Effect of Different Administered Doses of Capsaicin and Titanium Implant Osseointegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Surgical Procedures
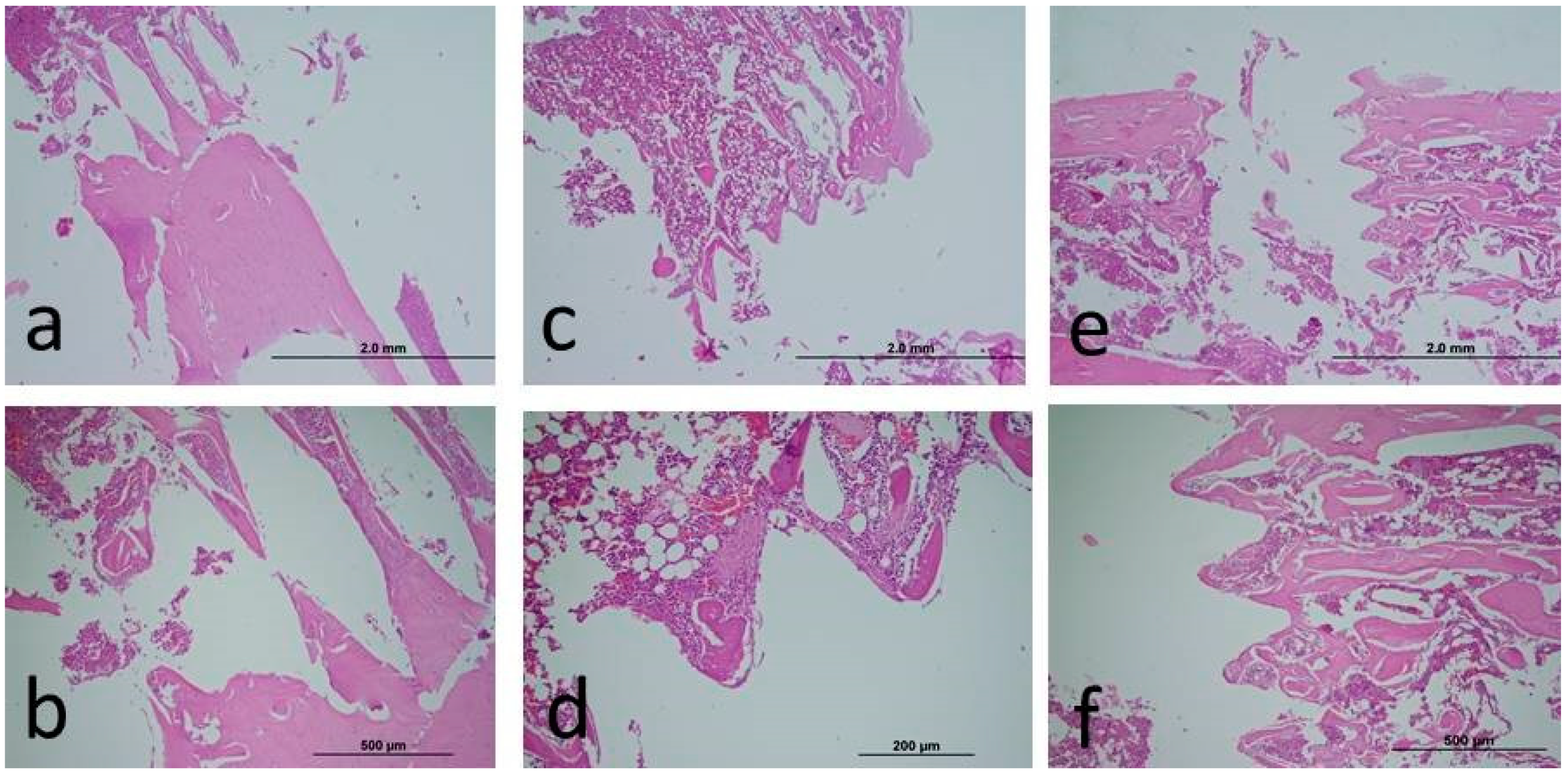
2.3. Histopathological Analysis
2.4. Biochemical Analysis
2.5. Semi-Quantitative Scoring of Histopathologic Parameters
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Togari, A. Adrenergic regulation of bone metabolism: Possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc. Res. Tech. 2002, 58, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Togari, A.; Arai, M.; Kondo, A. The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin. Ther. Targets 2005, 9, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Togari, A.; Arai, M. Pharmacological topics of bone metabolism: The physiological function of the sympathetic nervous system in modulating bone resorption. J. Pharmacol. Sci. 2008, 106, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Togari, A.; Arai, M.; Kondo, H. The neuro-osteogenic network: The sympathetic regulation of bone resorption. Jpn. Dent. Sci. Rev. 2012, 48, 61–70. [Google Scholar] [CrossRef]
- Ishizuka, K.; Hirukawa, K.; Nakamura, H.; Togari, A. Inhibitory effect of CGRP on osteoclast formation by mouse bone marrow cells treated with isoproterenol. Neurosci. Lett. 2005, 379, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, T.; Fujiyama, K.; Fujiyoshi, Y.; Inaguma, N.; Takano-Yamamoto, T. Inferior alveolar nerve transection inhibits the increase in osteoclast appearance during experimental tooth movement. Bone 2000, 26, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Hara-Irie, F.; Amizuka, N.; Ozawa, H. Immunohistochemical and ultrastructural localization of CGRP-positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone 1996, 18, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, M.; Konttinen, Y.T.; Santavirta, S.; Paavolainen, P.; Gu, X.H.; Terenghi, G.; Polak, J.M. Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience 1993, 54, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ahmad, T.; Spetea, M.; Ahmed, M.; Kreicbergs, A. Bone reinnervation after fracture: A study in the rat. J. Bone Miner. Res. 2001, 16, 1505–1510. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [PubMed]
- Newson, P.N.; van den Buuse, M.; Martin, S.; Lynch-Frame, A.; Chahl, L.A. Effects of neonatal treatment with the TRPV1 agonist, capsaicin, on adult rat brain and behaviour. Behav. Brain Res. 2014, 272, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Oh, J.M.; Valtschanoff, J.G. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005, 1047, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.; Llorens, A.; Baroukh, B.; Cherruau, M.; Saffar, J.L. Effects of capsaicin-induced sensory denervation on osteoclastic resorption in adult rats. Exp. Physiol. 2000, 85, 62–66. [Google Scholar] [PubMed]
- Offley, S.C.; Guo, T.Z.; Wei, T.; Clark, J.D.; Vogel, H.; Lindsey, D.P.; Jacobs, C.R.; Yao, W.; Lane, N.E.; Kingery, W.S. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J. Bone Miner. Res. 2005, 20, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.K.; Guo, X.; Lao, J.; Qin, Y.X. Effect of capsaicin-sensitive sensory neurons on bone architecture and mechanical properties in the rat hindlimb suspension model. J. Orthop. Translat. 2017, 10, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Kojo, T.; Wang, Y.H.; Ando, H.; Nakanishi, E.; Zhang, M.; Fukuyama, H.; Uchida, Y. Effect of loading on the development of nerve fibers around oral implants in the dog mandible. Clin. Oral Implant. Res. 2001, 12, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ye, J.; Zeng, X.; Gong, P. Effects of capsaicin-induced sensory denervation on early implant osseointegration in adult rats. R Soc. Open Sci. 2019, 6, 181082. [Google Scholar] [CrossRef] [PubMed]
