Transcatheter Mitral Valve Intervention: Current and Future Role of Multimodality Imaging for Device Selection and Periprocedural Guidance
Abstract
:1. Introduction
- ▪
- A-FMR. The main mechanism is represented by annular dysfunction or dilatation and atriogenic leaflet tethering with reduced leaflet remodeling, leading to an annulus–leaflet area imbalance. It usually involves patients with preserved left ventricular (LV) systolic function, particularly those suffering from atrial fibrillation (AF) [7,8]. Optimal treatment remains debated and includes rhythm control and different transcatheter and surgical procedures, with the latter potentially able to treat all the mechanisms of the disease: plication for left atrium (LA) enlargement, annuloplasty for annulus dilatation, patch augmentation for insufficient leaflet remodeling, and the Cox–Maze procedure for AF [9,10]. When feasible, MVr is probably associated with a better outcome than MVR [11].
- ▪
- V-FMR. The main mechanism is LV dilatation and/or systolic dysfunction with global or regional remodeling of LV and/or asynchrony, leading to symmetric or asymmetric tethering of mitral leaflets. The best treatment usually involves a comprehensive use of the “classical” heart failure management strategies: optimal medical treatment, myocardial revascularization if indicated, cardiac resynchronization therapy, and transcatheter edge-to-edge repair (TEER) [12].
2. Materials and Methods
3. Transcatheter Mitral Valve Intervention
- -
- Leaflet’s approximation: MitraClip (Abbott Cardiovascular, Plymouth, MN, USA), PASCAL (Edwards Lifesciences, Irvine, CA, USA);
- -
- Direct annuloplasty: Cardioband (Edwards Lifesciences, Irvine, CA, USA);
- -
- Indirect annuloplasty: Carillon Mitral Contour System (Cardiac Dimensions, Washington, DC, USA);
- -
- Chordal repair: HARPOON (Edwards Lifesciences, Irvine, CA, USA). NeoChord (NeoChord Inc. Louise Park, MN, USA).
- Vi-V: This approach encompasses the implantation of a new bioprosthesis within a degenerated mitral bioprosthesis and is the most used out of the three. It is feasible via both TS and TA approaches. ViV procedures have demonstrated excellent results in terms of procedural (74%) and technical (94.4%) success rates, with a low incidence of post-procedural adverse events: 2.2% significant left ventricular outflow obstruction (LVOTO), less than 1% conversion to surgery, 3.3% significant paravalvular leak (PVL), and 6.2% 30-day mortality.
- Vi-R: This approach is based on the implantation of a bioprosthesis following a failed MV annuloplasty. The procedural success rate (57.4%) is lower compared to ViV procedures, with an increased risk of adverse events: 5% LVOTO, 12.6% PVL, 12% requiring a second valve implantation, and 9.9% 30-day mortality. The higher incidence of peri-procedural complications, along with significant residual MR, partly accounts for this elevated mortality rate. It is noteworthy that in patients previously subjected to MVr with an annuloplasty ring, TEER should be prioritized as the first option [35].
- Vi-MAC: This procedure poses significant technical challenges and is associated with a high-risk profile in the target population. Procedural success rates are comparatively lower (41.4%), with a higher incidence of complications: 8.6% conversion to surgery, 39.7% LVOTO, 6.9% valve embolization, 13% significant residual MR, 34.5% 30-day mortality, and over 60% one-year mortality. Due to these complexities and risks, Vi-MAC procedures are considered the most challenging among the various TMVR approaches. There are two ongoing trial that will provide additional information on the feasibility of TMVR in this setting, one with the Tendyne device (ClinicalTrials.Gov: NCT 0.539458) and the other with the Intrepid TMVR system (APOLLO, ClinicalTrials.Gov: NCT03242642).
4. Pre-Procedural Evaluation
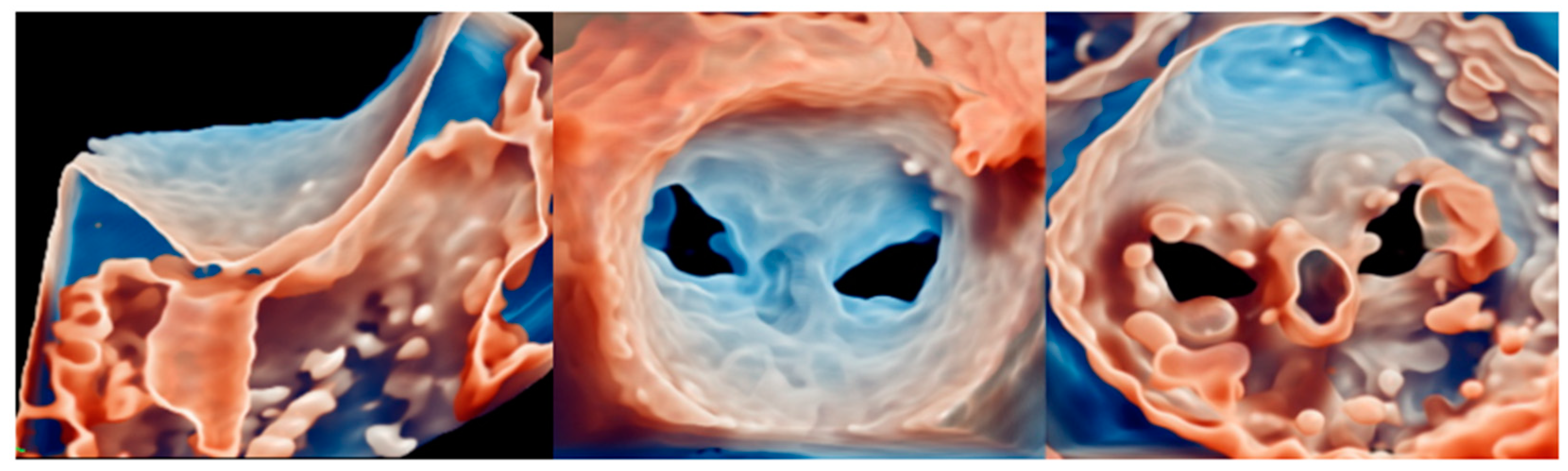
4.1. Transcatether Edge-to-Edge Repair
4.2. Indirect and Direct Annuloplasty
4.3. Chordal Repair
- “Type A” (isolated central posterior prolapse/flail);
- “Type B” (posterior multi-scallop prolapse/flail);
- “Type C” (anterior or bileaflet prolapse/flail);
- “Type D” (para-commissural prolapse/flail or any significant annular or leaflet disease, e.g., calcification).
