Clinical Outcomes after Intracorporeal versus Extracorporeal Anastomosis in Patients Undergoing Laparoscopic Right Hemicolectomy for Colon Cancer
Abstract
:1. Introduction
2. Materials and Methods
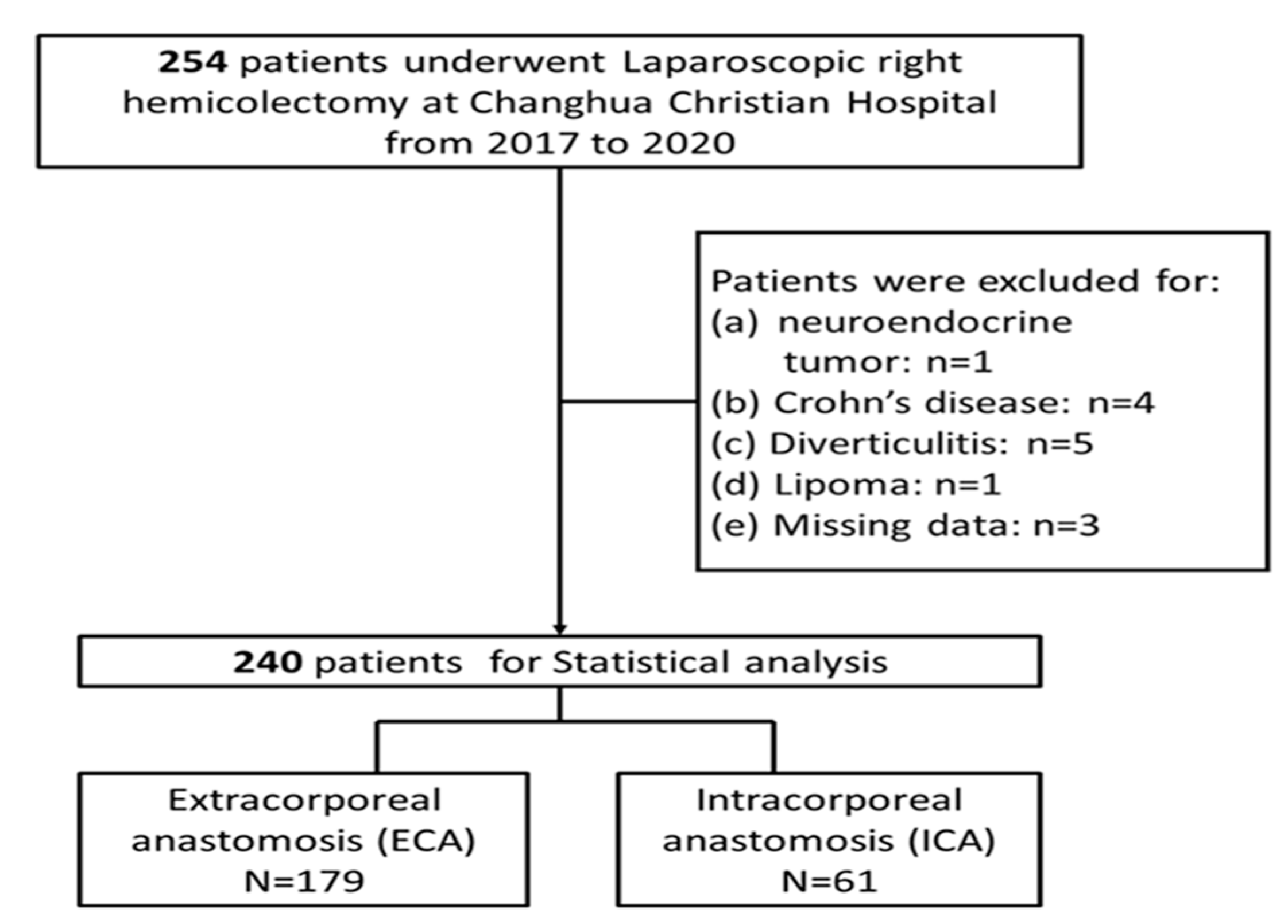
2.1. Study Design and Patient Selection
2.2. Surgical Procedures
2.3. Study Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Postoperative Outcomes
3.3. Long-Term Outcomes
3.4. Univariate Analysis of Factors Associated with OS and CSS
3.5. Multivariable Analysis of Factors Associated with OS and CSS
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Arhin, N.D.; Shen, C.; Bailey, C.E.; Matsuoka, L.K.; Hawkins, A.T.; Holowatyj, A.N.; Ciombor, K.K.; Hopkins, M.B.; Geiger, T.M.; Kam, A.E.; et al. Surgical resection and survival outcomes in metastatic young adult colorectal cancer patients. Cancer Med. 2021, 10, 4269–4281. [Google Scholar] [CrossRef]
- Murphy, C.C.; Sandler, R.S.; Sanoff, H.K.; Yang, Y.C.; Lund, J.L.; Baron, J.A. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin. Gastroenterol. Hepatol. 2017, 15, 903–909.e6. [Google Scholar] [CrossRef]
- Davis, D.M.; Marcet, J.E.; Frattini, J.C.; Prather, A.D.; Mateka, J.J.; Nfonsam, V.N. Is it time to lower the recommended screening age for colorectal cancer? J. Am. Coll. Surg. 2011, 213, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 1978–1998. [Google Scholar] [CrossRef]
- Al-Taher, M.; Okamoto, N.; Mutter, D.; Stassen, L.P.S.; Marescaux, J.; Diana, M.; Dallemagne, B. International survey among surgeons on laparoscopic right hemicolectomy: The gap between guidelines and reality. Surg. Endosc. 2022, 36, 5840–5853. [Google Scholar] [CrossRef]
- Portale, G.; Bartolotta, P.; Azzolina, D.; Gregori, D.; Fiscon, V. Laparoscopic right hemicolectomy with 2D or 3D video system technology: Systematic review and meta-analysis. Int. J. Colorectal Dis. 2023, 38, 34. [Google Scholar] [CrossRef]
- Schlinkert, R.T. Laparoscopic-assisted right hemicolectomy. Dis. Colon. Rectum 1991, 34, 1030–1031. [Google Scholar] [CrossRef]
- Jurowich, C.; Lichthardt, S.; Kastner, C.; Haubitz, I.; Prock, A.; Filser, J.; Germer, C.T.; Wiegering, A. Laparoscopic versus open right hemicolectomy in colon carcinoma: A propensity score analysis of the DGAV StuDoQ|ColonCancer registry. PLoS ONE 2019, 14, e0218829. [Google Scholar] [CrossRef]
