; Sánchez-Chapul, L.; Vargas-Cañas, S.; et al. Immunodetection analysis of muscular dystrophies in Mexico. Muscle Nerve 2012, 45, 338–345. [Google Scholar] [CrossRef]
- Nigro, V.; Piluso, G. Spectrum of muscular dystrophies associated with sarcolemmal-protein genetic defects. Biochim. Biophys. Acta 2015, 1852, 585–593. [Google Scholar] [CrossRef]
- Mah, J.K.; Korngut, L.; Fiest, K.M.; Dykeman, J.; Day, L.J.; Pringsheim, T.; Jette, N. A Systematic Review and Meta-analysis on the Epidemiology of the Muscular Dystrophies. Can. J. Neurol. Sci. Le J. Can. Des Sci. Neurol. 2016, 43, 163–177. [Google Scholar] [CrossRef]
- Ervasti, J.M.; Campbell, K.P. Dystrophin-associated glycoproteins: Their possible roles in the pathogenesis of Duchenne muscular dystrophy. Mol. Cell Biol. Hum. Dis. Ser. 1993, 3, 139–166. [Google Scholar] [CrossRef]
- Campbell, K.P. Three muscular dystrophies: Loss of cytoskeleton-extracellular matrix linkage. Cell 1995, 80, 675–679. [Google Scholar] [CrossRef]
- Stone, M.R.; O’Neill, A.; Catino, D.; Bloch, R.J. Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol. Biol. Cell 2005, 16, 4280–4293. [Google Scholar] [CrossRef]
- Nigro, V.; Savarese, M. Genetic basis of limb-girdle muscular dystrophies: The 2014 update. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2014, 33, 1–12. [Google Scholar]
- Moens, P.; Baatsen, P.H.; Maréchal, G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J. Muscle Res. Cell Motil. 1993, 14, 446–451. [Google Scholar] [CrossRef]
- Cohn, R.D.; Campbell, K.P. Molecular basis of muscular dystrophies. Muscle Nerve 2000, 23, 1456–1471. [Google Scholar] [CrossRef]
- Petrof, B.J. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Mol. Cell. Biochem. 1998, 179, 111–123. [Google Scholar] [CrossRef]
- Cotta, A.; Carvalho, E.; da-Cunha-Júnior, A.L.; Paim, J.F.; Navarro, M.M.; Valicek, J.; Menezes, M.M.; Nunes, S.V.; Xavier Neto, R.; Takata, R.I.; et al. Common recessive limb girdle muscular dystrophies differential diagnosis: Why and how? Arq. Neuro-Psiquiatr. 2014, 72, 721–734. [Google Scholar] [CrossRef]
- Peverelli, L.; Testolin, S.; Villa, L.; D’Amico, A.; Petrini, S.; Favero, C.; Magri, F.; Morandi, L.; Mora, M.; Mongini, T.; et al. Histologic muscular history in steroid-treated and untreated patients with Duchenne dystrophy. Neurology 2015, 85, 1886–1893. [Google Scholar] [CrossRef]
- Dubowitz, V.; Sewry, C.A.; Oldfors, A. Muscle Biopsy a Practical Approach, 3rd ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2007; p. 611. [Google Scholar]
- Peter, A.K.; Ko, C.Y.; Kim, M.H.; Hsu, N.; Ouchi, N.; Rhie, S.; Izumiya, Y.; Zeng, L.; Walsh, K.; Crosbie, R.H. Myogenic Akt signaling upregulates the utrophin-glycoprotein complex and promotes sarcolemma stability in muscular dystrophy. Hum. Mol. Genet. 2009, 18, 318–327. [Google Scholar] [CrossRef]
- Peter, A.K.; Crosbie, R.H. Hypertrophic response of Duchenne and limb-girdle muscular dystrophies is associated with activation of Akt pathway. Exp. Cell Res. 2006, 312, 2580–2591. [Google Scholar] [CrossRef]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.J.; Hutcheson, D.A.; Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011, 138, 3625–3637. [Google Scholar] [CrossRef]
- Rodgers, J.T.; King, K.Y.; Brett, J.O.; Cromie, M.J.; Charville, G.W.; Maguire, K.K.; Brunson, C.; Mastey, N.; Liu, L.; Tsai, C.R.; et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature 2014, 510, 393–396. [Google Scholar] [CrossRef]
- Fukada, S.; Uezumi, A.; Ikemoto, M.; Masuda, S.; Segawa, M.; Tanimura, N.; Yamamoto, H.; Miyagoe-Suzuki, Y.; Takeda, S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 2007, 25, 2448–2459. [Google Scholar] [CrossRef]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef]
- Biressi, S.; Miyabara, E.H.; Gopinath, S.D.; Carlig, P.M.; Rando, T.A. A Wnt-TGFβ2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Sci. Transl. Med. 2014, 6, 267ra176. [Google Scholar] [CrossRef]
- Ganassi, M.; Muntoni, F.; Zammit, P.S. Defining and identifying satellite cell-opathies within muscular dystrophies and myopathies. Exp. Cell Res. 2022, 411, 112906. [Google Scholar] [CrossRef]
- McDonald, C.M.; Muntoni, F.; Penematsa, V.; Jiang, J.; Kristensen, A.; Bibbiani, F.; Goodwin, E.; Gordish-Dressman, H.; Morgenroth, L.; Werner, C.; et al. Ataluren delays loss of ambulation and respiratory decline in nonsense mutation Duchenne muscular dystrophy patients. J. Comp. Eff. Res. 2022, 11, 139–155. [Google Scholar] [CrossRef]
- Mercuri, E.; Seferian, A.M.; Servais, L.; Deconinck, N.; Stevenson, H.; Ni, X.; Zhang, W.; East, L.; Yonren, S.; Muntoni, F. Safety, tolerability and pharmacokinetics of eteplirsen in young boys aged 6-48 months with Duchenne muscular dystrophy amenable to exon 51 skipping. Neuromuscul. Disord. NMD 2023, 33, 476–483. [Google Scholar] [CrossRef]