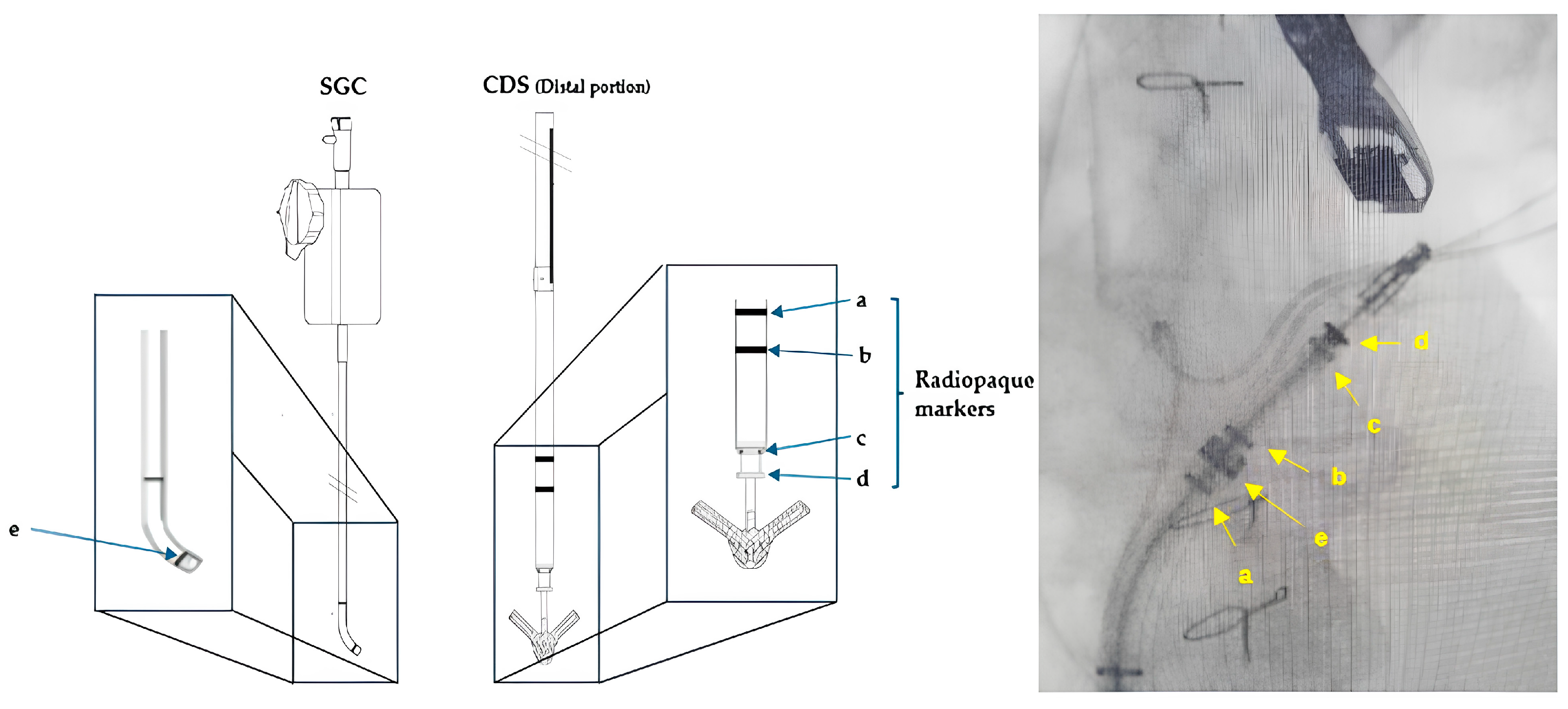
4.4. TMVR
5. Intra-Procedural Guidance
5.1. Transcatether Edge-to-Edge Repair
- (1)
- TSP and SGC advancement;
- (2)
- Straddling and steering of the device;
- (3)
- Orientation;
- (4)
- Grasping;
- (5)
- Final evaluation and release.
5.2. Indirect Annuloplasty
5.3. Direct Annuloplasty
5.4. Chordal Repair
5.5. TMVR
6. Post-Procedural Evaluation
6.1. TEER
6.2. Annuloplasty Devices
6.3. Chordal Repair
6.4. TMVR
7. Emerging Techniques and Potential Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ASD | Atrial septal defect |
| AV | Atrio-ventricular |
| cMDCT | Contrast multidetector-computed tomography |
| CS | Coronary sinus |
| FMR | Functional mitral regurgitation |
| GCV | Great cardiac vein |
| IAS | Interatrial septum |
| IE | Interventional echocardiographer |
| LA | Left atrium, left atrial |
| LCA | Left circumflex artery |
| LV | Left ventricle, left ventricular |
| LVOT | Left ventricular outflow |
| LVOTO | Left ventricular outflow obstruction |
| MA | Mitral annulus |
| MR | Mitral regurgitation |
| PMR | Primary mitral regurgitation |
| PVL | Paravalvular leak |
| PWD | Pulsed-wave Doppler |
| RAE | Radiation exposure |
| SHD | Structural heart disease |
| TEE | Transesophageal echocardiography |
| TEER | Transcatheter edge-to-edge repair |
| TMVI | Transcatheter mitral valve intervention |
| TMVr | Transcatheter mitral valve repair |
| TMVR | Transcatheter mitral valve replacement |
| TR | Tricuspid regurgitation |
| Vi-MAC | Valve in MAC |
| Vi-R | Valve in ring |
| Vi-V | Valve in valve |
| VR | Virtual reality |
| WMAs | Wall motion abnormalities |
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Ielasi, A.; Oppizzi, M.; Faggiano, P.; Ferri, L.; Calabrese, A.; Vizzardi, E.; Alfieri, O.; Margonato, A. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur. J. Heart Fail. 2009, 11, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Rusinaru, D.; Grigioni, F.; Michelena, H.I.; Vanoverschelde, J.L.; Avierinos, J.F.; Barbieri, A.; Pislaru, S.V.; Russo, A.; Pasquet, A.; et al. Long-term mortality associated with left ventricular dysfunction in mitral regurgitation due to flail leaflets: A multicenter analysis. Circ. Cardiovasc. Imaging 2014, 7, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Chaput, M.; Handschumacher, M.D.; Tournoux, F.; Hua, L.; Guerrero, J.L.; Vlahakes, G.J.; Levine, R.A. Mitral leaflet adaptation to ventricular remodeling: Occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008, 118, 845–852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sannino, A.; Smith, R.L., 2nd; Schiattarella, G.G.; Trimarco, B.; Esposito, G.; Grayburn, P.A. Survival and Cardiovascular Outcomes of Patients With Secondary Mitral Regurgitation: A Systematic Review and Meta-analysis. JAMA Cardiol. 2017, 2, 1130–1139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okamoto, C.; Okada, A.; Nishimura, K.; Moriuchi, K.; Amano, M.; Takahama, H.; Amaki, M.; Hasegawa, T.; Kanzaki, H.; Fujita, T.; et al. Prognostic comparison of atrial and ventricular functional mitral regurgitation. Open Heart 2021, 8, e001574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silbiger, J.J. Novel pathogenetic mechanisms and structural adaptations in ischemic mitral regurgitation. J. Am. Soc. Echocardiogr. 2013, 26, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Verhaert, D.; Rega, F.; Thomas, J.D.; Vandervoort, P.M. Atrial Functional Mitral Regurgitation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Farhan, S.; Silbiger, J.J.; Halperin, J.L.; Zhang, L.; Dukkipati, S.R.; Vogel, B.; Kini, A.; Sharma, S.; Lerakis, S. Pathophysiology, Echocardiographic Diagnosis, and Treatment of Atrial Functional Mitral Regurgitation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 2314–2330, Erratum in J. Am. Coll. Cardiol. 2023, 81, 711. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Domene, R.; Canovas, S.J. Going further of mitral ring annuloplasty: The role of surgery in atrial functional mitral regurgitation. J. Thorac. Dis. 2023, 15, 2381–2384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirji, S.A.; Cote, C.L.; Javadikasgari, H.; Malarczyk, A.; McGurk, S.; Kaneko, T. Atrial functional versus ventricular functional mitral regurgitation: Prognostic implications. J. Thorac. Cardiovasc. Surg. 2022, 164, 1808–1815.e4. