Tricuspid Annular Plane Systolic Excursion-to-Systolic Pulmonary Artery Pressure Ratio as a Prognostic Factor in Heart Transplant Patients
Abstract
1. Introduction
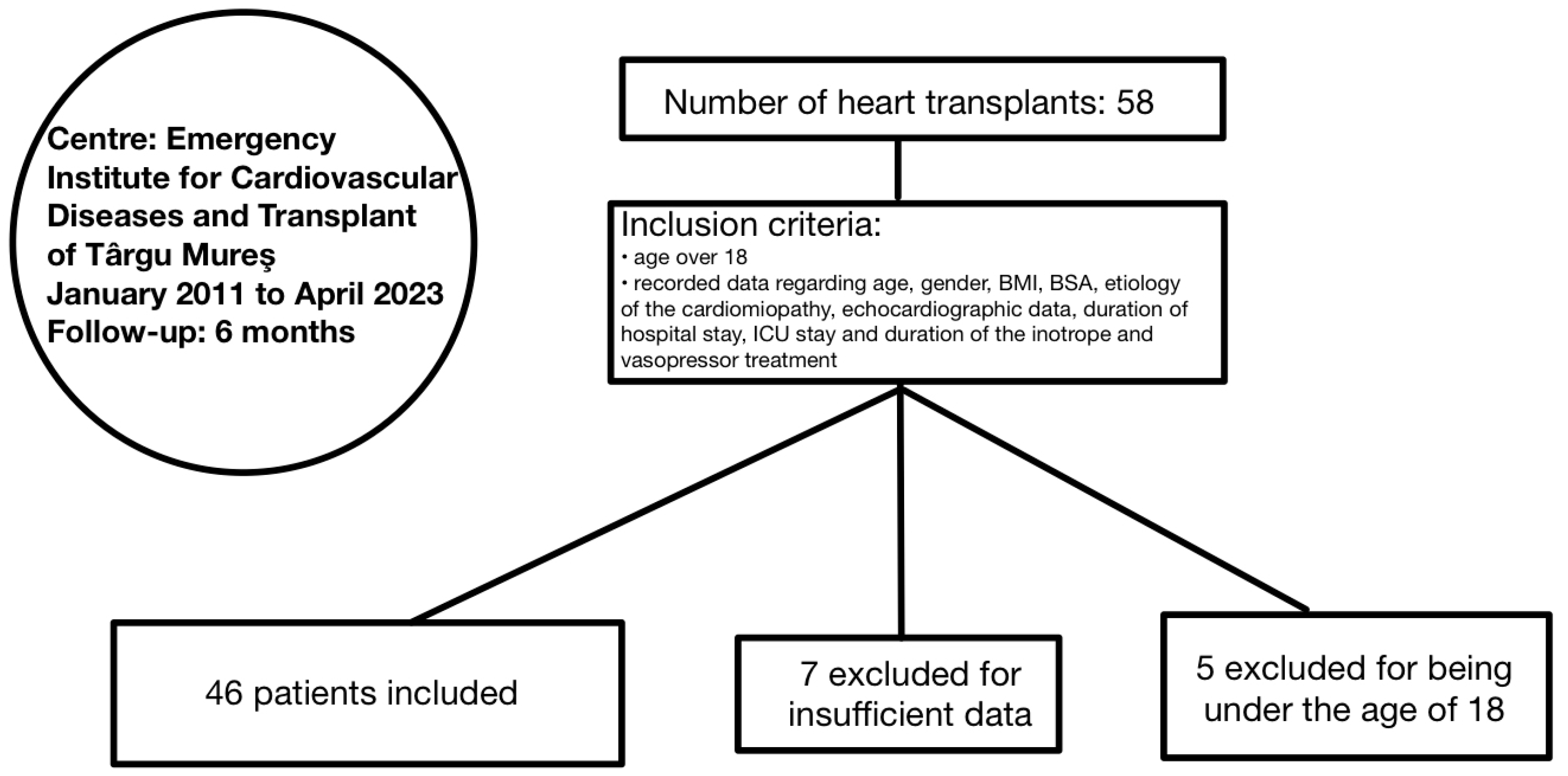
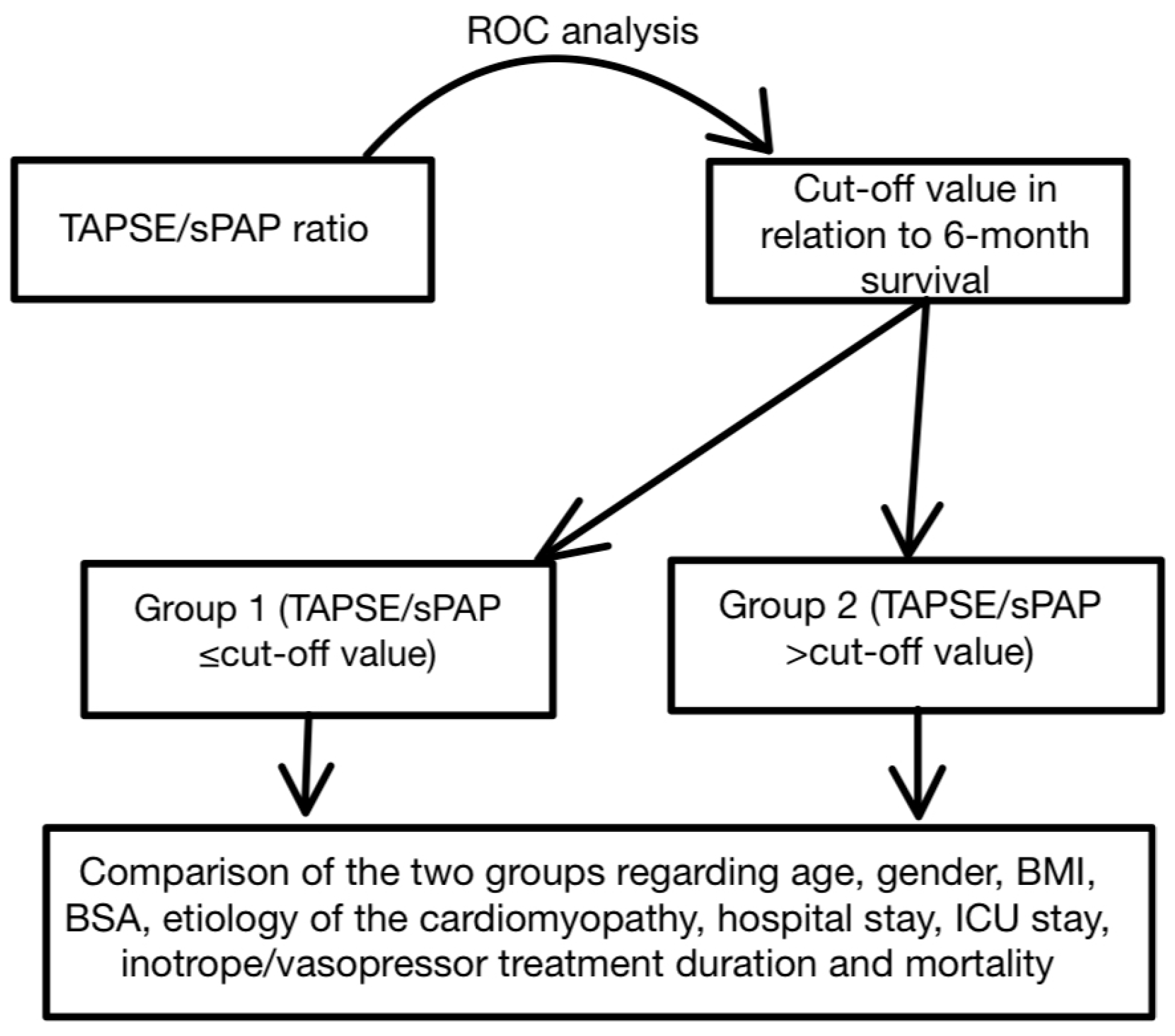
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brink, J.G.; Hassoulas, J. The first human heart transplant and further advances in cardiac transplantation at Groote Schuur Hospital and the University of Cape Town—With reference to: The operation. A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. Cardiovasc. J. Afr. 2009, 20, 31–35. [Google Scholar] [PubMed]
- Alraies, M.C.; Eckman, P. Adult heart transplant: Indications and outcomes. J. Thorac. Dis. 2014, 6, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.; Williams, P.; Mancini, D.; Lund, L.H. Selecting patients for heart transplantation: Comparison of the Heart Failure Survival Score (HFSS) and the Seattle Heart Failure Model (SHFM). J. Heart Lung Transplant. 2011, 30, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; Shah, A.; Griffith, B.P. Current status and outcomes in heart transplantation: A narrative review. Rev. Cardiovasc. Med. 2022, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Baba, D.-F.; Suciu, H.; Avram, C.; Gyorgy, M.; Danilesco, A.; Huma, L.; Sin, I.A. Elevated Levels of Neutrophil-to Monocyte Ratio Are Associated with the Initiation of Paroxysmal Documented Atrial Fibrillation in the First Two Months after Heart Transplantation: A Uni-Institutional Retrospective Study. J. Cardiovasc. Dev. Dis. 2023, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Baba, D.F.; Suciu, H.; Huma, L.; Avram, C.; Danilesco, A.; Moldovan, D.A.; Opincar, A.S.; Sin, A.I. Platelet-to-Albumin Ratio: The Prognostic Utility in the Prediction of 2-Month Postoperative Heart Transplant Complications. J. Cardiovasc. Dev. Dis. 2023, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Kavarana, M.N.; Savage, A.; O’Connell, R.; Rubinstein, C.S.; Flynn-Reeves, J.; Joshi, K.; Stroud, M.R.; Ikonomidis, J.S.; Bradley, S.M. Composite