- Voit, T.; Topaloglu, H.; Straub, V.; Muntoni, F.; Deconinck, N.; Campion, G.; De Kimpe, S.J.; Eagle, M.; Guglieri, M.; Hood, S.; et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): An exploratory, randomised, placebo-controlled phase 2 study. Lancet. Neurol. 2014, 13, 987–996. [Google Scholar] [CrossRef]
- Robinson-Hamm, J.N.; Gersbach, C.A. Gene therapies that restore dystrophin expression for the treatment of Duchenne muscular dystrophy. Hum. Genet. 2016, 135, 1029–1040. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. Lancet. Neurol. 2010, 9, 177–189. [Google Scholar] [CrossRef]
- Bonifati, M.D.; Ruzza, G.; Bonometto, P.; Berardinelli, A.; Gorni, K.; Orcesi, S.; Lanzi, G.; Angelini, C. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve 2000, 23, 1344–1347. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Scotton, C.; Passarelli, C.; Ferlini, A. Duchenne Muscular Dystrophy: From Diagnosis to Therapy. Molecules 2015, 20, 18168–18184. [Google Scholar] [CrossRef]
- Escolar, D.M.; Hache, L.P.; Clemens, P.R.; Cnaan, A.; McDonald, C.M.; Viswanathan, V.; Kornberg, A.J.; Bertorini, T.E.; Nevo, Y.; Lotze, T.; et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology 2011, 77, 444–452. [Google Scholar] [CrossRef]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 2016, Cd003725. [Google Scholar] [CrossRef]
- Manzur, A.Y.; Kinali, M.; Muntoni, F. Update on the management of Duchenne muscular dystrophy. Arch. Dis. Child. 2008, 93, 986–990. [Google Scholar] [CrossRef]
- Manzur, A.Y.; Kuntzer, T.; Pike, M.; Swan, A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2008, 1, Cd003725. [Google Scholar] [CrossRef]
- Thooyamani, A.S.; Mukhopadhyay, A. PDGFRα mediated survival of myofibroblasts inhibit satellite cell proliferation during aberrant regeneration of lacerated skeletal muscle. Sci. Rep. 2021, 11, 63. [Google Scholar] [CrossRef]
- Girardi, F.; Taleb, A.; Ebrahimi, M.; Datye, A.; Gamage, D.G.; Peccate, C.; Giordani, L.; Millay, D.P.; Gilbert, P.M.; Cadot, B.; et al. TGFβ signaling curbs cell fusion and muscle regeneration. Nat. Commun. 2021, 12, 750. [Google Scholar] [CrossRef]
- Angelini, C.; Fanin, M.; Menegazzo, E.; Freda, M.P.; Duggan, D.J.; Hoffman, E.P. Homozygous alpha-sarcoglycan mutation in two siblings: One asymptomatic and one steroid-responsive mild limb-girdle muscular dystrophy patient. Muscle Nerve 1998, 21, 769–775. [Google Scholar] [CrossRef]
- Connolly, A.M.; Pestronk, A.; Mehta, S.; Al-Lozi, M. Primary alpha-sarcoglycan deficiency responsive to immunosuppression over three years. Muscle Nerve 1998, 21, 1549–1553. [Google Scholar] [CrossRef]
- Wong-Kisiel, L.C.; Kuntz, N.L. Two siblings with limb-girdle muscular dystrophy type 2E responsive to deflazacort. Neuromuscul. Disord. NMD 2010, 20, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Wong-Kisiel, L.C.; Ji, T.; Renaud, D.L.; Kotagal, S.; Patterson, M.C.; Dalmau, J.; Mack, K.J. Response to immunotherapy in a 20-month-old boy with anti-NMDA receptor encephalitis. Neurology 2010, 74, 1550–1551. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.A.; Abath-Neto, O.; Maximino, J.R.; Chadi, G.; Zanoteli, E.; Reed, U.C. Clinical aspects of patients with sarcoglycanopathies under steroids therapy. Arq. Neuro-Psiquiatr. 2014, 72, 768–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darin, N.; Kroksmark, A.K.; Ahlander, A.C.; Moslemi, A.R.; Oldfors, A.; Tulinius, M. Inflammation and response to steroid treatment in limb-girdle muscular dystrophy 2I. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2007, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, C.; Escolar, D.; Brockington, M.; Clement, E.M.; Mein, R.; Jimenez-Mallebrera, C.; Torelli, S.; Feng, L.; Brown, S.C.; Sewry, C.A.; et al. Fukutin gene mutations in steroid-responsive limb girdle muscular dystrophy. Ann. Neurol. 2006, 60, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, L.; Tibaudo, L.; Pegoraro, E.; Bello, L.; Canton, M. Teaching an Old Molecule New Tricks: Drug Repositioning for Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2019, 20, 6053. [Google Scholar] [CrossRef]
- Péladeau, C.; Adam, N.; Bronicki, L.M.; Coriati, A.; Thabet, M.; Al-Rewashdy, H.; Vanstone, J.; Mears, A.; Renaud, J.M.; Holcik, M.; et al. Identification of therapeutics that target eEF1A2 and upregulate utrophin A translation in dystrophic muscles. Nat. Commun. 2020, 11, 1990. [Google Scholar] [CrossRef]
- Fontelonga, T.M.; Jordan, B.; Nunes, A.M.; Barraza-Flores, P.; Bolden, N.; Wuebbles, R.D.; Griner, L.M.; Hu, X.; Ferrer, M.; Marugan, J.; et al. Sunitinib promotes myogenic regeneration and mitigates disease progression in the mdx mouse model of Duchenne muscular dystrophy. Hum. Mol. Genet. 2019, 28, 2120–2132. [Google Scholar] [CrossRef]