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Mirabel, M.; Iung, B.; Baron, G.; Messika-Zeitoun, D.; Détaint, D.; Vanoverschelde, J.L.; Butchart, E.G.; Ravaud, P.; Vahanian, A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 2007, 28, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Kar, S.; Rinaldi, M.; Fail, P.; Hermiller, J.; Smalling, R.; Whitlow, P.L.; Gray, W.; Low, R.; Herrmann, H.C.; et al. Percutaneous mitral repair with the MitraClip system: Safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J. Am. Coll. Cardiol. 2009, 54, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; Kapadia, S.R.; et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N. Engl. J. Med. 2023, 388, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Doldi, P.M.; Stolz, L.; Kalbacher, D.; Köll, B.; Geyer, M.; Ludwig, S.; Orban, M.; Braun, D.; Weckbach, L.T.; Stocker, T.J.; et al. Right ventricular dysfunction predicts outcome after transcatheter mitral valve repair for primary mitral valve regurgitation. Eur. J. Heart Fail. 2022, 24, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Karam, N.; Stolz, L.; Orban, M.; Deseive, S.; Praz, F.; Kalbacher, D.; Westermann, D.; Braun, D.; Näbauer, M.; Neuss, M.; et al. Impact of Right Ventricular Dysfunction on Outcomes After Transcatheter Edge-to-Edge Repair for Secondary Mitral Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Vaturi, M.; Kaewkes, D.; Koren, O.; Koseki, K.; Solanki, A.; Natanzon, S.S.; Patel, V.; Skaf, S.; Makar, M.; et al. Prognostic Value of Baseline Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure Ratio in Mitral Transcatheter Edge-to-Edge Repair. J. Am. Soc. Echocardiogr. 2023, 36, 391–401.e19. [Google Scholar] [CrossRef] [PubMed]
- Szerlip, M.; Spargias, K.S.; Makkar, R.; Kar, S.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.C.; Smith, R.L.; Fam, N.P.; Rinaldi, M.J.; et al. 2-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. JACC Cardiovasc. Interv. 2021, 14, 1538–1548, Erratum in JACC Cardiovasc. Interv. 2022, 15, 1395. [Google Scholar] [CrossRef] [PubMed]
- Zahr, F.; Smith, R.L.; Gillam, L.D.; Chadderdon, S.; Makkar, R.; von Bardeleben, R.S.; Ruf, T.F.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. One-Year Outcomes From the CLASP IID Randomized Trial for Degenerative Mitral Regurgitation. JACC Cardiovasc. Interv. 2023, 16, S1936-8798(23)01358-4. [Google Scholar] [CrossRef] [PubMed]
- Schofer, J.; Siminiak, T.; Haude, M.; Herrman, J.P.; Vainer, J.; Wu, J.C.; Levy, W.C.; Mauri, L.; Feldman, T.; Kwong, R.Y.; et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: Results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009, 120, 326–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siminiak, T.; Wu, J.C.; Haude, M.; Hoppe, U.C.; Sadowski, J.; Lipiecki, J.; Fajadet, J.; Shah, A.M.; Feldman, T.; Kaye, D.M.; et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: Results of the TITAN Trial. Eur. J. Heart Fail. 2012, 14, 931–938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipiecki, J.; Siminiak, T.; Sievert, H.; Müller-Ehmsen, J.; Degen, H.; Wu, J.C.; Schandrin, C.; Kalmucki, P.; Hofmann, I.; Reuter, D.; et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: The TITAN II trial. Open Heart 2016, 3, e000411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Witte, K.K.; Lipiecki, J.; Siminiak, T.; Meredith, I.T.; Malkin, C.J.; Goldberg, S.L.; Stark, M.A.; von Bardeleben, R.S.; Cremer, P.C.; Jaber, W.A.; et al. The REDUCE FMR Trial: A Randomized Sham-Controlled Study of Percutaneous Mitral Annuloplasty in Functional Mitral Regurgitation. JACC Heart Fail. 2019, 7, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Lipiecki, J.; Kaye, D.M.; Witte, K.K.; Haude, M.; Kapadia, S.; Sievert, H.; Goldberg, S.L.; Levy, W.C.; Siminiak, T. Long-Term Survival Following Transcatheter Mitral Valve Repair: Pooled Analysis of Prospective Trials with the Carillon Device. Cardiovasc. Revasc. Med. 2020, 21, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Messika-Zeitoun, D.; Nickenig, G.; Latib, A.; Kuck, K.H.; Baldus, S.; Schueler, R.; La Canna, G.; Agricola, E.; Kreidel, F.; Huntgeburth, M.; et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur. Heart J. 2019, 40, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hegeman, R.M.J.J.; Gheorghe, L.L.; de Kroon, T.L.; van Putte, B.P.; Swaans, M.J.; Klein, P. State-of-the-Art Review: Technical and Imaging Considerations in Novel Transapical