Impact of Norepinephrine and Dopamine Infusion on Renal Arterial Resistive Index during Pre-Emptive Living Donor Kidney Transplantation: Propensity Score Matching Analysis
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Considerations
2.2. Study Population
2.3. Surgery and General Anesthesia
2.4. Norepinephrine and Dopamine Infusion during Surgery
2.5. Intraoperative RARI Measurement
2.6. Clinical Variables for PSM
2.7. Postoperative Variables
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Comparison of Perioperative Factors before and after PSM
3.3. RARI in the Dopamine and Norepinephrine Groups in PS-Matched Patients
3.4. Association between Norepinephrine Infusion and a High RARI during Pre-Emptive LDKT
3.5. Intraoperative Vital Signs and Brain Natriuretic Peptide Level in PSM Patients
3.6. Postoperative Kidney Graft Outcomes in PSM Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kohei, N.; Sawada, Y.; Hirai, T.; Omoto, K.; Ishida, H.; Tanabe, K. Influence of dialysis duration on the outcome of living kidney transplantation. Ther. Apher. Dial. 2014, 18, 481–488. [Google Scholar] [CrossRef]
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, S11–S103. [Google Scholar] [CrossRef]
- Andrews, P.A.; Burnapp, L. British Transplantation Society/Renal Association UK Guidelines for Living Donor Kidney Transplantation 2018: Summary of Updated Guidance. Transplantation 2018, 102, e307. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Snyder, J.J.; Matas, A.J.; Ellison, M.D.; Gill, J.S.; Kausz, A.T. Preemptive kidney transplantation: The advantage and the advantaged. J. Am. Soc. Nephrol. 2002, 13, 1358–1364. [Google Scholar] [CrossRef]
- Papalois, V.E.; Moss, A.; Gillingham, K.J.; Sutherland, D.E.; Matas, A.J.; Humar, A. Pre-emptive transplants for patients with renal failure: An argument against waiting until dialysis. Transplantation 2000, 70, 625–631. [Google Scholar] [CrossRef]
- Auneau-Enjalbert, L.; Hardouin, J.B.; Blanchin, M.; Giral, M.; Morelon, E.; Cassuto, E.; Meurette, A.; Sébille, V. Comparison of longitudinal quality of life outcomes in preemptive and dialyzed patients on waiting list for kidney transplantation. Qual. Life Res. 2020, 29, 959–970. [Google Scholar] [CrossRef]
- Huang, Y.; Samaniego, M. Preemptive kidney transplantation: Has it come of age? Nephrol. Ther. 2012, 8, 428–432. [Google Scholar] [CrossRef]
- Liem, Y.S.; Weimar, W. Early living-donor kidney transplantation: A review of the associated survival benefit. Transplantation 2009, 87, 317–318. [Google Scholar] [CrossRef]
- Dinavahi, R.; Akalin, E. Preemptive kidney transplantation in patients with diabetes mellitus. Endocrinol. Metab. Clin. N. Am. 2007, 36, 1039–1049. [Google Scholar] [CrossRef]
- Renew, J.R.; Pai, S.L. A simple protocol to improve safety and reduce cost in hemodialysis patients undergoing elective surgery. Middle East. J. Anaesthesiol. 2014, 22, 487–492. [Google Scholar]