- Vitiello, L.; Marabita, M.; Sorato, E.; Nogara, L.; Forestan, G.; Mouly, V.; Salviati, L.; Acosta, M.; Blaauw, B.; Canton, M. Drug Repurposing for Duchenne Muscular Dystrophy: The Monoamine Oxidase B Inhibitor Safinamide Ameliorates the Pathological Phenotype in mdx Mice and in Myogenic Cultures from DMD Patients. Front. Physiol. 2018, 9, 1087. [Google Scholar] [CrossRef]
- Kracht, K.D.; Eichorn, N.L.; Berlau, D.J. Perspectives on the advances in the pharmacotherapeutic management of Duchenne muscular dystrophy. Expert Opin. Pharmacother. 2022, 23, 1701–1710. [Google Scholar] [CrossRef]
- Hoch, L.; Bourg, N.; Degrugillier, F.; Bruge, C.; Benabides, M.; Pellier, E.; Tournois, J.; Mahé, G.; Maignan, N.; Dawe, J.; et al. Dual Blockade of Misfolded Alpha-Sarcoglycan Degradation by Bortezomib and Givinostat Combination. Front. Pharmacol. 2022, 13, 856804. [Google Scholar] [CrossRef]
- Alonso-Pérez, J.; Carrasco-Rozas, A.; Borrell-Pages, M.; Fernández-Simón, E.; Piñol-Jurado, P.; Badimon, L.; Wollin, L.; Lleixà, C.; Gallardo, E.; Olivé, M.; et al. Nintedanib Reduces Muscle Fibrosis and Improves Muscle Function of the Alpha-Sarcoglycan-Deficient Mice. Biomedicines 2022, 10, 2629. [Google Scholar] [CrossRef]
- Foltz, S.J.; Luan, J.; Call, J.A.; Patel, A.; Peissig, K.B.; Fortunato, M.J.; Beedle, A.M. Four-week rapamycin treatment improves muscular dystrophy in a fukutin-deficient mouse model of dystroglycanopathy. Skelet. Muscle 2016, 6, 20. [Google Scholar] [CrossRef]
- Wu, B.; Shah, S.N.; Lu, P.; Bollinger, L.E.; Blaeser, A.; Sparks, S.; Harper, A.D.; Lu, Q.L. Long-Term Treatment of Tamoxifen and Raloxifene Alleviates Dystrophic Phenotype and Enhances Muscle Functions of FKRP Dystroglycanopathy. Am. J. Pathol. 2018, 188, 1069–1080. [Google Scholar] [CrossRef]
- Harandi, V.M.; Oliveira, B.M.S.; Allamand, V.; Friberg, A.; Fontes-Oliveira, C.C.; Durbeej, M. Antioxidants Reduce Muscular Dystrophy in the dy(2J)/dy(2J) Mouse Model of Laminin α2 Chain-Deficient Muscular Dystrophy. Antioxidants 2020, 9, 244. [Google Scholar] [CrossRef]
- Girgenrath, M.; Beermann, M.L.; Vishnudas, V.K.; Homma, S.; Miller, J.B. Pathology is alleviated by doxycycline in a laminin-alpha2-null model of congenital muscular dystrophy. Ann. Neurol. 2009, 65, 47–56. [Google Scholar] [CrossRef]
- Elbaz, M.; Yanay, N.; Laban, S.; Rabie, M.; Mitrani-Rosenbaum, S.; Nevo, Y. Life or death by NFκB, Losartan promotes survival in dy2J/dy2J mouse of MDC1A. Cell Death Dis. 2015, 6, e1690. [Google Scholar] [CrossRef]
- Meinen, S.; Lin, S.; Ruegg, M.A. Angiotensin II type 1 receptor antagonists alleviate muscle pathology in the mouse model for laminin-α2-deficient congenital muscular dystrophy (MDC1A). Skelet. Muscle 2012, 2, 18. [Google Scholar] [CrossRef]
- Kanamori, H.; Naruse, G.; Yoshida, A.; Minatoguchi, S.; Watanabe, T.; Kawaguchi, T.; Yamada, Y.; Mikami, A.; Kawasaki, M.; Takemura, G.; et al. Metformin Enhances Autophagy and Provides Cardioprotection in δ-Sarcoglycan Deficiency-Induced Dilated Cardiomyopathy. Circulation. Heart Fail. 2019, 12, e005418. [Google Scholar] [CrossRef]
- De Los Santos, S.; Palma-Flores, C.; Zentella-Dehesa, A.; Canto, P.; Coral-Vázquez, R.M. (-)-Epicatechin inhibits development of dilated cardiomyopathy in δ sarcoglycan null mouse. Nutr. Metab. Cardiovasc. Dis. NMCD 2018, 28, 1188–1195. [Google Scholar] [CrossRef]
- Li, J.W.; Wang, X.Y.; Zhang, X.; Gao, L.; Wang, L.F.; Yin, X.H. (-)-Epicatechin protects against myocardial ischemia-induced cardiac injury via activation of the PTEN/PI3K/AKT pathway. Mol. Med. Rep. 2018, 17, 8300–8308. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.D.; Durbeej, M.; Moore, S.A.; Coral-Vazquez, R.; Prouty, S.; Campbell, K.P. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J. Clin. Investig. 2001, 107, R1–R7. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, S.; Sciorati, C.; D’Antona, G.; Innocenzi, A.; Covarello, D.; Galvez, B.G.; Perrotta, C.; Monopoli, A.; Sanvito, F.; Bottinelli, R.; et al. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc. Natl. Acad. Sci. USA 2007, 104, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Hoch, L.; Henriques, S.F.; Bruge, C.; Marsolier, J.; Benabides, M.; Bourg, N.; Tournois, J.; Mahé, G.; Morizur, L.; Jarrige, M.; et al. Identification of thiostrepton as a pharmacological approach to rescue misfolded alpha-sarcoglycan mutant proteins from degradation. Sci. Rep. 2019, 9, 6915. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; De los Santos, S.; Gonzalez-Basurto, S.; Canto, P.; Mendoza-Lorenzo, P.; Palma-Flores, C.; Ceballos-Reyes, G.; Villarreal, F.; Zentella-Dehesa, A.; Coral-Vazquez, R. (-)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic δ-sarcoglycan null mouse striated muscle. FEBS J. 2014, 281, 5567–5580. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meinen, S.; Barzaghi, P.; Sumanovski, L.T.; Courdier-Früh, I.; Rüegg, M.A.; Meier, T. Omigapil ameliorates the pathology of muscle dystrophy caused by laminin-alpha2 deficiency. J. Pharmacol. Exp. Ther. 2009, 331, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Nevo, Y.; Halevy, O.; Genin, O.; Moshe, I.; Turgeman, T.; Harel, M.; Biton, E.; Reif, S.; Pines, M. Fibrosis inhibition and muscle histopathology improvement in laminin-alpha2-deficient mice. Muscle Nerve 2010, 42, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Southern, W.M.; Nichenko, A.S.; Qualls, A.E.; Portman, K.; Gidon, A.; Beedle, A.M.; Call, J.A. Mitochondrial dysfunction in skeletal muscle of fukutin-deficient mice is resistant to exercise- and 5-aminoimidazole-4-carboxamide ribonucleotide-induced rescue. Exp. Physiol. 2020, 105, 1767–1777. [Google Scholar] [CrossRef]