risk factors predict survival after transplantation for congenital heart disease. J. Thorac. Cardiovasc. Surg. 2013, 146, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Nog, R.; Aggarwal Gupta, C.; Panza, J.A. Role of MicroRNA in Heart Transplant. Cardiol. Rev. 2022, 30, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.; Edwards, N.F.A.; Doyle, S.N.; Wong, Y.W.; Scalia, G.M.; Sabapathy, S.; Chan, J. Prognostic Value of Left and right ventricular deformation strain analysis on Acute Cellular rejection in Heart Transplant recipients: A 6-year outcome study. Int. J. Cardiovasc. Imaging 2022, 38, 2271–2281. [Google Scholar] [CrossRef]
- Fauvel, C.; Raitiere, O.; Boucly, A.; De Groote, P.; Renard, S.; Bertona, J.; Lamblin, N.; Artaud-Macari, E.; Viacroze, C.; Schleifer, D.; et al. Interest of TAPSE/sPAP ratio for noninvasive pulmonary arterial hypertension risk assessment. J. Heart Lung Transplant. 2022, 41, 1761–1772. [Google Scholar] [CrossRef]
- Aydlnyllmaz, F.; Guliyev, İ.; Özbeyaz, N.B.; Algül, E.; Aker, M.; Şahan, H.F.; Erzurum, M.; Felekoğlu, M.A.; Kalkan, K. Predicting hospitalization by TAPSE/SPAP and the role of spironolactone in asymptomatic heart failure patients. Biomark. Med. 2023, 17, 197–207. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Caminati, A.; Grasso, E.; Colleoni, M.; Nicolosi, G.L.; Lombardo, M.; Harari, S. TAPSE/SPAP ratio stratifies mortality risk in mild-to-moderate idiopathic pulmonary fibrosis. Int. J. Tuberc. Lung Dis. 2024, 28, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.C.; Rosato, E.; D’Angelo, A.; Cristiano, E.; Marchitti, S.; Volpe, M.; Rubattu, S.; Romaniello, A. The prognostic role of the echocardiographic tricuspid annular plane systolic excursion/systolic pulmonary arterial pressure (TAPSE/sPAP) ratio and its relationship with NT-proANP plasma level in systemic sclerosis. Front. Cardiovasc. Med. 2022, 9, 1021048. [Google Scholar] [CrossRef] [PubMed]