- Folsom, A.R.; Lutsey, P.L.; Astor, B.C.; Wattanakit, K.; Heckbert, S.R.; Cushman, M. Chronic kidney disease and venous thromboembolism: A prospective study. Nephrol. Dial. Transplant. 2010, 25, 3296–3301. [Google Scholar] [CrossRef]
- Schmid, S.; Jungwirth, B. Anaesthesia for renal transplant surgery: An update. Eur. J. Anaesthesiol. 2012, 29, 552–558. [Google Scholar] [CrossRef]
- Ricaurte, L.; Vargas, J.; Lozano, E.; Díaz, L. Anesthesia and kidney transplantation. Transplant. Proc. 2013, 45, 1386–1391. [Google Scholar] [CrossRef]
- Tena, B.; Vendrell, M. Perioperative considerations for kidney and pancreas-kidney transplantation. Best. Pract. Res. Clin. Anaesthesiol. 2020, 34, 3–14. [Google Scholar] [CrossRef]
- Mittel, A.M.; Wagener, G. Anesthesia for Kidney and Pancreas Transplantation. Anesthesiol. Clin. 2017, 35, 439–452. [Google Scholar] [CrossRef]
- Ciapetti, M.; di Valvasone, S.; di Filippo, A.; Cecchi, A.; Bonizzoli, M.; Peris, A. Low-dose dopamine in kidney transplantation. Transplant. Proc. 2009, 41, 4165–4168. [Google Scholar] [CrossRef]
- Di Giantomasso, D.; Morimatsu, H.; May, C.N.; Bellomo, R. Increasing renal blood flow: Low-dose dopamine or medium-dose norepinephrine. Chest 2004, 125, 2260–2267. [Google Scholar] [CrossRef]
- Marik, P.E. Low-dose dopamine: A systematic review. Intensive Care Med. 2002, 28, 877–883. [Google Scholar] [CrossRef]
- Kellum, J.A.; Decker, J.M. Use of dopamine in acute renal failure: A meta-analysis. Crit. Care Med. 2001, 29, 1526–1531. [Google Scholar] [CrossRef]
- Redfors, B.; Bragadottir, G.; Sellgren, J.; Swärd, K.; Ricksten, S.E. Effects of norepinephrine on renal perfusion, filtration and oxygenation in vasodilatory shock and acute kidney injury. Intensive Care Med. 2011, 37, 60–67. [Google Scholar] [CrossRef]
- Deruddre, S.; Cheisson, G.; Mazoit, J.X.; Vicaut, E.; Benhamou, D.; Duranteau, J. Renal arterial resistance in septic shock: Effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007, 33, 1557–1562. [Google Scholar] [CrossRef]
- Albanèse, J.; Leone, M.; Garnier, F.; Bourgoin, A.; Antonini, F.; Martin, C. Renal effects of norepinephrine in septic and nonseptic patients. Chest 2004, 126, 534–539. [Google Scholar] [CrossRef]
- Morimatsu, H.; Uchino, S.; Chung, J.; Bellomo, R.; Raman, J.; Buxton, B. Norepinephrine for hypotensive vasodilatation after cardiac surgery: Impact on renal function. Intensive Care Med. 2003, 29, 1106–1112. [Google Scholar] [CrossRef]
- Di Giantomasso, D.; Morimatsu, H.; May, C.N.; Bellomo, R. Intrarenal blood flow distribution in hyperdynamic septic shock: Effect of norepinephrine. Crit. Care Med. 2003, 31, 2509–2513. [Google Scholar] [CrossRef]