- Anania, G.; Arezzo, A.; Davies, R.J.; Marchetti, F.; Zhang, S.; Di Saverio, S.; Cirocchi, R.; Donini, A. A global systematic review and meta-analysis on laparoscopic vs open right hemicolectomy with complete mesocolic excision. Int. J. Colorectal Dis. 2021, 36, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Cirocchi, R.; Cesare Campanile, F.; Di Saverio, S.; Popivanov, G.; Carlini, L.; Pironi, D.; Tabola, R.; Vettoretto, N. Laparoscopic versus open colectomy for obstructing right colon cancer: A systematic review and meta-analysis. J. Visc. Surg. 2017, 154, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.F.; Cleary, R.K. Intracorporeal anastomosis versus extracorporeal anastomosis for minimally invasive colectomy. J. Gastrointest. Oncol. 2020, 11, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Cleary, R.K.; Kassir, A.; Johnson, C.S.; Bastawrous, A.L.; Soliman, M.K.; Marx, D.S.; Giordano, L.; Reidy, T.J.; Parra-Davila, E.; Obias, V.J.; et al. Intracorporeal versus extracorporeal anastomosis for minimally invasive right colectomy: A multi-center propensity score-matched comparison of outcomes. PLoS ONE 2018, 13, e0206277. [Google Scholar] [CrossRef]
- Ricci, C.; Casadei, R.; Alagna, V.; Zani, E.; Taffurelli, G.; Pacilio, C.A.; Minni, F. A critical and comprehensive systematic review and meta-analysis of studies comparing intracorporeal and extracorporeal anastomosis in laparoscopic right hemicolectomy. Langenbecks Arch. Surg. 2017, 402, 417–427. [Google Scholar] [CrossRef]
- Milone, M.; Elmore, U.; Vignali, A.; Gennarelli, N.; Manigrasso, M.; Burati, M.; Milone, F.; De Palma, G.D.; Delrio, P.; Rosati, R. Recovery after intracorporeal anastomosis in laparoscopic right hemicolectomy: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2018, 403, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tamini, N.; Bernasconi, D.; Ripamonti, L.; Lo Bianco, G.; Braga, M.; Nespoli, L. Clinical Validation of the Comprehensive Complication Index in Colon Cancer Surgery. Cancers 2021, 13, 1745. [Google Scholar] [CrossRef] [PubMed]
- Putter, H.; Schumacher, M.; van Houwelingen, H.C. On the relation between the cause-specific hazard and the subdistribution rate for competing risks data: The Fine-Gray model revisited. Biom. J. Biom. Z. 2020, 62, 790–807. [Google Scholar] [CrossRef] [PubMed]
- Cuk, P.; Buyukuslu, M.; Moller, S.; Verwaal, V.J.; Al-Najami, I.; Ellebaek, M.B. Intracorporeal versus extracorporeal anastomosis in segmental resections for colon cancer: A retrospective cohort study of 328 patients. Langenbecks Arch. Surg. 2023, 408, 219. [Google Scholar] [CrossRef]
- Hussain, Z.; Park, H. Inflammation and Impaired Gut Physiology in Post-operative Ileus: Mechanisms and the Treatment Options. J. Neurogastroenterol. Motil. 2022, 28, 517–530. [Google Scholar] [CrossRef]