- Maccallini, M.; Barge-Caballero, G.; Barge-Caballero, E.; López-Pérez, M.; Bilbao-Quesada, R.; González-Babarro, E.; Gómez-Otero, I.; López-López, A.; Gutiérrez-Feijoo, M.; Varela-Román, A.; et al. Prognostic value of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio in cardiac amyloidosis. Rev. Esp. Cardiol. 2024, in press. [CrossRef] [PubMed]
- He, Q.; Lin, Y.; Zhu, Y.; Gao, L.; Ji, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Usefulness of Right Ventricle-Pulmonary Artery Coupling in Cardiovascular Disease. J. Clin. Med. 2023, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Monitillo, F.; di Terlizzi, V.; Gioia, M.I.; Barone, R.; Grande, D.; Parisi, G.; Brunetti, N.D.; Iacoviello, M. Right Ventricular Function in Chronic Heart Failure: From the Diagnosis to the Therapeutic Approach. J. Cardiovasc. Dev. Dis. 2020, 7, 12. [Google Scholar] [CrossRef]
- Schmeißer, A.; Rauwolf, T.; Groscheck, T.; Fischbach, K.; Kropf, S.; Luani, B.; Tanev, I.; Hansen, M.; Meißler, S.; Schäfer, K.; et al. Predictors and prognosis of right ventricular function in pulmonary hypertension due to heart failure with reduced ejection fraction. ESC Heart Fail. 2021, 8, 2968–2981. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Villani, S.; Generati, G.; Ferraro, O.E.; Pellegrino, M.; Alfonzetti, E.; Labate, V.; Gaeta, M.; Sugimoto, T.; Bandera, F. Right Ventricular Contractile Reserve and Pulmonary Circulation Uncoupling During Exercise Challenge in Heart Failure: Pathophysiology and Clinical Phenotypes. JACC Heart Fail. 2016, 4, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.N.; Kobashigawa, J.A.; Givertz, M.M. Evolving Areas in Heart Transplantation. JACC Heart Fail. 2017, 5, 869–878. [Google Scholar] [CrossRef]
- Colalillo, A.; Hoffmann-Vold, A.M.; Pellicano, C.; Romaniello, A.; Gabrielli, A.; Hachulla, E.; Smith, V.; Simeón-Aznar, C.P.; Castellví, I.; Airò, P.; et al. The role of TAPSE/sPAP ratio in predicting pulmonary hypertension and mortality in the systemic sclerosis EUSTAR cohort. Autoimmun. Rev. 2023, 22, 103290. [Google Scholar] [CrossRef]