- Treggiari, M.M.; Romand, J.A.; Burgener, D.; Suter, P.M.; Aneman, A. Effect of increasing norepinephrine dosage on regional blood flow in a porcine model of endotoxin shock. Crit. Care Med. 2002, 30, 1334–1339. [Google Scholar] [CrossRef]
- Enhesari, A.; Mardpour, S.; Makki, Z.; Mardpour, S. Early ultrasound assessment of renal transplantation as the valuable biomarker of long lasting graft survival: A cross-sectional study. Iran. J. Radiol. 2014, 11, e11492. [Google Scholar] [CrossRef]
- Radermacher, J.; Mengel, M.; Ellis, S.; Stuht, S.; Hiss, M.; Schwarz, A.; Eisenberger, U.; Burg, M.; Luft, F.C.; Gwinner, W.; et al. The renal arterial resistance index and renal allograft survival. N. Engl. J. Med. 2003, 349, 115–124. [Google Scholar] [CrossRef]
- Krumme, B. Renal Doppler sonography—Update in clinical nephrology. Nephron Clin. Pract. 2006, 103, c24–c28. [Google Scholar] [CrossRef]
- Pape, L.; Mengel, M.; Offner, G.; Melter, M.; Ehrich, J.H.; Strehlau, J. Renal arterial resistance index and computerized quantification of fibrosis as a combined predictive tool in chronic allograft nephropathy. Pediatr. Transplant. 2004, 8, 565–570. [Google Scholar] [CrossRef]
- Heine, G.H.; Girndt, M.; Sester, U.; Köhler, H. No rise in renal Doppler resistance indices at peak serum levels of cyclosporin A in stable kidney transplant patients. Nephrol. Dial. Transplant. 2003, 18, 1639–1643. [Google Scholar] [CrossRef]
- Naesens, M.; Heylen, L.; Lerut, E.; Claes, K.; De Wever, L.; Claus, F.; Oyen, R.; Kuypers, D.; Evenepoel, P.; Bammens, B.; et al. Intrarenal resistive index after renal transplantation. N. Engl. J. Med. 2013, 369, 1797–1806. [Google Scholar] [CrossRef]
- Go, J.; Park, S.C.; Yun, S.S.; Ku, J.; Park, J.; Shim, J.W.; Lee, H.M.; Kim, Y.S.; Moon, Y.E.; Hong, S.H.; et al. Exposure to Hyperchloremia Is Associated with Poor Early Recovery of Kidney Graft Function after Living-Donor Kidney Transplantation: A Propensity Score-Matching Analysis. J. Clin. Med. 2019, 8, 955. [Google Scholar] [CrossRef]
- Regolisti, G.; Maggiore, U.; Cademartiri, C.; Belli, L.; Gherli, T.; Cabassi, A.; Morabito, S.; Castellano, G.; Gesualdo, L.; Fiaccadori, E. Renal resistive index by transesophageal and transparietal echo-doppler imaging for the prediction of acute kidney injury in patients undergoing major heart surgery. J. Nephrol. 2017, 30, 243–253. [Google Scholar] [CrossRef]
- Radermacher, J.; Ellis, S.; Haller, H. Renal resistance index and progression of renal disease. Hypertension 2002, 39, 699–703. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- Jaszczuk, S.; Natarajan, S.; Papalois, V. Anaesthetic Approach to Enhanced Recovery after Surgery for Kidney Transplantation: A Narrative Review. J. Clin. Med. 2022, 11, 3435. [Google Scholar] [CrossRef]
- Smudla, A.; Trimmel, D.; Szabó, G.; Fazakas, J. Systolic Blood Pressure Pattern: The Tick Mark Signal of Delayed Renal Graft Function. Transplant. Proc. 2019, 51, 1226–1230. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA 2016, 316, 509–518. [Google Scholar] [CrossRef]