and Port-Access Mitral Valve Chordal Repair for Degenerative Mitral Regurgitation. Front. Cardiovasc. Med. 2022, 9, 850700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colli, A.; Zucchetta, F.; Kliger, C.; Bellu, R.; Francone, M.; Sedati, P.; Jelnin, V.; Ruiz, C.E.; Manzan, E.; Besola, L.; et al. CT for the Transapical Off-Pump Mitral Valve Repair With Neochord Implantation Procedure. JACC Cardiovasc. Imaging 2017, 10, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.; Bizzotto, E.; Manzan, E.; Besola, L.; Pradegan, N.; Bellu, R.; Pittarello, D.; Gerosa, G. Patient-Specific Ventricular Access Site Selection for the NeoChord Mitral Valve Repair Procedure. Ann. Thorac. Surg. 2017, 104, e199–e202. [Google Scholar] [CrossRef] [PubMed]
- Seeburger, J.; Rinaldi, M.; Nielsen, S.L.; Salizzoni, S.; Lange, R.; Schoenburg, M.; Alfieri, O.; Borger, M.A.; Mohr, F.W.; Aidietis, A. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: The TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J. Am. Coll. Cardiol. 2014, 63, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Gammie, J.S.; Bartus, K.; Gackowski, A.; Szymanski, P.; Bilewska, A.; Kusmierczyk, M.; Kapelak, B.; Rzucidlo-Resil, J.; Duncan, A.; Yadav, R.; et al. Safety and performance of a novel transventricular beating heart mitral valve repair system: 1-year outcomes. Eur. J. Cardiothorac. Surg. 2021, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Taramasso, M. Mitral valve-in-valve, valve-in-ring, and valve-in-MAC: The Good, the Bad, and the Ugly. Eur. Heart J. 2019, 40, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.D.; Smith, T.W.; Boyd, W.D.; Rogers, J.H. Clipping the Ring: Transcatheter Edge-to-Ring Mitral Valve Repair in a Patient With Prior Mitral Annuloplasty Ring. JACC Cardiovasc. Interv. 2018, 11, e55–e58. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Gössl, M.; Babaliaros, V.; Rizik, D.; Conradi, L.; Bae, R.; Burke, R.F.; Schäfer, U.; Lisko, J.C.; Riley, R.D.; et al. Novel Transcatheter Mitral Valve Prosthesis for Patients With Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2019, 74, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Moat, N.; Badhwar, V.; Walters, D.; Paone, G.; Bethea, B.; Bae, R.; Dahle, G.; Mumtaz, M.; Grayburn, P.; et al. Initial Feasibility Study of a New Transcatheter Mitral Prosthesis: The First 100 Patients. J. Am. Coll. Cardiol. 2019, 73, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, P.; Plicht, B.; Schenk, I.M.; Janosi, R.A.; Erbel, R.; Buck, T. Direct assessment of size and shape of noncircular vena contracta area in functional versus organic mitral regurgitation using real-time three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.; Ingallina, G.; Ancona, F.; Gaspardone, C.; Biondi, F.; Margonato, D.; Morosato, M.; Belli, M.; Tavernese, A.; Stella, S.; et al. Quantification of Mitral Regurgitation in Mitral Valve Prolapse by Three-Dimensional Vena Contracta Area: Derived Cutoff Values and Comparison With Two-Dimensional Multiparametric Approach. J. Am. Soc. Echocardiogr. 2024, 37, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; Kizilbash, A.M.; Afridi, I.; Franco, F.; Peshock, R.M.; Grayburn, P.A. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: Comparison with cine magnetic resonance imaging. J. Am. Coll. Cardiol. 1998, 32, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Barletta, V.; Hinojar, R.; Carbonell, A.; González-Gómez, A.; Fabiani, I.; Di Bello, V.; Jiménez-Nacher, J.J.; Zamorano, J.; Fernández-Golfín, C. Three-dimensional full automated software in the evaluation of the left ventricle function: From theory to clinical practice. Int. J. Cardiovasc. Imaging 2018, 34, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Hausleiter, J.; Stocker, T.J.; Adamo, M.; Karam, N.; Swaans, M.J.; Praz, F. Mitral valve transcatheter edge-to-edge repair. EuroIntervention 2023, 18, 957–976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agricola, E.; Ancona, F.; Bartel, T.; Brochet, E.; Dweck, M.; Faletra, F.; Lancellotti, P.; Mahmoud-Elsayed, H.; Marsan, N.A.; Maurovich-Hovart, P.; et al. Multimodality imaging for patient selection, procedural guidance, and follow-up of transcatheter interventions for structural heart disease: A consensus document of the EACVI Task Force on Interventional Cardiovascular Imaging: Part 1: Access routes, transcatheter aortic valve implantation, and transcatheter mitral valve interventions. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e209–e268. [Google Scholar] [CrossRef] [PubMed]