- Colalillo, A.; Grimaldi, M.C.; Vaiarello, V.; Pellicano, C.; Leodori, G.; Gigante, A.; Romaniello, A.; Rosato, E. In systemic sclerosis, the TAPSE/sPAP ratio can be used in addition to the DETECT algorithm for pulmonary arterial hypertension diagnosis. Rheumatology 2022, 61, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, C.; Dillinger, J.G.; Bouleti, C.; Trimaille, A.; Tron, C.; Chaussade, A.S.; Thuaire, C.; Delmas, C.; Boccara, A.; Roule, V.; et al. TAPSE/sPAP prognostic value for In-Hospital Adverse Events in Patients Hospitalized for Acute Coronary Syndrome. Eur. Heart J. Cardiovasc. Imaging 2024, jeae110. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Smith, J.; Grigoryan, K.; Lysne, V.; Rajani, R.; Chambers, J.B. The tricuspid annular plane systolic excursion to systolic pulmonary artery pressure index: Association with all-cause mortality in patients with moderate or severe tricuspid regurgitation. Int. J. Cardiol. 2020, 317, 176–180. [Google Scholar] [CrossRef] [PubMed]
- L’Official, G.; Vely, M.; Kosmala, W.; Galli, E.; Guerin, A.; Chen, E.; Sportouch, C.; Dreyfus, J.; Oger, E.; Donal, E. Isolated functional tricuspid regurgitation: How to define patients at risk for event? ESC Heart Fail. 2023, 10, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, A.; Tomaniak, M.; Gumiężna, K.; Kurzyna, P.; Bednarek, A.; Skulimowska, J.; Pedzich, E.; Kapłon-Cieślicka, A.; Rdzanek, A.; Ścisło, P. Clinical and echocardiographic characterization of patients hospitalized for severe tricuspid valve regurgitation: A single tertiary-care center experience with two-year follow-up. Pol. Arch. Intern. Med. 2024, 15, 16752. [Google Scholar] [CrossRef]