- Marik, P.E.; Mohedin, M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA 1994, 272, 1354–1357. [Google Scholar] [CrossRef]
- Segal, J.M.; Phang, P.T.; Walley, K.R. Low-dose dopamine hastens onset of gut ischemia in a porcine model of hemorrhagic shock. J. Appl. Physiol. 1992, 73, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.P.; Korner, P.I.; Selig, S.E. Mechanisms involved in the renal responses to intravenous and renal artery infusions of noradrenaline in conscious dogs. J. Physiol. 1981, 321, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cronin, R.E.; Erickson, A.M.; de Torrente, A.; McDonald, K.M.; Schrier, R.W. Norepinephrine-induced acute renal failure: A reversible ischemic model of acute renal failure. Kidney Int. 1978, 14, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Cronin, R.E.; de Torrente, A.; Miller, P.D.; Bulger, R.E.; Burke, T.J.; Schrier, R.W. Pathogenic mechanisms in early norepinephrine-induced acute renal failure: Functional and histological correlates of protection. Kidney Int. 1978, 14, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Llinás, M.T.; López, R.; Rodríguez, F.; Roig, F.; Salazar, F.J. Role of COX-2-derived metabolites in regulation of the renal hemodynamic response to norepinephrine. Am. J. Physiol. Renal Physiol. 2001, 281, F975–F982. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Deichmann, P.C. Afferent arteriolar responses to ANG II involve activation of PLA2 and modulation by lipoxygenase and P-450 pathways. Am. J. Physiol. 1997, 273, F274–F282. [Google Scholar] [CrossRef] [PubMed]
- Chatziantoniou, C.; Arendshorst, W.J. Prostaglandin interactions with angiotensin, norepinephrine, and thromboxane in rat renal vasculature. Am. J. Physiol. 1992, 262, F68–F76. [Google Scholar] [CrossRef] [PubMed]
- Pelayo, J.C. Renal adrenergic effector mechanisms: Glomerular sites for prostaglandin interaction. Am. J. Physiol. 1988, 254, F184–F190. [Google Scholar] [CrossRef]
- Inscho, E.W.; Carmines, P.K.; Navar, L.G. Prostaglandin influences on afferent arteriolar responses to vasoconstrictor agonists. Am. J. Physiol. 1990, 259, F157–F163. [Google Scholar] [CrossRef]
- LeDoux, D.; Astiz, M.E.; Carpati, C.M.; Rackow, E.C. Effects of perfusion pressure on tissue perfusion in septic shock. Crit. Care Med. 2000, 28, 2729–2732. [Google Scholar] [CrossRef]