- Harr, J.N.; Juo, Y.Y.; Luka, S.; Agarwal, S.; Brody, F.; Obias, V. Incisional and port-site hernias following robotic colorectal surgery. Surg. Endosc. 2016, 30, 3505–3510. [Google Scholar] [CrossRef]
- Samia, H.; Lawrence, J.; Nobel, T.; Stein, S.; Champagne, B.J.; Delaney, C.P. Extraction site location and incisional hernias after laparoscopic colorectal surgery: Should we be avoiding the midline? Am. J. Surg. 2013, 205, 264–267, discussion 268. [Google Scholar] [CrossRef]
- Jarry, C.; Carcamo, L.; Gonzalez, J.J.; Bellolio, F.; Miguieles, R.; Urrejola, G.; Zuniga, A.; Crovari, F.; Molina, M.E.; Larach, J.T. Implementation of intracorporeal anastomosis in laparoscopic right colectomy is safe and associated with a shorter hospital stay. Updates Surg. 2021, 73, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Anania, G.; Tamburini, N.; Sanzi, M.; Schimera, A.; Bombardini, C.; Resta, G.; Marino, S.; Valpiani, G.; Valentini, A.; Cavallesco, G. Extracorporeal versus intracorporeal anastomosis in laparoscopic right hemicolectomy for cancer. Minim. Invasive Ther. Allied Technol. 2022, 31, 112–118. [Google Scholar] [CrossRef] [PubMed]
- van Oostendorp, S.; Elfrink, A.; Borstlap, W.; Schoonmade, L.; Sietses, C.; Meijerink, J.; Tuynman, J. Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: A systematic review and meta-analysis. Surg. Endosc. 2017, 31, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Cai, Z.; Lu, J.; Yang, Y.; Xu, Q.; Wang, N.; He, L.; Hu, X.; Fingerhut, A.; Zheng, M.; et al. Pathological and perioperative outcomes of extracorporeal versus intracorporeal anastomosis in laparoscopic transverse colon cancer resection: Retrospective multicentre study. BJS Open 2023, 7, zrad045. [Google Scholar] [CrossRef]
- Biondi, A.; Di Mauro, G.; Morici, R.; Sangiorgio, G.; Vacante, M.; Basile, F. Intracorporeal versus Extracorporeal Anastomosis for Laparoscopic Right Hemicolectomy: Short-Term Outcomes. J. Clin. Med. 2021, 10, 5967. [Google Scholar] [CrossRef] [PubMed]
- Hoyuela, C.; Guillaumes, S.; Ardid, J.; Hidalgo, N.J.; Bachero, I.; Trias, M.; Martrat, A. The impact of intracorporeal anastomosis in right laparoscopic colectomy in the surgical site infections and the hospital stay: A cohort study. Updates Surg. 2021, 73, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.K.; Chern, Y.J.; Lin, Y.C.; Hsu, Y.J.; Chiang, J.M.; Tsai, W.S.; Hsieh, P.S.; Hung, H.Y.; Yeh, C.Y.; You, J.F. Short- and medium-term outcomes of intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: A propensity score-matched study. World J. Surg. Oncol. 2021, 19, 6. [Google Scholar] [CrossRef]