- Mena, C.; Wencker, D.; Krumholz, H.M.; McNamara, R.L. Detection of Heart Transplant Rejection in Adults by Echocardiographic Diastolic Indices: A Systematic Review of the Literature. J. Am. Soc. Echocardiogr. 2006, 19, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Al-Ani, A.; Gude, E.; Gullestad, L.; Aakhus, S. Echocardiographic evaluation of left ventricular filling pressure in heart transplant recipients. Scand. Cardiovasc. J. 2014, 48, 349–356. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; Fusi, C.; Landra, F.; Barilli, M.; Lisi, M.; Mandoli, G.E.; D’Ascenzi, F.; Focardi, M.; Valente, S.; Cameli, M. Diastolic function in heart transplant: From physiology to echocardiographic assessment and prognosis. Front. Cardiovasc. Med. 2022, 9, 969270. [Google Scholar] [CrossRef]
- Buddhe, S.; Richmond, M.E.; Gilbreth, J.; Lai, W.W. Longitudinal Strain by Speckle Tracking Echocardiography in Pediatric Heart Transplant Recipients. Congenit. Heart Dis. 2015, 10, 362–370. [Google Scholar] [CrossRef]
- Baba, D.F.; Suciu, H.; Avram, C.; Danilesco, A.; Moldovan, D.A.; Rauta, R.C.; Huma, L.; Sin, I.A. The Role of Preoperative Chronic Statin Therapy in Heart Transplant Receipts-A Retrospective Single-Center Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 3471. [Google Scholar] [CrossRef]
- Roshanali, F.; Mandegar, M.H.; Bagheri, J.; Sarzaeem, M.R.; Chitsaz, S.; Alaeddini, F.; Saidi, B. Echo rejection score: New echocardiographic approach to diagnosis of heart transplant rejection. Eur. J. Cardio-Thorac. Surg. 2010, 38, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Putzer, G.J.B.; Cooper, D.; Keehn, C.; Asante-Korang, A.; Boucek, M.M.; Boucek, R.J. An improved echocardiographic rejection-surveillance strategy following pediatric heart transplantation. J. Heart Lung Transplant. 2000, 19, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.C.P.L.; Rodrigues, A.C.T.; Vieira, M.L.C.; Fischer, C.H.; Monaco, C.G.; Filho, E.B.L.; Bacal, F.; Caixeta, A.; Morhy, S.S. Evaluation of the myocardial deformation in the diagnosis of rejection after heart transplantation. Front. Cardiovasc. Med. 2022, 9, 991016. [Google Scholar] [CrossRef]