- Martin, C.; Papazian, L.; Perrin, G.; Saux, P.; Gouin, F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest 1993, 103, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Desjars, P.; Pinaud, M.; Bugnon, D.; Tasseau, F. Norepinephrine therapy has no deleterious renal effects in human septic shock. Crit. Care Med. 1989, 17, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Smail, N.; Cabral, A.; Rogiers, P.; Vincent, J.L. Effects of norepinephrine on regional blood flow and oxygen extraction capabilities during endotoxic shock. Am. J. Respir. Crit. Care Med. 1997, 155, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Viviand, X.; Leone, M.; Thirion, X. Effect of norepinephrine on the outcome of septic shock. Crit. Care Med. 2000, 28, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Owen, V.S.; Rosgen, B.K.; Cherak, S.J.; Ferland, A.; Stelfox, H.T.; Fiest, K.M.; Niven, D.J. Adverse events associated with administration of vasopressor medications through a peripheral intravenous catheter: A systematic review and meta-analysis. Crit. Care 2021, 25, 146. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Vasopressor therapy in critically ill patients with shock. Intensive Care Med. 2019, 45, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Darmon, M.; Schortgen, F.; Vargas, F.; Liazydi, A.; Schlemmer, B.; Brun-Buisson, C.; Brochard, L. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 2011, 37, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kajal, K.; Chauhan, R.; Negi, S.L.; Gourav, K.P.; Panda, P.; Mahajan, S.; Sarna, R. Intraoperative evaluation of renal resistive index with transesophageal echocardiography for the assessment of acute renal injury in patients undergoing coronary artery bypass grafting surgery: A prospective observational study. Ann. Card. Anaesth. 2022, 25, 158–163. [Google Scholar] [PubMed]
- Marty, P.; Szatjnic, S.; Ferre, F.; Conil, J.M.; Mayeur, N.; Fourcade, O.; Silva, S.; Minville, V. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery: A prospective observational study. Eur. J. Anaesthesiol. 2015, 32, 37–43. [Google Scholar] [CrossRef]
- Pravisani, R.; Baccarani, U.; Langiano, N.; Meroi, F.; Avital, I.; Bove, T.; Adani, G.L. Predictive Value of Intraoperative Doppler Flowmetry for Delayed Graft Function in Kidney Transplantation: A Pilot Study. Transplant. Proc. 2020, 52, 1556–1558. [Google Scholar] [CrossRef]
- Król, R.; Chudek, J.; Kolonko, A.; Ziaja, J.; Pawlicki, J.; Wiecek, A.; Cierpka, L. Intraoperative resistance index measured with transsonic flowmeter on kidney graft artery can predict early and long-term graft function. Transplant. Proc. 2011, 43, 2926–2929. [Google Scholar] [CrossRef] [PubMed]

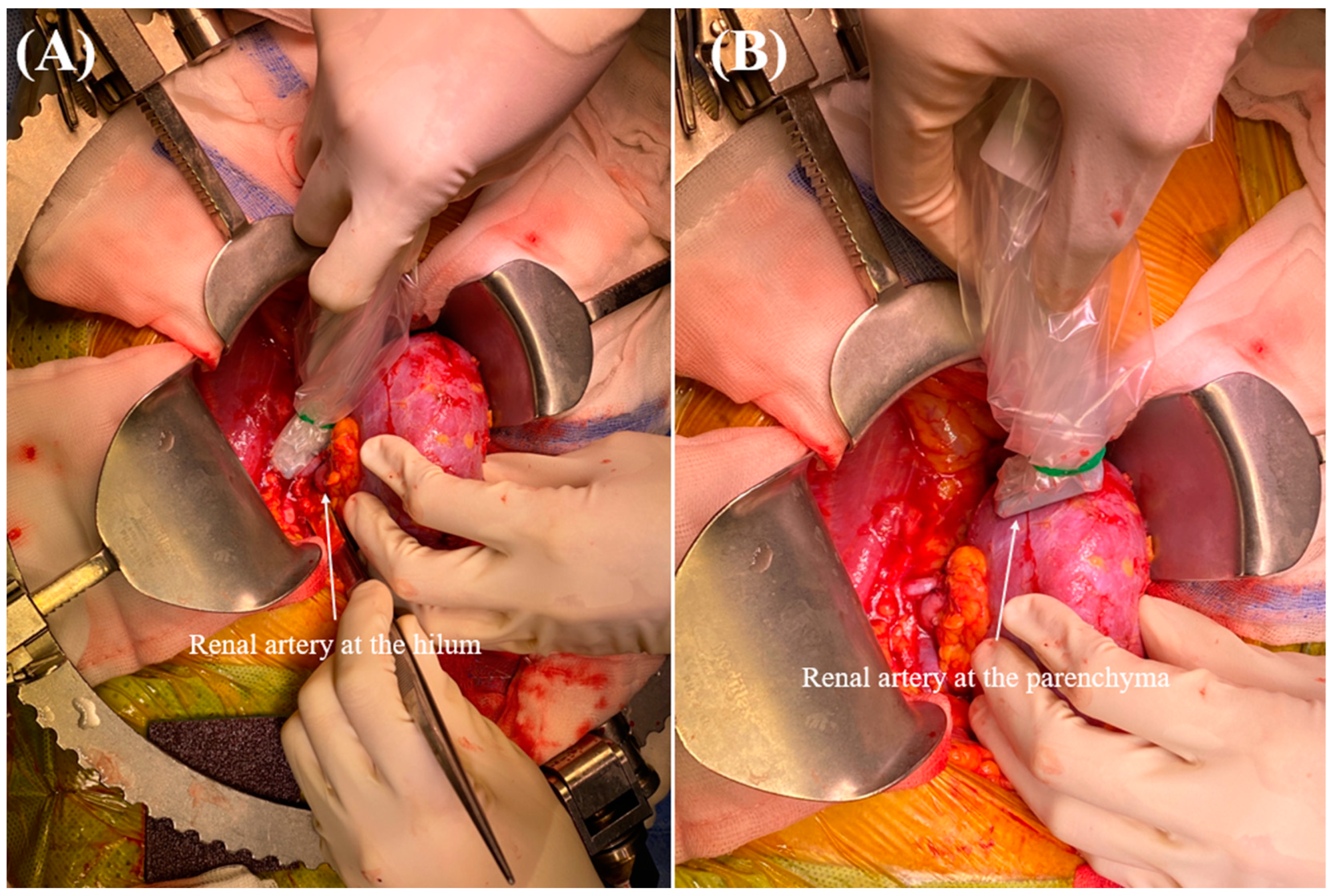
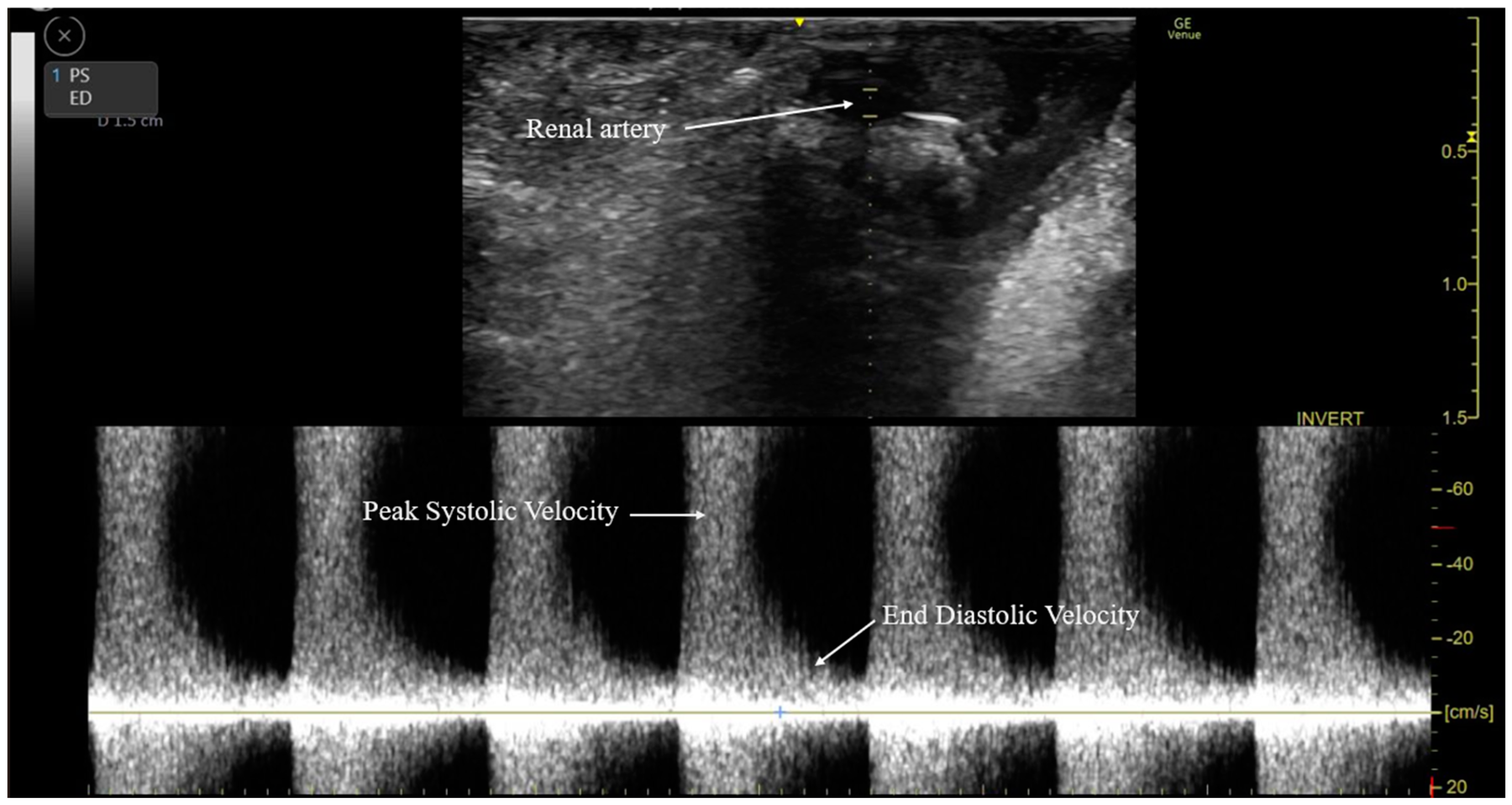
| Before Propensity Score Matching | After Propensity Score Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Dopamine | Norepinephrine | p | SD | Dopamine | Norepinephrine | p | SD |
| n | 177 | 170 | 167 | 167 | ||||
| Preoperative recipient factors | ||||||||
| Sex (female) | 85 (48.0%) | 88 (51,8%) | 0.486 | 0.075 | 81 (48.5%) | 86 (51.5%) | 0.584 | 0.06 |
| Age (years) | 49.9 ± 12.7 | 49.5 ± 10.9 | 0.734 | −0.04 | 49.7 ± 12.9 | 49.5 ± 10.9 | 0.905 | −0.014 |