- Rampoldi, E.; Meola, G.; Conti, A.M.; Velicogna, M.; Larizza, L. A comparative analysis of collagen III, IV, laminin and fibronectin in Duchenne muscular dystrophy biopsies and cell cultures. Eur. J. Cell Biol. 1986, 42, 27–34. [Google Scholar]
- Cáceres, S.; Cuellar, C.; Casar, J.C.; Garrido, J.; Schaefer, L.; Kresse, H.; Brandan, E. Synthesis of proteoglycans is augmented in dystrophic mdx mouse skeletal muscle. Eur. J. Cell Biol. 2000, 79, 173–181. [Google Scholar] [CrossRef]
- Fadic, R.; Mezzano, V.; Alvarez, K.; Cabrera, D.; Holmgren, J.; Brandan, E. Increase in decorin and biglycan in Duchenne Muscular Dystrophy: Role of fibroblasts as cell source of these proteoglycans in the disease. J. Cell. Mol. Med. 2006, 10, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Beyer, C.; Palumbo-Zerr, K.; Zhang, Y.; Ramming, A.; Distler, A.; Gelse, K.; Distler, O.; Schett, G.; Wollin, L.; et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, K.E.; Zhong, J.; Papakonstantinou, E.; Karakiulakis, G.; Tamm, M.; Seidel, P.; Sun, Q.; Mandal, J.; Lardinois, D.; Lambers, C.; et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir. Res. 2014, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, M.; Machahua-Huamani, C.; Vicens-Zygmunt, V.; Llatjós, R.; Escobar, I.; Sala-Llinas, E.; Luburich-Hernaiz, P.; Dorca, J.; Montes-Worboys, A. Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm. Med. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Carthy, J.M.; Sundqvist, A.; Heldin, A.; van Dam, H.; Kletsas, D.; Heldin, C.H.; Moustakas, A. Tamoxifen Inhibits TGF-β-Mediated Activation of Myofibroblasts by Blocking Non-Smad Signaling Through ERK1/2. J. Cell. Physiol. 2015, 230, 3084–3092. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Ichikawa, T.; Liu, X.; Kobayashi, T.; Wang, X.Q.; Kawasaki, S.; Togo, S.; Kamio, K.; Mao, L.; Ann, Y.; et al. N-acetyl-L-cysteine inhibits TGF-beta1-induced profibrotic responses in fibroblasts. Pulm. Pharmacol. Ther. 2009, 22, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Lahme, B.; Tihaa, L.; Weiskirchen, R.; Gressner, A.M. N-acetyl-L-cysteine suppresses TGF-beta signaling at distinct molecular steps: The biochemical and biological efficacy of a multifunctional, antifibrotic drug. Biochem. Pharmacol. 2005, 70, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, Z.; Zu, C.; Ma, J.; Lu, S.; Zhong, J.; Zhang, S. Losartan Attenuates Myocardial Endothelial-To-Mesenchymal Transition in Spontaneous Hypertensive Rats via Inhibiting TGF-β/Smad Signaling. PLoS ONE 2016, 11, e0155730. [Google Scholar] [CrossRef]
- Penke, L.R.K.; Speth, J.; Wettlaufer, S.; Draijer, C.; Peters-Golden, M. Bortezomib Inhibits Lung Fibrosis and Fibroblast Activation without Proteasome Inhibition. Am. J. Respir. Cell Mol. Biol. 2022, 66, 23–37. [Google Scholar] [CrossRef]
- Accorsi, A.; Cramer, M.L.; Girgenrath, M. Fibrogenesis in LAMA2-Related Muscular Dystrophy Is a Central Tenet of Disease Etiology. Front. Mol. Neurosci. 2020, 13, 3. [Google Scholar] [CrossRef]
- Sato, S.; Kawamura, H.; Takemoto, M.; Maezawa, Y.; Fujimoto, M.; Shimoyama, T.; Koshizaka, M.; Tsurutani, Y.; Watanabe, A.; Ueda, S.; et al. Halofuginone prevents extracellular matrix deposition in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2009, 379, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fei, H.; Wang, Z.; Zhu, T. Low-dose halofuginone inhibits the synthesis of type I collagen without influencing type II collagen in the extracellular matrix of chondrocytes. Mol. Med. Rep. 2017, 16, 3290–3298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, K.H.; Hsu, H.H.; Lee, C.C.; Yen, T.H.; Ko, Y.C.; Yang, C.W.; Hung, C.C. The AMPK agonist AICAR inhibits TGF-β1 induced activation of kidney myofibroblasts. PLoS ONE 2014, 9, e106554. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, K.A.; Trial, J.; Entman, M.L. Aicar treatment reduces interstitial fibrosis in aging mice: Suppression of the inflammatory fibroblast. J. Mol. Cell. Cardiol. 2017, 111, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, X.; Guo, Z.; Fan, Y.; Tian, F.; Li, T.; Pan, Q.; Liu, F.; Liang, X. The Dual Effect and Mechanism of (-)-epicatechin on Hypoxic-induced Proliferation and Apoptosis of Cardiac Fibroblasts. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Massagué, J.; Xi, Q. TGF-β control of stem cell differentiation genes. FEBS Lett. 2012, 586, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.; Jenkins, R.H.; Fraser, D.J. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J. Pathol. 2013, 229, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, R.; Overstreet, J.M.; Higgins, P.J. TGF-β signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cell. Signal. 2013, 25, 264–268. [Google Scholar] [CrossRef]