- Mingo-Santos, S.; Moñivas-Palomero, V.; Garcia-Lunar, I.; Mitroi, C.D.; Goirigolzarri-Artaza, J.; Rivero, B.; Oteo, J.F.; Castedo, E.; González-Mirelis, J.; Cavero, M.A.; et al. Usefulness of Two-Dimensional Strain Parameters to Diagnose Acute Rejection after Heart Transplantation. J. Am. Soc. Echocardiogr. 2015, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Ciliberto, G.R. Doppler echocardiography after heart transplantation. Ital. Heart J Suppl. 2000, 1, 1411–1416. [Google Scholar] [PubMed]
- Resende, M.V.C.; Vieira, M.L.C.; Bacal, F.; Andrade, J.L.; Stolf, N.A.; Bocchi, E.A. Tissue doppler echocardiography in the diagnosis of heart transplantation rejection. Arq. Bras. Cardiol. 2011, 97, 8–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, C.A.; Naish, J.H.; Shaw, S.M.; Yonan, N.; Williams, S.G.; Clark, D.; Bishop, P.W.; Ainslie, M.P.; Borg, A.; Coutts, G.; et al. Multiparametric cardiovascular magnetic resonance surveillance of acute cardiac allograft rejection and characterisation of transplantation-associated myocardial injury: A pilot study. J. Cardiovasc. Magn. Reson. 2014, 16, 52. [Google Scholar] [CrossRef]
- Foroutan, F.; Alba, A.C.; Guyatt, G.; Duero Posada, J.; Ng Fat Hing, N.; Arseneau, E.; Meade, M.; Hanna, S.; Badiwala, M.; Ross, H. Predictors of 1-year mortality in heart transplant recipients: A systematic review and meta-analysis. Heart 2018, 104, 151–160. [Google Scholar] [CrossRef]
- Wever-Pinzon, O.; Edwards, L.B.; Taylor, D.O.; Kfoury, A.G.; Drakos, S.G.; Selzman, C.H.; Fang, J.C.; Lund, L.H.; Stehlik, J. Association of recipient age and causes of heart transplant mortality: Implications for personalization of post-transplant management—An analysis of the International Society for Heart and Lung Transplantation Registry. J. Heart Lung Transplant. 2017, 36, 407–417. [Google Scholar] [CrossRef]
- Pazdernik, M.; Wichterle, D.; Chet, Z.; Bedanova, H.; Kautzner, J.; Melenovsky, V.; Karmazin, V.; Malek, I.; Stiavnicky, P.; Tomasek, A.; et al. Heart rate and early progression of cardiac allograft vasculopathy: A prospective study using highly automated 3-D optical coherence tomography analysis. Clin. Transplant. 2020, 34, e13773. [Google Scholar] [CrossRef]