- Yerit, K.C.; Posch, M.; Seemann, M.; Hainich, S.; Dörtbudak, O.; Turhani, D.; Ozyuvaci, H.; Watzinger, F.; Ewers, R. Implant survival in mandibles of irradiated oral cancer patients. Clin. Oral Implant. Res. 2006, 17, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L.; Turner, R.; Elde, R. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience 1991, 44, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Nevins, M.L. Dental Implant Considerations in the Diabetic Patient. Periodontology 2000, 23, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Aljateeli, M.; Wang, H.L. Implant Microdesigns and Their Impact on Osseointegration. Implant. Dent. 2013, 22, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Kim, M.; Yoon, S.W.; Lee, C.H. Short-term control of capsaicin on blood and oxidative stress of rats in vivo. Phytother. Res. 2003, 17, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Dumitrache, M.D.; Jieanu, A.S.; Scheau, C.; Badarau, I.A.; Popescu, G.D.A.; Caruntu, A.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review). Exp. Ther. Med. 2021, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I.; Kocyigit, A.; Gonel, A.; Arslan, E.; Durgun, M. The Protective Effect of Naringenin-Oxime on Cisplatin-Induced Toxicity in Rats. Biochem. Res. Int. 2017, 2017, 9478958. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method formeasuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Q.; Sculean, A.; Buser, D.; Pippenger, B.E.; Dard, M.; Shirakata, Y.; Chandad, F.; Zhang, Y. Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Investig. 2016, 20, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; DeConde, A.S.; Lee, M.; Walthers, C.M.; Sepahdari, A.R.; Elashoff, D.; Grogan, T.; Bezouglaia, O.; Tetradis, S.; John, M.S.; et al. Biomimetic scaffolds facilitate healing of critical-sized segmental mandibular defects. Am. J. Otolaryngol. 2015, 36, 1–6. [Google Scholar] [CrossRef]
- Erdmann, N.; Bondarenko, A.; Hewicker-Trautwein, M.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Meyer-Lindenberg, A. Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: A comparative study in rabbits. Biomed. Eng. Online 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.P.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Fini, M.; Martini, D.; Orsini, E.; Leonardi, L.; Ruggeri, A.; Giavaresi, G.; Ottani, V. Biological fixation of endosseous implants. Micron 2005, 36, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Matsuda, Y.; Sato, K.; de Jong, P.R.; Bertin, S.; Tabeta, K.; Yamazaki, K. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci. Rep. 2016, 6, 29294. [Google Scholar] [CrossRef] [PubMed]
- Granholm, S.; Henning, P.; Lerner, U.H. Comparisons between the effects of calcitonin receptor-stimulating peptide and intermedin and other peptides in the calcitonin family on bone resorption and osteoclastogenesis. J. Cell. Biochem. 2011, 112, 3300–3312. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhang, G.; Tan, Y.H. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol. Sin. 2013, 34, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Smith, N.C.; Garbarg, M.; Levi, R. Histamine H3-receptor-mediated inhibition of calcitonin gene-related peptide release from cardiac C fibers. A regulatory negative-feedback loop. Circ. Res. 1996, 78, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, K.; Yayama, K.; Hayashi, T.; Matsushita, H.; Yamaguchi, T.; Shigeno, T.; Ogitani, Y.; Hirayama, M.; Kato, T.; Fukada, S.