- Bou Saleh, N.; Voron, T.; De'Angelis, N.; Franco, I.; Canoui-Poitrine, F.; Mutter, D.; Brunetti, F.; Gagniere, J.; Memeo, R.; Pezet, D.; et al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right hemicolectomy: Results from the CLIMHET study group. Tech. Coloproctol. 2020, 24, 585–592. [Google Scholar] [CrossRef]
- Allaix, M.E.; Degiuli, M.; Bonino, M.A.; Arezzo, A.; Mistrangelo, M.; Passera, R.; Morino, M. Intracorporeal or Extracorporeal Ileocolic Anastomosis After Laparoscopic Right Colectomy: A Double-blinded Randomized Controlled Trial. Ann. Surg. 2019, 270, 762–767. [Google Scholar] [CrossRef]
- Ozawa, H.; Toyota, N.; Sakamoto, J.; Nakanishi, H.; Nakanishi, R.; Fujita, S. Mid-term outcomes of intracorporeal versus extracorporeal anastomosis after laparoscopic colectomy: A propensity score-matched cohort study from a single institution. Surg. Today 2023, 53, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, Y.; Feng, B.; Su, X.; Sun, Y.; Xu, L.; Lu, J.; Zhang, G.; Wu, A.; Kang, L.; et al. Intracorporeal Anastomosis Versus Extracorporeal Anastomosis in Laparoscopic Right Colectomy: An Observational Cohort Study. World J. Surg. 2023, 47, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Ichhpuniani, S.; McKechnie, T.; Lee, J.; Biro, J.; Lee, Y.; Park, L.; Doumouras, A.; Hong, D.; Eskicioglu, C. Lymph Node Ratio as a Predictor of Survival for Colon Cancer: A Systematic Review and Meta-Analysis. Am. Surg. 2024, 90, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Beirat, A.F.; Amarin, J.Z.; Suradi, H.H.; Qwaider, Y.Z.; Muhanna, A.; Maraqa, B.; Al-Ani, A.; Al-Hussaini, M. Lymph node ratio is a more robust predictor of overall survival than N stage in stage III colorectal adenocarcinoma. Diagn. Pathol. 2024, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Baqar, A.R.; Wilkins, S.; Staples, M.; Angus Lee, C.H.; Oliva, K.; McMurrick, P. The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres. Int. J. Surg. 2019, 64, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, M.; Martling, A.; Veres, K.; Horvath-Puho, E.; Wille-Jorgensen, P.; Hoirup Petersen, S.; Laurberg, S.; Sorensen, H.T.; Syk, I.; Group, C.S. No benefit of more intense follow-up after surgery for colorectal cancer in the risk group with elevated CEA levels—An analysis within the COLOFOL randomized clinical trial. Eur. J. Surg. Oncol. 2021, 47, 2053–2059. [Google Scholar] [CrossRef]