- Cater, R.; Taylor, J. The experiences of heart transplant recipients’ spouses during the pretransplant waiting period: Integrative review. J. Clin. Nurs. 2017, 26, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Burker, E.J.; Evon, D.M.; Ascari, J.C.; Loiselle, M.M.; Finkel, J.B.; Mill, M.R. Relationship between Coping and Depression in Heart Transplant Candidates and Their Spouses. Prog. Transpl. 2006, 16, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A. Small studies: Strengths and limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef]
- Bacușcă, A.E.; Burlacu, A.; Tinică, G.; Enache, M.; Ţărus, A.; Gavriluţă, C.; Brinza, C.; Hanganu, B.; Ioan, B.G. Organ Procurement, Donation, and Transplant Awareness in an Urban Eastern European Region: A General Population Survey. Ann. Transpl. 2022, 27, e938016. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.; Karner-Huţuleac, A.; Ioan, B. Factors of the willingness to consent to the donation of a deceased family member’s organs among the Romanian urban population. Transpl. Proc. 2013, 45, 3178–3182. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean | SD |
|---|---|---|
| Age (years) | 45.19 | 10.50 |
| BMI (kg/sqm) | 24.78 | 4.08 |
| BSA (sqm) | 1.92 | 0.22 |
| TAPSE (mm) | 17.06 | 3.17 |
| sPAP (mmHg) | 32.67 | 7.90 |
| TAPSE/sPAP (mm/mmHg) | 0.56 | 0.19 |
| Hospital stay (days) | 56.89 | 65.17 |
| ICU stay (days) | 49.23 | 61.57 |
| i.v. inotropes/vasopressors (days) | 8.39 | 12.82 |
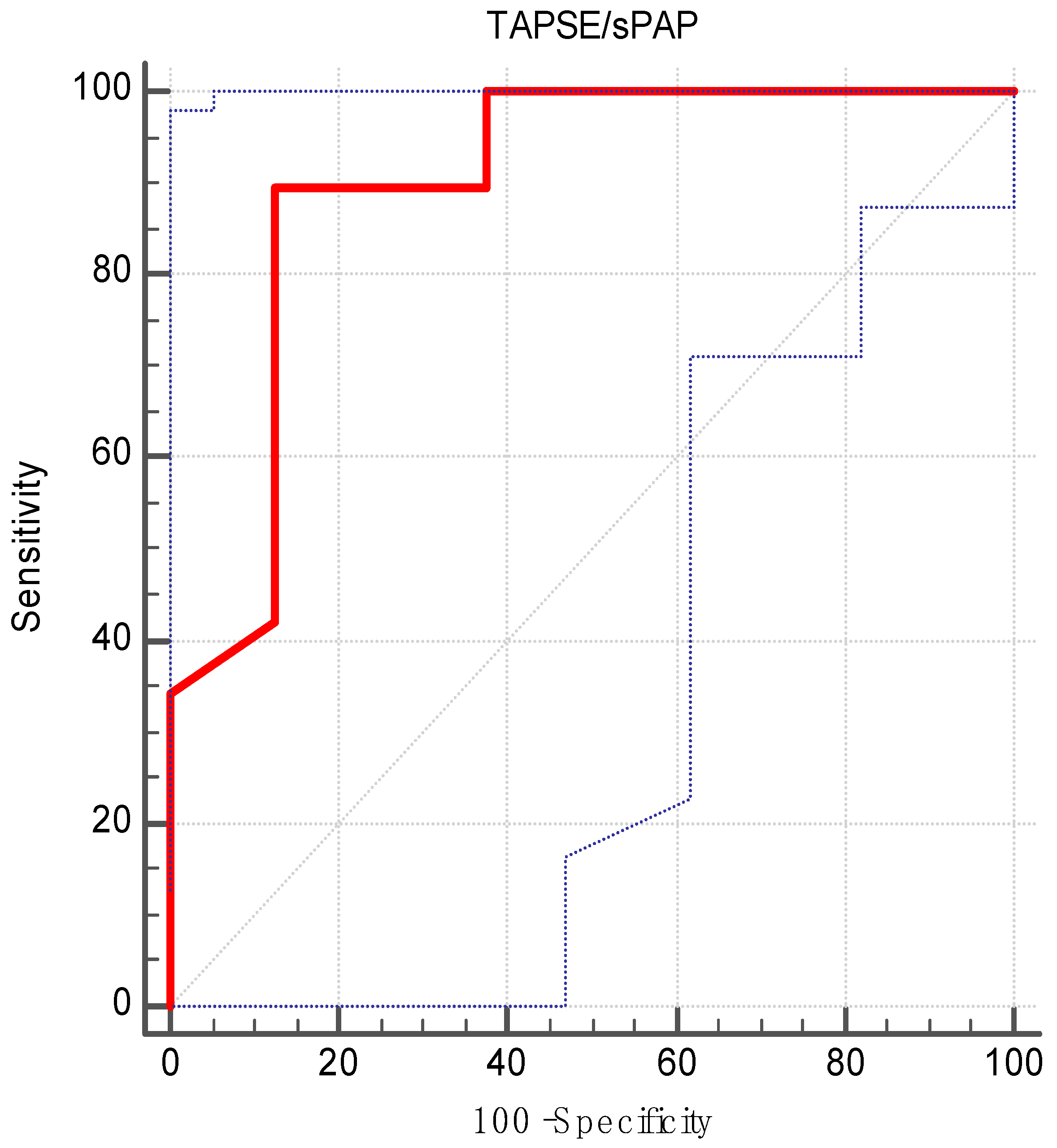
| AUC | 0.89 |
| Standard error | 0.07 |
| 95% CI | 0.77–0.96 |
| z statistic | 5.11 |
| p value | <0.001 |
| Maximum Youden‘s index | 0.76 |
| Associated criterion | >0.47 |
| Sensitivity | 89.47 |
| Specificity | 87.50 |
| Parameter | Group 1 (n = 11) | Group 2 (n = 35) | p Value |
|---|---|---|---|
| Age, mean (SD)/median (years) | 40.45 (13.31)/37 | 46.68 (9.18)/47 | 0.08 * |