- Lesevic, H.; Karl, M.; Braun, D.; Barthel, P.; Orban, M.; Pache, J.; Hadamitzky, M.; Mehilli, J.; Stecher, L.; Massberg, S.; et al. Long-Term Outcomes After MitraClip Implantation According to the Presence or Absence of EVEREST Inclusion Criteria. Am. J. Cardiol. 2017, 119, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Windecker, S.; Kapadia, S. PASCAL: A New Addition to the Armamentarium of Transcatheter Repair Systems for Mitral Leaflet Approximation. JACC Cardiovasc. Interv. 2019, 12, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Koell, B.; Orban, M.; Weimann, J.; Kassar, M.; Karam, N.; Neuss, M.; Petrescu, A.; Iliadis, C.; Unterhuber, M.; Adamo, M.; et al. Outcomes Stratified by Adapted Inclusion Criteria After Mitral Edge-to-Edge Repair. J. Am. Coll. Cardiol. 2021, 78, 2408–2421. [Google Scholar] [CrossRef] [PubMed]
- Piroli, F.; Boccellino, A.; Ingallina, G.; Rolando, M.; Melillo, F.; Ancona, F.; Stella, S.; Biondi, F.; Palmisano, A.; Esposito, A.; et al. Feasibility and reliability of comprehensive three-dimensional transoesophageal echocardiography screening process for transcatheter mitral valve replacement. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1043–1051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.H.; Kim, E.Y.; Jin, G.Y.; Choi, J.B. A Review of the Use of Cardiac Computed Tomography for Evaluating the Mitral Valve before and after Mitral Valve Repair. Korean J. Radiol. 2017, 18, 773–785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koo, H.J.; Yang, D.H.; Oh, S.Y.; Kang, J.W.; Kim, D.H.; Song, J.K.; Lee, J.W.; Chung, C.H.; Lim, T.H. Demonstration of mitral valve prolapse with CT for planning of mitral valve repair. Radiographics 2014, 34, 1537–1552. [Google Scholar] [CrossRef] [PubMed]
- Pulerwitz, T.C.; Khalique, O.K.; Leb, J.; Hahn, R.T.; Nazif, T.M.; Leon, M.B.; George, I.; Vahl, T.P.; D’Souza, B.; Bapat, V.N.; et al. Optimizing Cardiac CT Protocols for Comprehensive Acquisition Prior to Percutaneous MV and TV Repair/Replacement. JACC Cardiovasc. Imaging 2020, 13, 836–850. [Google Scholar] [CrossRef]
- Ranganath, P.; Moore, A.; Guerrero, M.; Collins, J.; Foley, T.; Williamson, E.; Rajiah, P. CT for Pre- and Postprocedural Evaluation of Transcatheter Mitral Valve Replacement. Radiographics 2020, 40, 1528–1553. [Google Scholar] [CrossRef] [PubMed]
- Heiser, L.; Gohmann, R.F.; Noack, T.; Renatus, K.; Lurz, P.; Thiele, H.; Seitz, P.; Gutberlet, M. CT Planning prior to Transcatheter Mitral Valve Replacement (TMVR). Rofo 2022, 194, e1, Erratum in Rofo 2022, 194, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Takahashi, Y.; Fujii, H.; Sakon, Y.; Izuta, S.; Kitada, R.; Morisaki, A.; Yoshida, H.; Ehara, S.; Shibata, T. Computed tomography to identify risk factors for left circumflex artery injury during mitral surgery. Eur. J. Cardiothorac. Surg. 2022, 61, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Feirer, N.; Kornyeva, A.; Lang, M.; Sideris, K.; Voss, B.; Krane, M.; Lange, R.; Vitanova, K. Non-robotic minimally invasive mitral valve repair: A 20-year single-centre experience. Eur. J. Cardiothorac. Surg. 2022, 62, ezac223. [Google Scholar] [CrossRef] [PubMed]
- Bergonzoni, E.; D’Onofrio, A.; Mastro, F.; Gerosa, G. Microinvasive mitral valve repair with transapical mitral neochordae implantation. Front. Cardiovasc. Med. 2023, 10, 1166892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
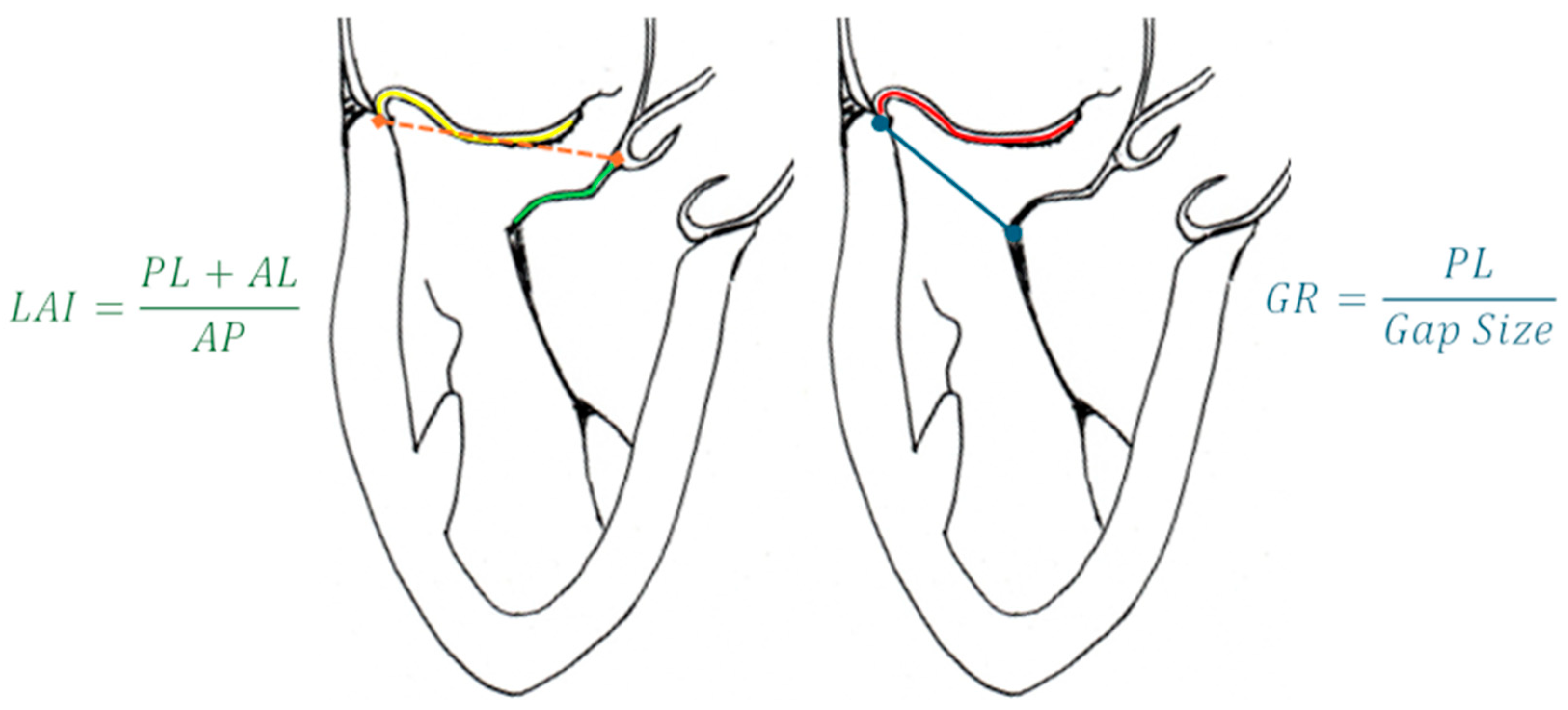
- Colli, A.; Besola, L.; Montagner, M.; Azzolina, D.; Soriani, N.; Manzan, E.; Bizzotto, E.; Zucchetta, F.; Bellu, R.; Pittarello, D.; et al. Prognostic impact of leaflet-to-annulus index in patients treated with transapical off-pump echo-guided mitral valve repair with NeoChord implantation. Int. J. Cardiol. 2018, 257, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Feuchtner, G.M.; Alkadhi, H.; Karlo, C.; Sarwar, A.; Meier, A.; Dichtl, W.; Leschka, S.; Blankstein, R.; Gruenenfelder, J.; Stolzmann, P.; et al. Cardiac CT angiography for the diagnosis of mitral valve prolapse: Comparison with echocardiography1. Radiology 2010, 254, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Nicoletti, V.; Colantoni, C.; Monti, C.B.; Pannone, L.; Vignale, D.; Darvizeh, F.; Agricola, E.; Schaffino, S.; De Cobelli, F.; et al. Dynamic changes of mitral valve annulus geometry at preprocedural CT: Relationship with functional classes of regurgitation. Eur. Radiol. Exp. 2021, 5, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suh, Y.J.; Lee, S.; Chang, B.C.; Shim, C.Y.; Hong, G.R.; Choi, B.W.; Kim, Y.J. Utility of Cardiac CT for Preoperative Evaluation of Mitral Regurgitation: Morphological Evaluation of Mitral Valve and Prediction of Valve Replacement. Korean J. Radiol. 