- Munoz-Montano, W.R.; Lopez-Basave, H.N.; Castillo-Morales, A.; Castillo-Morales, C.; Sanchez-Trejo, K.; Catalan, R.; Diaz-Romero, C.; Lino-Silva, L.S.; Maliachi-Diaz, A.; Ruiz-Garcia, E.; et al. Persistent high levels of carcinoembryonic antigen after tumor resection are associated with poorer survival outcomes in patients with resected colon cancer. BMC Cancer 2023, 23, 678. [Google Scholar] [CrossRef]
| All Patients | ICA | ECA | p-Value | |
|---|---|---|---|---|
| n = 240 | n = 61 | n = 179 | ||
| Age, years | 65.9 ± 13.7 | 65.6 ± 14.3 | 65.9 ± 13.6 | 0.867 |
| Sex | 0.455 | |||
| Female | 124 (51.7) | 29 (47.5) | 95 (53.1) | |
| Male | 116 (48.3) | 32 (52.5) | 84 (46.9) | |
| BMI, kg/m2 | 24.6 ± 4.3 | 25.2 ± 5.5 | 24.3 ± 3.9 | 0.238 |
| Comorbidities | ||||
| DM | 51 (21.3) | 15 (24.6) | 36 (20.1) | 0.460 |
| HTN | 86 (35.8) | 25 (41.0) | 61 (34.1) | 0.331 |
| ESRD | 7 (2.9) | 2 (3.3) | 5 (2.8) | >0.999 |
| CVA | 10 (4.2) | 3 (4.9) | 7 (3.9) | 0.717 |
| Preoperative CEA (ng/mL) * | 3 (1.9–8.8) | 2.85 (1.9–8.65) | 3.2 (1.8–8.8) | 0.762 |
| <=5 | 148 (0.6) | 39 (0.7) | 109 (0.6) | 0.707 |
| >5 | 87 (0.4) | 21 (0.4) | 66 (0.4) | |
| ASA | 0.204 | |||
| I | 9 (3.8) | 0 (0.0) | 9 (5.0) | |
| II | 101 (42.1) | 28 (45.9) | 73 (40.8) | |
| III | 130 (54.2) | 33 (54.1) | 97 (54.2) | |
| Surgical Type | 0.443 | |||
| Scheduled | 214 (89.2) | 56 (91.8) | 158 (88.3) | |
| Emergent | 26 (10.8) | 5 (8.2) | 21 (11.7) | |
| Site | 0.097 | |||
| Appendix | 16 (6.7) | 7 (11.5) | 9 (5.0) | |
| Cecum | 41 (17.1) | 14 (23.0) | 27 (15.1) | |
| Ascending colon | 118 (49.2) | 28 (45.9) | 90 (50.3) | |
| Transverse colon | 65 (27.1) | 12 (19.7) | 53 (29.6) | |
| With liver resection | 5 (2.1) | 0 (0.0) | 5 (2.8) | 0.333 |
| T stage | 0.022 | |||
| T0 | 31 (12.9) | 13 (21.3) | 18 (10.1) | |
| T1 | 22 (9.2) | 8 (13.1) | 14 (7.8) | |
| T2 | 31 (12.9) | 11 (18.0) | 20 (11.2) | |
| T3 | 132 (55.0) | 25 (41.0) | 107 (59.8) | |
| T4 | 24 (10.0) | 4 (6.6) | 20 (11.2) | |
| N stage | 0.130 | |||
| N0 | 153 (63.8) | 45 (73.8) | 108 (60.3) | |
| N1 | 52 (21.7) | 11 (18.0) | 41 (22.9) | |
| N2 | 35 (14.6) | 5 (8.2) | 30 (16.8) | |
| M stage | 0.281 | |||
| M0 | 211 (87.9) | 56 (91.8) | 155 (86.6) | |
| M1 | 29 (12.1) | 5 (8.2) | 24 (13.4) | |
| Pathologic stage | 0.056 | |||
| 0 | 31 (12.9) | 13 (21.3) | 18 (10.1) | |
| I | 47 (19.6) | 16 (26.2) | 31 (17.3) | |
| II | 67 (27.9) | 14 (23.0) | 53 (29.6) | |
| III | 66 (27.5) | 13 (21.3) | 53 (29.6) | |
| IV | 29 (12.1) | 5 (8.2) | 24 (13.4) | |
| Tumor size, cm | 4.6 ± 2.4 | 3.9 ± 2.3 | 4.8 ± 2.3 | 0.009 |
| All Patients | ICA | ECA | p-Value | |
|---|---|---|---|---|