- Elbaz, M.; Yanay, N.; Aga-Mizrachi, S.; Brunschwig, Z.; Kassis, I.; Ettinger, K.; Barak, V.; Nevo, Y. Losartan, a therapeutic candidate in congenital muscular dystrophy: Studies in the dy(2J) /dy(2J) mouse. Ann. Neurol. 2012, 71, 699–708. [Google Scholar] [CrossRef]
- Ardite, E.; Perdiguero, E.; Vidal, B.; Gutarra, S.; Serrano, A.L.; Muñoz-Cánoves, P. PAI-1-regulated miR-21 defines a novel age-associated fibrogenic pathway in muscular dystrophy. J. Cell Biol. 2012, 196, 163–175. [Google Scholar] [CrossRef]
- Dong, Y.; Lakhia, R.; Thomas, S.S.; Dong, Y.; Wang, X.H.; Silva, K.A.; Zhang, L. Interactions between p-Akt and Smad3 in injured muscles initiate myogenesis or fibrogenesis. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E367–E375. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.N.; Andres-Mateos, E.; Marx, R.; Mejias, R.; Van Erp, C.; Simmers, J.L.; Walston, J.D.; Ward, C.W.; Cohn, R.D. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Transl. Med. 2011, 3, 82ra37. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Kim, D.Y.; Kim, A.Y.; Lee, E.J.; Kim, S.H.; Lee, M.M.; Sung, S.E.; Park, J.K.; Jeong, K.S. Chronic effects of losartan on the muscles and the serologic profiles of mdx mice. Life Sci. 2015, 143, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Brietz, A.; Schuch, K.V.; Wangorsch, G.; Lorenz, K.; Dandekar, T. Analyzing ERK 1/2 signalling and targets. Mol. Biosyst. 2016, 12, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Wetzker, R.; Böhmer, F.D. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat. Rev. Mol. Cell Biol. 2003, 4, 651–657. [Google Scholar] [CrossRef]
- Chang, F.; Steelman, L.S.; Lee, J.T.; Shelton, J.G.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia 2003, 17, 1263–1293. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 1995, 80, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Yamada, M.; Kunida, K.; Yasuda, S.; Matsuda, M. Processive phosphorylation of ERK MAP kinase in mammalian cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12675–12680. [Google Scholar] [CrossRef] [PubMed]
- Chambard, J.C.; Lefloch, R.; Pouysségur, J.; Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta 2007, 1773, 1299–1310. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, K.; Huang, M.; Qiu, Q.; Xiao, Y.; Shi, M.; Zou, Y.; Yang, X.; Xu, H.; Liang, L. Halofuginone inhibits TNF-α-induced the migration and proliferation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Int. Immunopharmacol. 2017, 43, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Haycock, J.W.; MacNeil, S.; Jones, P.; Harris, J.B.; Mantle, D. Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport 1996, 8, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Wehling-Henricks, M. The role of free radicals in the pathophysiology of muscular dystrophy. J. Appl. Physiol. 2007, 102, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Tasca, E.; Nascimbeni, A.C.; Fanin, M. Muscle fatigue, nNOS and muscle fiber atrophy in limb girdle muscular dystrophy. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2014, 33, 119–126. [Google Scholar]
- Tidball, J.G.; Wehling-Henricks, M. Evolving therapeutic strategies for Duchenne muscular dystrophy: Targeting downstream events. Pediatr. Res. 2004, 56, 831–841. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Tidball, J.G. Expression of a muscle-specific, nitric oxide synthase transgene prevents muscle membrane injury and reduces muscle inflammation during modified muscle use in mice. J. Physiol. 2003, 550, 347–356. [Google Scholar] [CrossRef]
- Terrill, J.R.; Radley-Crabb, H.G.; Iwasaki, T.; Lemckert, F.A.; Arthur, P.G.; Grounds, M.D. Oxidative stress and pathology in muscular dystrophies: Focus on protein thiol oxidation and dysferlinopathies. FEBS J. 2013, 280, 4149–4164. [Google Scholar] [CrossRef] [PubMed]
- Disatnik, M.H.; Dhawan, J.; Yu, Y.; Beal, M.F.; Whirl, M.M.; Franco, A.A.; Rando, T.A. Evidence of oxidative stress in mdx mouse muscle: Studies of the pre-necrotic state. J. Neurol. Sci. 1998, 161, 77–84. [Google Scholar] [CrossRef]
- Whitehead, N.P.; Pham, C.; Gervasio, O.L.; Allen, D.G. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J. Physiol. 2008, 586, 2003–2014. [Google Scholar] [CrossRef]