2019, 20, 352–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, B.; Kocyigit, D.; Wang, T.K.M.; Tan, C.D.; Rodriguez, E.R.; Pettersson, G.B.; Unai, S.; Griffin, B.P. Mitral annular calcification and valvular dysfunction: Multimodality imaging evaluation, grading, and management. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e111–e122. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Kang, J.W.; Oh, S.Y.; Kim, D.H.; Song, J.M.; Kang, D.H.; Song, J.K.; Kim, J.B.; Jung, S.H.; Choo, S.J.; et al. Cardiac computed tomography for the localization of mitral valve prolapse: Scallop-by-scallop comparisons with echocardiography and intraoperative findings. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, P.; Anastasius, M.; Huang, A.L.; Blanke, P.; Leipsic, J. Transcatheter Mitral Valve Repair and Replacement: Current Evidence for Intervention and the Role of CT in Preprocedural Planning-A Review for Radiologists and Cardiologists Alike. Radiol. Cardiothorac. Imaging 2020, 2, e190106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faggioni, L.; Gabelloni, M.; Accogli, S.; Angelillis, M.; Costa, G.; Spontoni, P.; Petronio, A.S.; Caramella, D. Preprocedural planning of transcatheter mitral valve interventions by multidetector CT: What the radiologist needs to know. Eur. J. Radiol. Open 2018, 5, 131–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiarito, M.; Sanz-Sanchez, J.; Pighi, M.; Cannata, F.; Rubbio, A.P.; Munafò, A.; Cao, D.; Roccasalva, F.; Pini, D.; Pagnotta, P.A.; et al. Edge-to-edge percutaneous mitral repair for functional ischaemic and non-ischaemic mitral regurgitation: A systematic review and meta-analysis. ESC Heart Fail. 2022, 9, 3177–3187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanke, P.; Dvir, D.; Naoum, C.; Cheung, A.; Ye, J.; Thériault-Lauzier, P.; Spaziano, M.; Boone, R.H.; Wood, D.A.; Piazza, N.; et al. Prediction of fluoroscopic angulation and coronary sinus location by CT in the context of transcatheter mitral valve implantation. J. Cardiovasc. Comput. Tomogr. 2015, 9, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Thériault-Lauzier, P.; Andalib, A.; Martucci, G.; Mylotte, D.; Cecere, R.; Lange, R.; Tchétché, D.; Modine, T.; van Mieghem, N.; Windecker, S.; et al. Fluoroscopic anatomy of left-sided heart structures for transcatheter interventions: Insight from multislice computed tomography. JACC Cardiovasc. Interv. 2014, 7, 947–957. [Google Scholar] [CrossRef] [PubMed]
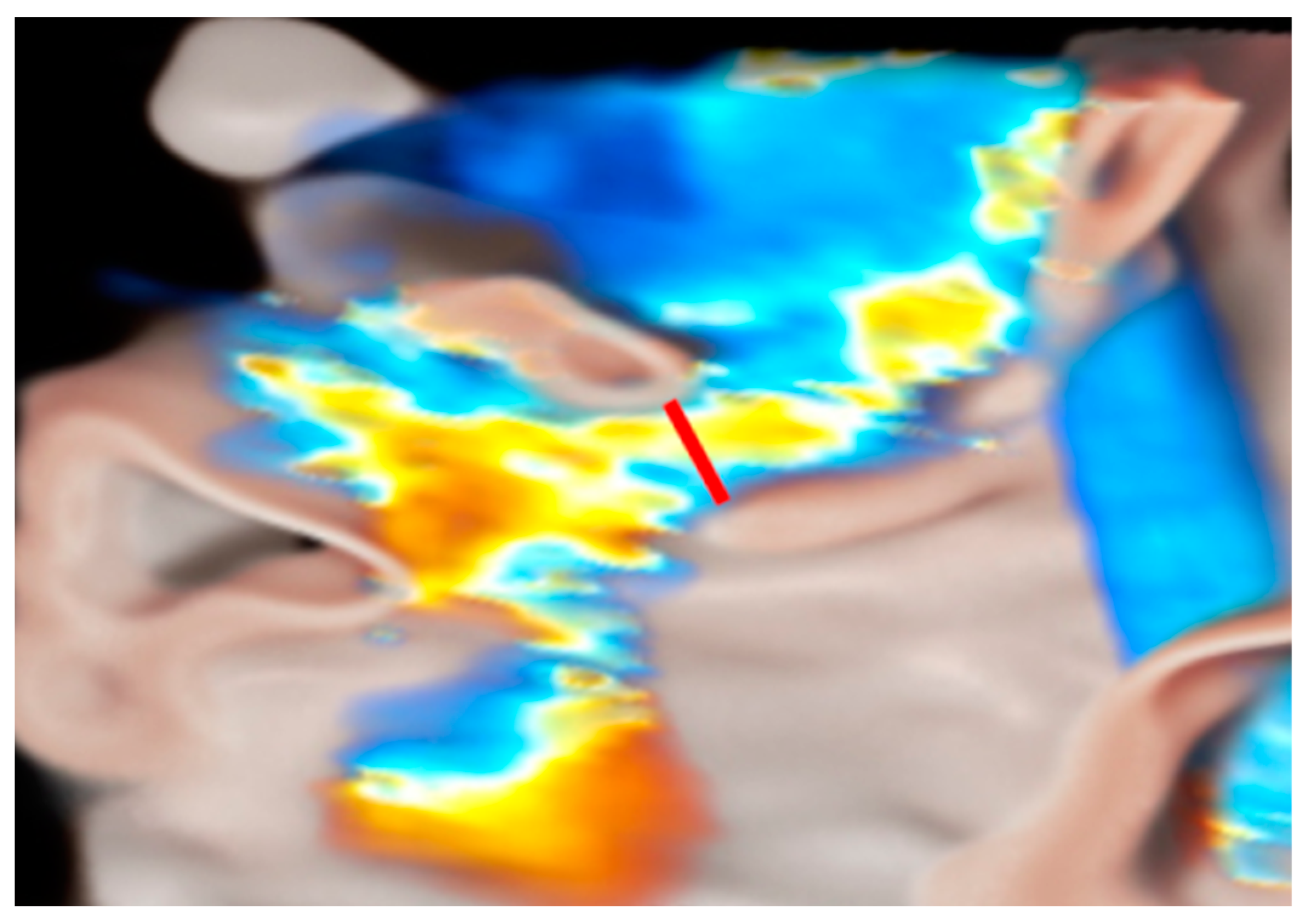
- Ingallina, G.; Rampa, L.; Dicandia, M.; Boccellino, A.; Melillo, F.; Stella, S.; Ancona, F.; Biondi, F.; Fiore, G.; Slavich, M.; et al. Anesthesia-Induced Intraprocedural Downgrading of Mitral Regurgitation During Transcatheter Edge-to-Edge Repair. Am. J. Cardiol. 2023, 190, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, H.; Yoshida, J.; Hayashi, A.; Nagaura, T.; Yamaguchi, S.; Rader, F.; Siegel, R.J.; Kar, S.; Shiota, T. Usefulness of Intraprocedural Pulmonary Venous Flow for Predicting Recurrent Mitral Regurgitation and Clinical Outcomes After Percutaneous Mitral Valve Repair With the MitraClip. JACC Cardiovasc. Interv. 2019, 12, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Kong, P.; Wang, S.; Duan, F.; Zhang, F.; Xie, Y.; Li, Z.; Li, W.; Pan, X. Feasibility of a Percutaneous and Non-Fluoroscopic Procedure for Transcatheter Mitral Valve Edge-to-Edge Repair. Rev. Cardiovasc. Med. 2023, 24, 346. [Google Scholar] [CrossRef]