| n = 240 | n = 61 | n = 179 | ||
| Postoperative outcomes | ||||
| Number of positive lymph nodes | 0.067 | |||
| 0 | 156 (65.0) | 47 (77.0) | 109 (60.9) | |
| 1–3 | 49 (20.4) | 9 (14.8) | 40 (22.3) | |
| ≥4 | 35 (14.6) | 5 (8.2) | 30 (16.8) | |
| Number of harvested lymph nodes | 0.492 | |||
| <12 | 7 (2.9) | 1 (1.6) | 6 (3.4) | |
| ≥12 | 233 (97.1) | 60 (98.4) | 173 (96.6) | |
| Lymph node ratio | 0.07 ± 0.17 | 0.04 ± 0.14 | 0.08 ± 0.17 | 0.070 |
| Operation duration, min | 150 (120–180) | 120 (110–155) | 150 (130–180) | <0.001 |
| Estimated blood loss, mL | 30 (30–50) | 30 (10–30) | 30 (30–50) | <0.001 |
| Postoperative CEA (ng/mL) * | 2.3 (1.6–4) | 2 (1.7–3.4) | 2.35 (1.6–4.1) | 0.566 |
| ≤5 | 175 (86.2) | 42 (93.3) | 133 (84.2) | 0.116 |
| >5 | 28 (13.8) | 3 (6.7) | 25 (15.8) | |
| Complication | 59 (24.6) | 16 (26.2) | 43 (24.0) | 0.730 |
| Anastomotic leakage | 8 (3.3) | 0 (0.0) | 8 (4.5) | 0.208 |
| Minor/Major | 6/2 | 0/ 0 | 6/2 | |
| Surgical site infection | 15 (6.3) | 3 (4.9) | 12 (6.7) | 0.766 |
| Minor/need operation | 7/ 8 | 1/ 2 | 6/ 6 | |
| Ileus | 26 (10.8) | 4 (6.6) | 22 (12.3) | 0.213 |
| Ureteral injury | 1 (0.4) | 0 (0.0) | 1 (0.6) | >0.999 |
| Intraabdominal abscess | 5 (2.1) | 0 (0.0) | 5 (2.8) | 0.333 |
| Other complications | 26 (10.8) | 10 (16.4) | 16 (8.9) | 0.106 |
| Clavien–Dindo Classification | 0.698 | |||
| None | 181 (75.4) | 45 (73.8) | 136 (76.0) | |
| Grade I | 17 (7.1) | 4 (6.6) | 13 (7.3) | |
| Grade II | 24 (10.0) | 8 (13.1) | 16 (8.9) | |
| Grade III | 13 (5.4) | 2 (3.3) | 11 (6.1) | |
| Grade IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Grade V | 5 (2.1) | 2 (3.3) | 3 (1.7) | |
| 30-day readmission | 5 (2.1) | 1 (1.6) | 4 (2.2) | >0.999 |
| Long-term outcomes | ||||
| Follow-up, months | 49.3 (38.4–60.0) | 51.3 (37.8–59.0) | 48.4 (38.7–60.7) | 0.967 |
| Recurrence | 35 (14.6) | 11 (18.0) | 24 (13.4) | 0.377 |
| All-cause death | 53 (22.1) | 12 (19.7) | 41 (22.9) | 0.599 |
| Cancer-specific death | 23 (9.6) | 4 (6.6) | 19 (10.6) | 0.353 |
| OS | CSS | |||
|---|---|---|---|---|
| Crude HR (95%CI) | p-Value | Crude SHR (95%CI) | p-Value | |
| Anastomosis (ICA vs. ECA) | 0.91 (0.46–1.69) | 0.778 | 0.62 (0.21–1.78) | 0.370 |
| Age, years | 1.05 (1.03–1.08) | <0.001 | 1.01 (0.98–1.04) | 0.588 |
| Sex (male vs. female) | 1.05 (0.61–1.81) | 0.865 | 0.44 (0.18–1.07) | 0.071 |
| BMI | 0.99 (0.92–1.05) | 0.718 | 1.03 (0.94–1.12) | 0.550 |
| DM | 1.87 (1.01–3.32) | 0.038 | 1.37 (0.54–3.53) | 0.508 |
| HTN | 1.51 (0.87–2.61) | 0.138 | 0.97 (0.41–2.30) | 0.946 |
| ESRD | 2.19 (0.53–5.97) | 0.188 | 1.45 (0.18–11.44) | 0.722 |