- Messina, S.; Altavilla, D.; Aguennouz, M.; Seminara, P.; Minutoli, L.; Monici, M.C.; Bitto, A.; Mazzeo, A.; Marini, H.; Squadrito, F.; et al. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am. J. Pathol. 2006, 168, 918–926. [Google Scholar] [CrossRef]
- Ismail, H.M.; Scapozza, L.; Ruegg, U.T.; Dorchies, O.M. Diapocynin, a dimer of the NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic muscle. PLoS ONE 2014, 9, e110708. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Deodato, B.; Campo, G.M.; Arlotta, M.; Miano, M.; Squadrito, G.; Saitta, A.; Cucinotta, D.; Ceccarelli, S.; Ferlito, M.; et al. IRFI 042, a novel dual vitamin E-like antioxidant, inhibits activation of nuclear factor-kappaB and reduces the inflammatory response in myocardial ischemia-reperfusion injury. Cardiovasc. Res. 2000, 47, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.M.; Zimmer, M.; Offman, E.; Grant, T.; Jirousek, M. A Novel NF-κB Inhibitor, Edasalonexent (CAT-1004), in Development as a Disease-Modifying Treatment for Patients with Duchenne Muscular Dystrophy: Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics in Adult Subjects. J. Clin. Pharmacol. 2017, 57, 627–639. [Google Scholar] [CrossRef]
- Acharyya, S.; Villalta, S.A.; Bakkar, N.; Bupha-Intr, T.; Janssen, P.M.; Carathers, M.; Li, Z.W.; Beg, A.A.; Ghosh, S.; Sahenk, Z.; et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Investig. 2007, 117, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, B.Y.; Peterson, C.A.; Horbinski, C.; Crofford, L.J. Implications of glucocorticoid therapy in idiopathic inflammatory myopathies. Nat. Reviews. Rheumatol. 2012, 8, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.R.; Ryan, M.J.; Alway, S.E. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 751–764. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Squire, S.; Raymackers, J.M.; Vandebrouck, C.; Potter, A.; Tinsley, J.; Fisher, R.; Gillis, J.M.; Davies, K.E. Prevention of pathology in mdx mice by expression of utrophin: Analysis using an inducible transgenic expression system. Hum. Mol. Genet. 2002, 11, 3333–3344. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.S.; Delgado-Diaz, D.C.; Carson, J.; Fayad, R.; Wilson, L.B.; Kostek, M.C. Resveratrol improves muscle function but not oxidative capacity in young mdx mice. Can. J. Physiol. Pharmacol. 2014, 92, 243–251. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef]
- Farup, J.; Madaro, L.; Puri, P.L.; Mikkelsen, U.R. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 2015, 6, e1830. [Google Scholar] [CrossRef] [PubMed]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Mallouk, N.; Jacquemond, V.; Allard, B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4950–4955. [Google Scholar] [CrossRef] [PubMed]
- Culligan, K.G.; Mackey, A.J.; Finn, D.M.; Maguire, P.B.; Ohlendieck, K. Role of dystrophin isoforms and associated proteins in muscular dystrophy (review). Int. J. Mol. Med. 1998, 2, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; Goonasekera, S.A.; Sargent, M.A.; Maillet, M.; Aronow, B.J.; Molkentin, J.D. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 19023–19028. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W., Jr. Capacitative calcium entry: Sensing the calcium stores. J. Cell Biol. 2005, 169, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W., Jr. Capacitative calcium entry revisited. Cell Calcium 1990, 11, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Buchanan, J.; Luik, R.M.; Lewis, R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006, 174, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Lyfenko, A.D.; Dirksen, R.T. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J. Physiol. 2008, 586, 4815–4824. [Google Scholar] [CrossRef]
- Zhao, X.; Moloughney, J.G.; Zhang, S.; Komazaki, S.; Weisleder, N. Orai1 mediates exacerbated Ca(2+) entry in dystrophic skeletal muscle. PLoS ONE 2012, 7, e49862. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, K.J.; Calabria, E.; Pallafacchina, G.; Ciciliot, S.; Serrano, A.L.; Argentini, C.; Kalhovde, J.M.; Lømo, T.; Schiaffino, S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc. Natl. Acad. Sci. USA 2004, 101, 10590–10595. [Google Scholar] [CrossRef] [PubMed]
- Demonbreun, A.R.; Lapidos, K.A.; Heretis, K.; Levin, S.; Dale, R.; Pytel, P.; Svensson, E.C.; McNally, E.M. Myoferlin regulation by NFAT in muscle injury, regeneration and repair. J. Cell Sci. 2010, 123, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, J.V.; Harrison, M.A.; Carbonetto, S.; Chin, E.; Michel, R.N.; Jasmin, B.J. Stimulation of calcineurin signaling attenuates the dystrophic pathology in mdx mice. Hum. Mol. Genet. 2004, 13, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Stupka, N.; Schertzer, J.D.; Bassel-Duby, R.; Olson, E.N.; Lynch, G.S. Stimulation of calcineurin Aalpha activity attenuates muscle pathophysiology in mdx dystrophic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R983–R992. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kay, D.I.; Rudra, R.T.; Chen, B.M.; Hsu, N.; Izumiya, Y.; Martinez, L.; Spencer, M.J.; Walsh, K.; Grinnell, A.D.; et al. Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function in dystrophin-deficient mdx mice. Hum. Mol. Genet. 2011, 20, 1324–1338. [Google Scholar] [CrossRef]