- Pascual, I.; Pozzoli, A.; Taramasso, M.; Maisano, F.; Ho, E.C. Fusion imaging for transcatheter mitral and tricuspid interventions. Ann. Transl. Med. 2020, 8, 965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zoghbi, W.A.; Jone, P.N.; Chamsi-Pasha, M.A.; Chen, T.; Collins, K.A.; Desai, M.Y.; Grayburn, P.; Groves, D.W.; Hahn, R.T.; Little, S.H.; et al. Guidelines for the Evaluation of Prosthetic Valve Function With Cardiovascular Imaging: A Report From the American Society of Echocardiography Developed in Collaboration With the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2024, 37, 2–63. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, L.; Ielasi, A.; Rensing, B.J.W.M.; Eefting, F.D.; Timmers, L.; Latib, A.; Swaans, M.J. Complications Following Percutaneous Mitral Valve Repair. Front. Cardiovasc. Med. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perl, L.; Kheifets, M.; Guido, A.; Agricola, E.; Denti, P.; Wild, M.G.; Praz, F.; Rubbio, A.P.; Bedogni, F.; De Marco, F.; et al. Acute Reduction in Left Ventricular Function Following Transcatheter Mitral Edge-to-Edge Repair. J. Am. Heart Assoc. 2023, 12, e029735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Utsunomiya, H.; Itabashi, Y.; Kobayashi, S.; Rader, F.; Hussaini, A.; Makar, M.; Trento, A.; Siegel, R.J.; Kar, S.; Shiota, T. Effect of Percutaneous Edge-to-Edge Repair on Mitral Valve Area and Its Association With Pulmonary Hypertension and Outcomes. Am. J. Cardiol. 2017, 120, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Paukovitsch, M.; Schneider, L.M.; Reichart, C.; Nita, N.; Rottbauer, W.; Keßler, M.; Markovic, S. Prevalence of iatrogenic atrial septal defects (iASD) after mitral valve (MV) transcatheter edge-to-edge repair (TEER) in the long-term follow-up. Open Heart 2021, 8, e001732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beri, N.; Singh, G.D.; Smith, T.W.; Fan, D.; Boyd, W.D.; Rogers, J.H. Iatrogenic atrial septal defect closure after transseptal mitral valve interventions: Indications and outcomes. Catheter. Cardiovasc. Interv. 2019, 94, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kargoli, F.; Pagnesi, M.; Rahgozar, K.; Goldberg, Y.; Ho, E.; Chau, M.; Colombo, A.; Latib, A. Current Devices and Complications Related to Transcatheter Mitral Valve Replacement: The Bumpy Road to the Top. Front. Cardiovasc. Med. 2021, 8, 639058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bapat, V.V.; Khaliel, F.; Ihleberg, L. Delayed migration of Sapien valve following a transcatheter mitral valve-in-valve implantation. Catheter. Cardiovasc. Interv. 2014, 83, E150–E154. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.C.; Assafin, M.; Sugiura, T.; Granada, J.F.; Chau, M.; Latib, A. 3-dimensional intracardiac echocardiography for structural heart interventions. Front. Cardiovasc. Med. 2023, 10, 1180299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cubeddu, R.J.; Sarkar, A.; Navas, V.; Navia, J.L. ‘Minimalist approach’ for transcatheter mitral valve replacement using intracardiac echocardiography and conscious sedation: A case series. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penicka, M.; Vecera, J.; Mirica, D.C.; Kotrc, M.; Kockova, R.; Van Camp, G. Prognostic Implications of Magnetic Resonance-Derived Quantification in Asymptomatic Patients With Organic Mitral Regurgitation: Comparison With Doppler Echocardiography-Derived Integrative Approach. Circulation 2018, 137, 1349–1360. [Google Scholar] [CrossRef]
- Baessato, F.; Fusini, L.; Muratori, M.; Tamborini, G.; Ghulam Ali, S.; Mantegazza, V.; Baggiano, A.; Mushtaq, S.; Pepi, M.; Patti, G.; et al. Echocardiography vs. CMR in the Quantification of Chronic Mitral Regurgitation: A Happy Marriage or Stormy Divorce? J. Cardiovasc. Dev. Dis. 2023, 10, 150. [Google Scholar] [CrossRef]
- Constant Dit Beaufils, A.-L.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.-M.; et al. Replacement Myocardial Fibrosis in Patients With Mitral Valve Prolapse: Relation to Mitral Regurgitation, Ventricular Remodeling, and Arrhythmia. Circulation 2021, 143, 1763–1774. [Google Scholar] [CrossRef]
- Tokodi, M.; Németh, E.; Lakatos, B.K.; Kispál, E.; Tősér, Z.; Staub, L.; Rácz, K.; Soltész, Á.; Szigeti, S.; Varga, T.; et al. Right ventricular mechanical pattern in patients undergoing mitral valve surgery: A predictor of post-operative dysfunction? ESC Heart Fail. 2020, 7, 1246–1256. [Google Scholar] [CrossRef]