| CVA | 2.53 (0.76–6.23) | 0.075 | 1.22 (0.15–9.74) | 0.850 |
| Anatomic Site | ||||
| Appendix | Ref | |||
| Ascending colon | 0.93 (0.32–3.95) | 0.910 | 0.73 (0.16–3.25) | 0.677 |
| Cecum | 1.12 (0.33–5.06) | 0.862 | 0.53 (0.09–3.08) | 0.477 |
| Transverse colon | 1.30 (0.44–5.58) | 0.671 | 0.63 (0.13–3.09) | 0.572 |
| pStage | NA | |||
| 0 | Ref | |||
| I | 1.58 (0.33–11.35) | 0.595 | ||
| II | 1.97 (0.50–13.06) | 0.389 | ||
| III | 4.11 (1.12–26.80) | 0.067 | ||
| IV | 17.91 (4.99–116.0) | <0.001 | ||
| Tumor size, cm | 1.10 (0.99–1.22) | 0.070 | 1.14 (1.03–1.27) | 0.011 |
| Post-op CEA (>5 vs. ≤5 ng/mL) | 4.92 (2.43–9.50) | <0.001 | 5.62 (2.31–13.69) | <0.001 |
| Complication | 2.09 (1.18–3.64) | 0.010 | 1.35 (0.55–3.35) | 0.512 |
| Lymph node ratio | 1.03 (1.02–1.04) | <0.001 | 1.05 (1.04–1.06) | <0.001 |
| OS | aHR (95%CI) | p-Value |
| Anastomosis (ICA vs. ECA) | 0.65 (0.25–1.44) | 0.378 |
| Age | 1.04 (1.01–1.07) | 0.005 |
| DM | 0.92 (0.42–1.87) | 0.821 |
| pStage (III–IV vs. 0–II) * | 3.99 (1.68–10.57) | 0.002 |
| Post-OP CEA (>5 vs. ≤5 ng/mL) | 1.95 (0.80–4.39) | 0.091 |
| Complication (Yes vs. No) | 1.17 (0.53–2.45) | 0.668 |
| Lymph node ratio | 1.03 (1.02–1.04) | <0.001 |
| CSS | aSHR (95%CI) | p-Value |
| Anastomosis (ICA vs. ECA) | 0.41 (0.10–1.66) | 0.211 |
| Tumor size | 0.98 (0.79–1.23) | 0.885 |
| Post-OP CEA (>5 vs. ≤5 ng/mL) | 1.75 (0.51–6.08) | 0.375 |
| Lymph node ratio | 1.05 (1.04–1.07) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-Y.; Cheng, B.; Sheu, G.-T. Clinical Outcomes after Intracorporeal versus Extracorporeal Anastomosis in Patients Undergoing Laparoscopic Right Hemicolectomy for Colon Cancer. Medicina 2024, 60, 1073. https://doi.org/10.3390/medicina60071073
Chang Y-Y, Cheng B, Sheu G-T. Clinical Outcomes after Intracorporeal versus Extracorporeal Anastomosis in Patients Undergoing Laparoscopic Right Hemicolectomy for Colon Cancer. Medicina. 2024; 60(7):1073. https://doi.org/10.3390/medicina60071073
Chicago/Turabian StyleChang, Yu-Yao, Bill Cheng, and Gwo-Tarng Sheu. 2024. "Clinical Outcomes after Intracorporeal versus Extracorporeal Anastomosis in Patients Undergoing Laparoscopic Right Hemicolectomy for Colon Cancer" Medicina 60, no. 7: 1073. https://doi.org/10.3390/medicina60071073
APA StyleChang, Y.-Y., Cheng, B., & Sheu, G.-T. (2024). Clinical Outcomes after Intracorporeal versus Extracorporeal Anastomosis in Patients Undergoing Laparoscopic Right Hemicolectomy for Colon Cancer. Medicina, 60(7), 1073. https://doi.org/10.3390/medicina60071073