- Peter, A.K.; Miller, G.; Crosbie, R.H. Disrupted mechanical stability of the dystrophin-glycoprotein complex causes severe muscular dystrophy in sarcospan transgenic mice. J. Cell Sci. 2007, 120, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Boppart, M.D.; Burkin, D.J.; Kaufman, S.J. Activation of AKT signaling promotes cell growth and survival in α7β1 integrin-mediated alleviation of muscular dystrophy. Biochim. Et Biophys. Acta 2011, 1812, 439–446. [Google Scholar] [CrossRef]
- Gurpur, P.B.; Liu, J.; Burkin, D.J.; Kaufman, S.J. Valproic acid activates the PI3K/Akt/mTOR pathway in muscle and ameliorates pathology in a mouse model of Duchenne muscular dystrophy. Am. J. Pathol. 2009, 174, 999–1008. [Google Scholar] [CrossRef]
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Vieira, N.M.; Eisenberg, I.; Marshall, J.L.; Gasperini, M.J.; Lek, A.; Myers, J.A.; Estrella, E.A.; et al. MicroRNA-486-dependent modulation of DOCK3/PTEN/AKT signaling pathways improves muscular dystrophy-associated symptoms. J. Clin. Investig. 2014, 124, 2651–2667. [Google Scholar] [CrossRef] [PubMed]
- Roffe, S.; Hagai, Y.; Pines, M.; Halevy, O. Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells: Effect on myotube fusion. Exp. Cell Res. 2010, 316, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
| Disease | Protein | Dose (Time Treatment) | Original Indication | Study Model | Outcomes | Pathways Modified | Reference |
|---|---|---|---|---|---|---|---|
| Sarcoglycanopathy | α-SG | Bortezomib and Givinostat (5 nM and 10 uM/ 24 h) | Multiple myeloma and DMD | R77C-α-SGmCh (independent triplicate) | R77C-α-SG membrane expression rescue | Blocks autophagy and proteasome | [52] |
| Nintedanib (50 mg/kg twice daily/10 weeks) | Pulmonary fibrosis | Sgca-/- mice (n = 21 †,¥) | Results in a functional muscle improvement, attenuating muscle fibrosis and inflammation | Reduction in the expression of some genes that are also involved in inflammation | [53] | ||
| Secondary Dystroglycanopathies | FKRP | Rapamycin (2 mg/kg once a day/4 weeks) | Immunosuppression | Myf5/Fktn conditional KO (n = 12 ¥) | Reduction of fibrosis and muscle damage improves muscle function | Partial regulation of autophagy and increased mTORC1 activation | [54] |
| Tamoxifen and raloxifene (50 mg/kg and 100 mg/kg/1 year) | Breast cancer and osteoporosis | P448L mice (n = 10 †) | Reduces muscle degeneration, reduces fibrosis, improves muscle functions, respiratory functions, and bone density | Inhibition of TGF-β and PAI-1, inhibition of fibroblast proliferation, inhibition of protein kinase C and NF-kB pathway | [55] | ||
| Congenital muscular dystrophy type 1A (MDC1A) | Laminin-α2 | N-acetyl-L-cysteine (NAC) and Vitamin E (150 mg/kg and 40 mg/kg, six times a week for 22 and 14 days, respectively) | Acetaminophen overdose, cystic fibrosis, and antioxidant activity | Homozygous dy2J/dy2J mice (n = 5 §) | Preserves muscle strength, reduces central nuclei, apoptosis, inflammation, fibrosis, and oxidative stress | Inhibits the upregulation of Fn1 gene expression in a differential manner in muscles | [56] |
| Doxycycline (6 mg/mL 6 weeks) | Antibiotic | Heterozygous Lama2dy-W/+ (n = 9) | Increases the median lifespan, increases body weight, delays hindlimb paralysis, reduces inflammation, inhibits apoptosis, delays the appearance of functional defects in motor nerves | Increases Akt phosphorylation, inactivation of Bax, and decreases caspase-3 activity | [57] | ||
| Losartan 0.6 g/l, 12 weeks | Hypertension | Homozygous dy2J/dy2J (n = 12 †) | Promotes survival, improves muscle strength, reduces fibrosis | Inhibits TGF-β and MAPK, increases TNF-α and mRNA expression of TRAF1, TRAF2, CIAP2, and FTH-1, increases BCL2, decreases caspase-3 | [58,59] |
| Disease | Protein | Drug Name | Dose (Treatment Time) | Study Model | Skeletal Muscle Effect | Reference |
|---|---|---|---|---|---|---|
| Sarcoglycanopathy | α-SG | HCT 1026 and nitric oxide | 30 mg/kg (for up to 12 months) | α-SG-null mice (n = 9) | Inhibition of inflammation. Preservation of satellite cell number and activity. | [64] |
| Thiostrepton | 3 μM (24 h) | R77C-α-SGmCh (independent triplicate) | Rescue of different mutations in α-SG. Membrane rescue. | [65] | ||
| δ-SG | (−)-Epicatechin | 1 mg/kg (twice a day for 2 weeks) | B6.129-Sgcdtm1Mcn/J mice (n = 5) | Recovery of reduced/ oxidized glutathione. Enhanced superoxide dismutase 2, catalase, and citrate synthase activities. | [66] | |