| Body mass index (kg/m2) | 23.3 ± 3.9 | 23.7 ± 4.1 | 0.298 | 0.109 | 23.4 ± 3.9 | 23.8 ± 4.1 | 0.368 | 0.096 |
| Comorbidity | ||||||||
| Diabetes mellitus | 62 (35.0%) | 57 (33.5%) | 0.769 | −0.032 | 59 (35.3%) | 55 (32.9%) | 0.644 | −0.051 |
| Hypertension | 87 (49.2%) | 74 (43.5%) | 0.294 | −0.113 | 83 (49.7%) | 72 (43.1%) | 0.227 | −0.132 |
| Echocardiography | ||||||||
| Ejection fraction (%) | 61.1 ± 5.5 | 59.8 ± 7.0 | 0.043 | −0.196 | 61.0 ± 5.5 | 60.0 ± 6.7 | 0.16 | −0.136 |
| Left ventricular mass index (g/m2) | 129.9 ± 83.8 | 127.4 ± 44.2 | 0.728 | −0.056 | 123.4 ± 36.2 | 127.6 ± 44.6 | 0.35 | 0.094 |
| Vital sign | ||||||||
| Systolic blood pressure (mmHg) | 133.7 ± 13.2 | 131.0 ± 13.5 | 0.059 | −0.201 | 133.4 ± 13.2 | 130.9 ± 13.5 | 0.092 | −0.183 |
| Diastolic blood pressure (mmHg) | 82.0 ± 9.8 | 81.0 ± 9.3 | 0.327 | −0.108 | 81.8 ± 9.9 | 81.0 ± 9.3 | 0.437 | −0.088 |
| Heart rate (beats/min) | 78.5 ± 9.2 | 78.6 ± 9.4 | 0.859 | 0.019 | 78.8 ± 9.3 | 78.6 ± 9.3 | 0.874 | −0.017 |
| Hourly urine output (mL/kg/h) | 2.1 ± 1.4 | 2.0 ± 1.3 | 0.638 | −0.052 | 2.1 ± 1.3 | 2.0 ± 1.3 | 0.799 | −0.029 |
| Brain natriuretic peptide (pg/mL) | 245.0 ± 483.8 | 278.6 ± 718.4 | 0.61 | 0.047 | 226.6 ± 442.2 | 278.6 ± 723.6 | 0.429 | 0.072 |
| High-sensitivity troponin I (pg/mL) | 45.5 ± 71.0 | 62.3 ± 199.0 | 0.291 | 0.085 | 45.6 ± 72.6 | 46.4 ± 84.7 | 0.923 | 0.004 |
| Corrected QT interval (ms) | 451.1 ± 29.3 | 450.5 ± 31.0 | 0.843 | −0.021 | 451.0 ± 29.5 | 450.2 ± 31.0 | 0.793 | −0.028 |
| Laboratory variables | ||||||||
| White blood cell count (×109/L) | 6.7 ± 3.1 | 6.8 ± 2.6 | 0.88 | 0.018 | 6.7 ± 3.2 | 6.8 ± 2.6 | 0.834 | 0.026 |
| Neutrophil (%) | 72.7 ± 13.1 | 71.4 ± 15.1 | 0.411 | −0.083 | 72.2 ± 13.1 | 71.5 ± 15.2 | 0.633 | −0.049 |
| Lymphocyte (%) | 17.8 ± 9.0 | 18.4 ± 9.0 | 0.59 | 0.058 | 18.0 ± 9.1 | 18.3 ± 9.0 | 0.748 | 0.036 |
| Hemoglobin (g/dL) | 10.7 ± 1.4 | 10.4 ± 1.4 | 0.034 | −0.23 | 10.6 ± 1.4 | 10.4 ± 1.4 | 0.113 | −0.173 |
| Albumin (g/dL) | 4.1 ± 0.4 | 4.0 ± 0.5 | 0.115 | −0.163 | 4.1 ± 0.4 | 4.0 ± 0.5 | 0.239 | −0.123 |
| Sodium (mEq/L) | 136.5 ± 3.9 | 137.2 ± 4.6 | 0.112 | 0.158 | 136.7 ± 3.7 | 137.2 ± 4.7 | 0.309 | 0.101 |
| Potassium (mEq/L) | 4.6 ± 0.5 | 4.5 ± 0.5 | 0.234 | −0.125 | 4.6 ± 0.5 | 4.6 ± 0.5 | 0.322 | −0.107 |
| Chloride (mEq/L) | 99.9 ± 5.6 | 100.4 ± 6.3 | 0.381 | 0.089 | 100.2 ± 5.6 | 100.4 ± 6.4 | 0.728 | 0.036 |
| Platelet count (×109/L) | 182.3 ± 61.4 | 186.5 ± 66.2 | 0.544 | 0.063 | 183.3 ± 61.7 | 187.6 ± 66.1 | 0.546 | 0.064 |
| International normalized ratio | 1.1 ± 0.9 | 1.1 ± 0.3 | 0.583 | −0.127 | 1.1 ± 0.9 | 1.1 ± 0.3 | 0.597 | −0.129 |
| Creatinine (mg/dL) | 7.7 ± 2.7 | 8.1 ± 2.7 | 0.22 | 0.132 | 7.7 ± 2.7 | 8.1 ± 2.6 | 0.261 | 0.123 |
| Intraoperative recipient factors | ||||||||