| Gender (male), n (%) | 10 (90.90) | 30 (85.71) | 1.00 † |
| BMI, mean (SD)/median (kg/sqm) | 24.39 (4.46)/24.6 | 24.90 (4.02)/24.6 | 0.65 ** |
| BSA, mean (SD)/median (sqm) | 1.93 (0.23)/1.96 | 1.92 (0.23)/1.96 | 0.84 ** |
| Non-ischemic cardiomyopathy, n (%) | 9 (81.81) | 25 (71.42) | 0.70 † |
| Hospital stay, mean (SD)/median (days) | 36.36 (21.75)/37 | 63.34 (72.82)/39 | 0.28 ** |
| ICU stay, mean (SD)/median (days) | 32.54 (21.11)/32 | 54.85 (69.05)/35 | 0.27 ** |
| i.v. inotropes/vasopressors, mean (SD)/median (days) | 14.36 (22.59)/5 | 6.51 (7.24)/4 | 0.06 ** |
| Mortality, n (%) | 7 (63.63) | 1 (2.85) | <0.001 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huma, L.; Suciu, H.; Avram, C.; Suteu, R.-A.; Danilesco, A.; Baba, D.-F.; Moldovan, D.-A.; Sin, A.-I. Tricuspid Annular Plane Systolic Excursion-to-Systolic Pulmonary Artery Pressure Ratio as a Prognostic Factor in Heart Transplant Patients. Medicina 2024, 60, 1078. https://doi.org/10.3390/medicina60071078
Huma L, Suciu H, Avram C, Suteu R-A, Danilesco A, Baba D-F, Moldovan D-A, Sin A-I. Tricuspid Annular Plane Systolic Excursion-to-Systolic Pulmonary Artery Pressure Ratio as a Prognostic Factor in Heart Transplant Patients. Medicina. 2024; 60(7):1078. https://doi.org/10.3390/medicina60071078
Chicago/Turabian StyleHuma, Laurentiu, Horatiu Suciu, Calin Avram, Radu-Adrian Suteu, Alina Danilesco, Dragos-Florin Baba, Diana-Andreea Moldovan, and Anca-Ileana Sin. 2024. "Tricuspid Annular Plane Systolic Excursion-to-Systolic Pulmonary Artery Pressure Ratio as a Prognostic Factor in Heart Transplant Patients" Medicina 60, no. 7: 1078. https://doi.org/10.3390/medicina60071078
APA StyleHuma, L., Suciu, H., Avram, C., Suteu, R.-A., Danilesco, A., Baba, D.-F., Moldovan, D.-A., & Sin, A.-I. (2024). Tricuspid Annular Plane Systolic Excursion-to-Systolic Pulmonary Artery Pressure Ratio as a Prognostic Factor in Heart Transplant Patients. Medicina, 60(7), 1078. https://doi.org/10.3390/medicina60071078