| Secondary Dystroglycanopathies | FKRP | AICAR | 500 mg/kg (4 weeks) | Myf5/Fktn knockout (n = 7 †,§) | Recovery of muscle contractile function. Enhancing autophagy. Induces satellite cell proliferation. | [69] |
| Congenital muscular dystrophy type 1A (MDC1A) | Laminin-α2 | Halofuginone | 5 μg (three times a week for 5 or 15 weeks) | dy2J/dy2J mice (n = 5) | Inhibited muscle fibrosis. Reduced collagen levels. Reduced number of centrally nuclear myofibers. Increased myofiber diameter. | [68] |
| Omigapil | 0.1 or 1 mg/kg | dyw/dyw mice (n = 13 ¥) | Reduces apoptosis and fibrotic tissue. | [67] |
| Drug/Molecule | Experimental Model | Mechanism of Action/Effect | Reference |
|---|---|---|---|
| AICAR | NRK-49F rat renal fibroblast cell line | Partially mediated through the activation of AMPKα-1. | [84,85] |
| Downregulation of ERK 1/2. | |||
| Mesenchymal fibroblasts derived from Hearts rats | Mesenchymal fibroblasts derived from Hearts rats. | ||
| NAC | Hepatic stellate cells isolated from male rats | Interferes with TGF-β. | |
| Sprague Dawley rats | The phosphorylation of receptor Smads by TβRI is abolished. | [77,78] | |
| Decrease in ligand affinity of the type III receptor (TβRIII) complex. | |||
| Human fetal lung fibroblast (HFL-1) cells | Inhibits the increase in TGF-β1. | ||
| Inactivates TGF-β1 itself and inhibits binding to its receptor. | |||
| Rapamycin and | Lung primary fibroblasts | Inhibits ECM protein expression. | |
| Pirfenidone | Inhibits the synthesis of profibrotic markers induced by TGF-β. | [75] | |
| Tamoxifen | Primary human skin fibroblasts (AG1523) | Inhibits ERK1/2 signaling. | |
| Human breast fibroblasts (HBF1 and HBF12) | Inhibits the activation of fibroblasts. | [76] | |
| Nintedanib | Biopsies from patients with systemic sclerosis (SSc) | Reduces TGF-β-induced fibrosis. | [73,74] |
| Reduced proliferation and migration of fibroblasts, as well as myofibroblast differentiation and collagen release. | |||
| Primary human lung fibroblasts | Antiproliferative capacity. | ||
| Receptor tyrosine kinase inhibitor. | |||
| (−)-Epicatechin | Cardiac fibroblasts | Decreases TGF-β1 levels. | [86] |
| Decreases fibronectin, urea, proline, and total collagen protein levels. | |||
| Restores levels of estrogen receptor (GPER). | |||
| Effects on the SMAD/TGF-β1 pathway, decreases SMAD levels. | |||
| Halofuginone | Tight skin mouse (TSK) model for scleroderma | Blocked the phosphorylation and subsequent activation of Smad3 downstream of TGFβ signaling. | [82,83] |
| Fibroblasts isolated from rat renal papillae | Inhibition of proliferation. | ||
| Inhibition of PDGF. | |||
| Bortezomib | Adult human lung fibroblast lines | Inhibits FGF-2-induced fibroblast proliferation. | |
| (CCL-210 and MRC5) | Prevents TGF-β-induced fibroblast differentiation. | ||
| Inhibits key kinases activated by TGF-b and FGF-2. | [80] | ||
| Losartan | C57BL/6 J mice, unilateral ureteral obstruction (UUO) | Degradation of TβRI through expression of Smurf1 and Smurf2. | |
| Spontaneously hypertensive rats (SHRs) | Inhibition of the classical TGF-β/Smad pathway. | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Angulo, A.; Landa-Solís, C.; Escobar-Cedillo, R.E.; Estrada-Mena, F.J.; Sánchez-Chapul, L.; Gómez-Díaz, B.; Carrillo-Mora, P.; Avilés-Arnaut, H.; Jiménez-Hernández, L.; Jiménez-Hernández, D.A.; et al. Pharmacological Treatments and Therapeutic Targets in Muscle Dystrophies Generated by Alterations in Dystrophin-Associated Proteins. Medicina 2024, 60, 1060. https://doi.org/10.3390/medicina60071060
Luna-Angulo A, Landa-Solís C, Escobar-Cedillo RE, Estrada-Mena FJ, Sánchez-Chapul L, Gómez-Díaz B, Carrillo-Mora P, Avilés-Arnaut H, Jiménez-Hernández L, Jiménez-Hernández DA, et al. Pharmacological Treatments and Therapeutic Targets in Muscle Dystrophies Generated by Alterations in Dystrophin-Associated Proteins. Medicina. 2024; 60(7):1060. https://doi.org/10.3390/medicina60071060
Chicago/Turabian StyleLuna-Angulo, Alexandra, Carlos Landa-Solís, Rosa Elena Escobar-Cedillo, Francisco Javier Estrada-Mena, Laura Sánchez-Chapul, Benjamín Gómez-Díaz, Paul Carrillo-Mora, Hamlet Avilés-Arnaut, Livier Jiménez-Hernández, Dulce Adeí Jiménez-Hernández, and et al. 2024. "Pharmacological Treatments and Therapeutic Targets in Muscle Dystrophies Generated by Alterations in Dystrophin-Associated Proteins" Medicina 60, no. 7: 1060. https://doi.org/10.3390/medicina60071060
APA StyleLuna-Angulo, A., Landa-Solís, C., Escobar-Cedillo, R. E., Estrada-Mena, F. J., Sánchez-Chapul, L., Gómez-Díaz, B., Carrillo-Mora, P., Avilés-Arnaut, H., Jiménez-Hernández, L., Jiménez-Hernández, D. A., & Miranda-Duarte, A. (2024). Pharmacological Treatments and Therapeutic Targets in Muscle Dystrophies Generated by Alterations in Dystrophin-Associated Proteins. Medicina, 60(7), 1060. https://doi.org/10.3390/medicina60071060