| Operation time (min) | 226.3 ± 44.3 | 230.0 ± 43.2 | 0.432 | 0.085 | 227.6 ± 44.8 | 230.1 ± 43.5 | 0.601 | 0.058 |
| Hourly fluid infusion (mL/kg/h) | 9.6 ± 3.7 | 10.0 ± 3.2 | 0.247 | 0.133 | 9.7 ± 3.7 | 10.0 ± 3.2 | 0.469 | 0.086 |
| Hemorrhage (mL) | 171.1 ± 69.6 | 160.5 ± 66.8 | 0.151 | −0.158 | 171.3 ± 68.6 | 160.4 ± 66.2 | 0.143 | −0.162 |
| Donor and graft factors | ||||||||
| Sex (female) | 85 (48.0%) | 88 (51.8%) | 0.486 | 0.075 | 81 (48.5%) | 86 (51.5%) | 0.584 | 0.06 |
| Age (years) | 49.7 ± 13.0 | 47.0 ± 12.4 | 0.049 | −0.217 | 49.1 ± 13.1 | 46.9 ± 12.5 | 0.117 | −0.177 |
| Body mass index (kg/m2) | 24.1 ± 3.2 | 24.1 ± 3.1 | 0.894 | 0.015 | 24.1 ± 3.2 | 24.1 ± 3.1 | 0.944 | 0.008 |
| Hemoglobin (g/dL) | 13.8 ± 1.1 | 13.8 ± 1.2 | 0.917 | 0.011 | 13.8 ± 1.1 | 13.8 ± 1.2 | 0.903 | 0.013 |
| Graft weight (g) | 185.2 ± 41.3 | 177.4 ± 37.6 | 0.063 | −0.21 | 183.9 ± 41.2 | 177.1 ± 37.8 | 0.12 | −0.18 |
| Total ischemic time (min) | 57.7 ± 15.0 | 59.9 ± 21.5 | 0.261 | 0.104 | 58.3 ± 15.2 | 59.9 ± 21.7 | 0.434 | 0.074 |
| Group | Dopamine | Norepinephrine | p |
|---|---|---|---|
| 167 | 167 | ||
| In the operating room (after renal vascular and ureter anastomosis) | |||
| Renal arterial resistive index | |||
| at renal hilum | 0.77 ± 0.11 | 0.66 ± 0.13 | <0.001 |
| at renal parenchyma | 0.71 ± 0.1 | 0.6 ± 0.1 | <0.001 |
| Renal arterial resistive index (>0.8) | |||
| at renal hilum | 66 (39.5%) | 20 (12.0%) | <0.001 |
| at renal parenchyma | 25 (15.0%) | 3 (1.8%) | <0.001 |
| In the ward (on postoperative 7) | |||
| Renal arterial resistive index | |||
| at renal hilum | 0.64 ± 0.11 | 0.64 ± 0.05 | 0.945 |
| at renal parenchyma | 0.56 ± 0.11 | 0.55 ± 0.05 | 0.382 |
| Renal arterial resistive index (>0.8) | |||
| at renal hilum | 4 (2.4%) | 0 (0.0%) | 0.123 |
| at renal parenchyma | 3 (1.8%) | 0 (0.0%) | 0.248 |
| β | Odds Ratio | 95% CI | p | |
|---|---|---|---|---|
| In the operating room (after renal vascular and ureter anastomosis) | ||||
| Norepinephrine adjusted for PS | ||||
| Renal arterial resistive index (>0.8) | ||||
| at renal hilum | −1.543 | 0.214 | 0.12–0.382 | <0.001 |
| at renal parenchyma | −2.301 | 0.1 | 0.029–0.348 | <0.001 |
| Group | Dopamine | Norepinephrine | p |
|---|---|---|---|
| 167 | 167 | ||
| At the beginning of the surgery | |||
| Systolic blood pressure (mmHg) | 126.5 ± 13.4 | 124.8 ± 9.8 | 0.185 |
| Diastolic blood pressure (mmHg) | 77.6 ± 11.0 | 75.4 ± 13.3 | 0.091 |
| Mean blood pressure (mmHg) | 93.9 ± 10.3 | 91.8 ± 11.0 | 0.075 |
| Heart rate (beats/min) | 73.2 ± 9.3 | 74.5 ± 7.4 | 0.158 |
| Central venous pressure (mmHg) | 5.6 ± 2.0 | 5.7 ± 1.8 | 0.841 |
| Brain natriuretic peptide (pg/mL) | 147.8 (94.8–251.2) | 139.6 (81.9–295.9) | 0.583 |
| After vascular graft and ureteral anastomosis | |||
| Systolic blood pressure (mmHg) | 145.7 ± 6.9 | 144.5 ± 6.7 | 0.137 |