- Crowhurst, J.A.; Scalia, G.M.; Whitby, M.; Murdoch, D.; Robinson, B.J.; Turner, A.; Johnston, L.; Margale, S.; Natani, S.; Clarke, A.; et al. Radiation Exposure of Operators Performing Transesophageal Echocardiography During Percutaneous Structural Cardiac Interventions. J. Am. Coll. Cardiol. 2018, 71, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.E., 3rd; Hall, M.J.; Iturbe, J.M.; Condado, J.F.; Kamioka, N.; Howell, S.; Thourani, V.H.; Clements, S.D.; Babaliaros, V.C.; Lerakis, S. Radioprotective strategies for interventional echocardiographers during structural heart interventions. Catheter. Cardiovasc. Interv. 2019, 93, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.G.; Meindl, C.; Hamerle, M.; Schach, C.; Maier, L.S.; Debl, K.; Birner, C.; Unsöld, B. Reduction of radiation exposure during transcatheter edge-to-edge mitral valve repair. Catheter. Cardiovasc. Interv. 2022, 99, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Melillo, F.; Fisicaro, A.; Stella, S.; Ancona, F.; Capogrosso, C.; Ingallina, G.; Maccagni, D.; Romano, V.; Ruggeri, S.; Godino, C.; et al. Systematic Fluoroscopic-Echocardiographic Fusion Imaging Protocol for Transcatheter Edge-to-Edge Mitral Valve Repair Intraprocedural Monitoring. J. Am. Soc. Echocardiogr. 2021, 34, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Faza, N.N.; Harb, S.C.; Wang, D.D.; van den Dorpel, M.M.P.; Van Mieghem, N.; Little, S.H. Physical and Computational Modeling for Transcatheter Structural Heart Interventions. JACC Cardiovasc. Imaging 2024, 17, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Faza, N.N.; Özden Tok, Ö.; Hahn, R.T. Imaging in Structural Heart Disease: The Evolution of a New Subspecialty. JACC Case Rep. 2019, 1, 440–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Złahoda-Huzior, A.; Januska, J.; Hecko, J.; Khokhar, A.; Dudek, D. Virtual reality-assisted heart team consultation for complex structural heart intervention. Eur. Heart J. Case Rep. 2022, 7, ytac477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veulemans, V.; Hellhammer, K.; Polzin, A.; Bönner, F.; Zeus, T.; Kelm, M. Current and future aspects of multimodal and fusion imaging in structural and coronary heart disease. Clin. Res. Cardiol. 2018, 107 (Suppl. 2), 49–54. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, Y.; Yao, J.; Mahadevan, V.S.; Gruber, D.; Arnaout, R.; Gentzsch, W.; Guccione, J.M.; Kassab, G.S. Mitral Valve Atlas for Artificial Intelligence Predictions of MitraClip Intervention Outcomes. Front. Cardiovasc. Med. 2021, 8, 759675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dabiri, Y.; Mahadevan, V.S.; Guccione, J.M.; Kassab, G.S. Machine learning used for simulation of MitraClip intervention: A proof-of-concept study. Front. Genet. 2023, 14, 1142446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Little, S.H.; Rigolin, V.H.; Garcia-Sayan, E.; Hahn, R.T.; Hung, J.; Mackensen, G.B.; Mankad, S.; Quader, N.; Saric, M. Recommendations for Special Competency in Echocardiographic Guidance of Structural Heart Disease Interventions: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 350–365. [Google Scholar] [CrossRef] [PubMed]








| Model | Number | AP (mm) | IC (mm) | Perimeter (mm) | EOA (cm2) |
|---|---|---|---|---|---|
| SP | 33S | 32.5 | 43.5 | 130 | 3.0 |
| 33M | 32.5 | 46.5 | 136 | ||
| 33L | 32.5 | 50.5 | 144 | ||
| 35M | 34.5 | 48.5 | 144 | ||
| 37S | 36.5 | 46.5 | 144 | ||
| 37L | 36.5 | 52.5 | 156 | ||
| 39M | 38.5 | 50.5 | 156 | ||
| 41S | 40.5 | 47.5 | 154 | ||
| LP | 29S | 29.0 | 42.5 | 119 | 2.2 |
| 29L | 29.0 | 47.5 | 129 | ||
| 33S | 32.5 | 43.5 | 130 | ||
| 35M | 34.5 | 48.5 | 144 | ||
| 37M | 36.5 | 49.5 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brugiatelli, L.; Rolando, M.; Lofiego, C.; Fogante, M.; Capodaglio, I.; Patani, F.; Tofoni, P.; Maurizi, K.; Nazziconi, M.; Massari, A.; et al. Transcatheter Mitral Valve Intervention: Current and Future Role of Multimodality Imaging for Device Selection and Periprocedural Guidance. Medicina 2024, 60, 1082. https://doi.org/10.3390/medicina60071082
Brugiatelli L, Rolando M, Lofiego C, Fogante M, Capodaglio I, Patani F, Tofoni P, Maurizi K, Nazziconi M, Massari A, et al. Transcatheter Mitral Valve Intervention: Current and Future Role of Multimodality Imaging for Device Selection and Periprocedural Guidance. Medicina. 2024; 60(7):1082. https://doi.org/10.3390/medicina60071082
Chicago/Turabian StyleBrugiatelli, Leonardo, Marco Rolando, Carla Lofiego, Marco Fogante, Irene Capodaglio, Francesca Patani, Paolo Tofoni, Kevin Maurizi, Marco Nazziconi, Arianna Massari, and et al. 2024. "Transcatheter Mitral Valve Intervention: Current and Future Role of Multimodality Imaging for Device Selection and Periprocedural Guidance" Medicina 60, no. 7: 1082. https://doi.org/10.3390/medicina60071082