| Diastolic blood pressure (mmHg) | 80.0 ± 7.8 | 80.1 ± 6.9 | 0.888 |
| Mean blood pressure (mmHg) | 94.5 ± 10.1 | 93.5 ± 9.0 | 0.526 |
| Heart rate (beats/min) | 96.5 ± 10.5 | 79.9 ± 11.2 | <0.001 |
| Central venous pressure (mmHg) | 11.7 ± 2.1 | 11.9 ± 3.0 | 0.425 |
| At the end of the surgery | |||
| Systolic blood pressure (mmHg) | 132.9 ± 16.7 | 131.5 ± 15.6 | 0.428 |
| Diastolic blood pressure (mmHg) | 75.2 ± 10.6 | 74.9 ± 9.6 | 0.787 |
| Mean blood pressure (mmHg) | 94.5 ± 10.1 | 93.8 ± 9.0 | 0.526 |
| Heart rate (beats/min) | 96.2 ± 15.0 | 88.8 ± 14.2 | <0.001 |
| Central venous pressure (mmHg) | 7.3 ± 2.9 | 7.4 ± 3.3 | 0.793 |
| Brain natriuretic peptide (pg/mL) | 119.0 (67.7–198.6) | 101.5 (62.2–174.4) | 0.14 |
| Group | Dopamine | Norepinephrine | p |
|---|---|---|---|
| 167 | 167 | ||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | |||
| Postoperative day 1 | 25.1 ± 17.4 | 30.0 ± 13.3 | 0.004 |
| Postoperative day 2 | 60.2 ± 30.0 | 60.8 ± 31.8 | 0.841 |
| Postoperative day 3 | 70.7 ± 32.2 | 69.4 ± 32.1 | 0.702 |
| Postoperative day 7 | 84.3 ± 34.3 | 80.9 ± 31.2 | 0.34 |
| Hourly urine output (mL/kg/h) | |||
| Postoperative day 1 | 36.5 ± 14.4 | 41.8 ± 16.9 | 0.002 |
| Postoperative day 2 | 29.0 ± 11.9 | 29.3 ± 10.6 | 0.804 |
| Postoperative day 3 | 26.4 ± 10.8 | 26.5 ± 9.5 | 0.949 |
| Postoperative day 7 | 15.4 ± 5.8 | 15.3 ± 7.5 | 0.921 |
| Rescue dialysis therapy | 8 (4.8%) | 11 (6.6%) | 0.479 |
| ICU stay (day) | 2 (2–2) | 2 (2–2) | 0.206 |
| Hospital stay (day) | 13 (12–14) | 13 (12–14) | 0.476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, J.; Kwon, H.; Park, H.; Park, S.C.; Yun, S.S.; Chae, M.S. Impact of Norepinephrine and Dopamine Infusion on Renal Arterial Resistive Index during Pre-Emptive Living Donor Kidney Transplantation: Propensity Score Matching Analysis. Medicina 2024, 60, 1066. https://doi.org/10.3390/medicina60071066
Huh J, Kwon H, Park H, Park SC, Yun SS, Chae MS. Impact of Norepinephrine and Dopamine Infusion on Renal Arterial Resistive Index during Pre-Emptive Living Donor Kidney Transplantation: Propensity Score Matching Analysis. Medicina. 2024; 60(7):1066. https://doi.org/10.3390/medicina60071066
Chicago/Turabian StyleHuh, Jaewon, Hyejin Kwon, Hunwoo Park, Sun Cheol Park, Sang Seob Yun, and Min Suk Chae. 2024. "Impact of Norepinephrine and Dopamine Infusion on Renal Arterial Resistive Index during Pre-Emptive Living Donor Kidney Transplantation: Propensity Score Matching Analysis" Medicina 60, no. 7: 1066. https://doi.org/10.3390/medicina60071066
APA StyleHuh, J., Kwon, H., Park, H., Park, S. C., Yun, S. S., & Chae, M. S. (2024). Impact of Norepinephrine and Dopamine Infusion on Renal Arterial Resistive Index during Pre-Emptive Living Donor Kidney Transplantation: Propensity Score Matching Analysis. Medicina, 60(7), 1066. https://doi.org/10.3390/medicina60071066