-I.; et al. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc. Natl. Acad. Sci. USA 2007, 104, 16702–16707. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, G.; Liu, W.; Liu, Y.; Ding, Y. The influence of the sensory neurotransmitter calcitonin gene-related peptide on bone marrow mesenchymal stem cells from ovariectomized rats. J. Bone Miner. Metab. 2017, 35, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.J.; Barfield, M.A.; Barton, S.; Dong, Y. Understanding Reactive Oxygen Species in Bone Regeneration: A Glance at Potential Therapeutics and Bioengineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 836764. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, F.; Keçeci, T. The effect of capsaicin on TBARS and TAS in rats with hypothyroidism. J. Istanb. Vet. Sci. 2022, 6, 98–104. [Google Scholar]
- Chaudhary, A.; Gour, J.K.; Rizvi, S.I. Capsaicin has potent anti-oxidative effects in vivo through a mechanism which is non-receptor mediated. Arch. Physiol. Biochem. 2022, 128, 141–147. [Google Scholar] [CrossRef]
- Manjunatha, H.; Srinivasan, K. Hypolipidemic and antioxidant effects of curcumin and capsaicin in high-fat-fed rats. Can. J. Physiol. Pharmacol. 2007, 85, 588–596. [Google Scholar] [CrossRef]
- Marco, F.; Milena, F.; Gianluca, G.; Vittoria, O. Peri-implant osteogenesis in health and osteoporosis. Micron 2005, 36, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of gene expression in osteoblasts. Biofactors 2010, 36, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.J.; Low, S.A.; Ramseier, N.T.; Hadap, R.V.; Young, N.A.; Wang, M.; Low, P.S. Analysis of the bone fracture targeting properties of osteotropic ligands. J. Control Release 2021, 329, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, V.; Venugopalan, S.; Ganapathy, D.; Ramadoss, R.; Kumar, S.M.; Kannan, R.K.; Jayakumar, A.; Duraisamy, R. Effect of Dietary Amino Acids L-Arginine and Lysine on Implant Osseointegration. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. S1), S106–S109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amengual-Peñafiel, L.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Jara-Sepúlveda, M.C.; Parada-Pozas, L.; Cartes-Velásquez, R. Coupling between Osseointegration and Mechanotransduction to Maintain Foreign Body Equilibrium in the Long-Term: A Comprehensive Overview. J. Clin. Med. 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Lin, Y.; Pang, Q.; Shen, W.; Chen, X.; Tu, F. The Synergistic Effect of TRPV1 on Oxidative Stress-Induced Autophagy and Apoptosis in Microglia. Anal. Cell. Pathol. 2021, 2021, 7955791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yazğan, Y.; Nazıroğlu, M. Ovariectomy-Induced Mitochondrial Oxidative Stress, Apoptosis, and Calcium Ion Influx Through TRPA1, TRPM2, and TRPV1 Are Prevented by 17β-Estradiol, Tamoxifen, and Raloxifene in the Hippocampus and Dorsal Root Ganglion of Rats. Mol. Neurobiol. 2017, 54, 7620–7638. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; A Rosen, T.; Gilbert, H.; Skinner, K.; E Raumann, B.; I Basbaum, A.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Liu, M.; He, Y.; Xiao, E.; Zhao, L.; Zhang, T.; Yang, H.-Q.; Zhang, Y. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci. Rep. 2017, 22, 42385. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, L.; Raucci, M.G.; Vadalà, G.; Ambrosio, L.; Papalia, R.; Denaro, V. Innovative biomaterials for the treat-ment of bone cancer. Int. J. Mol. Sci. 2021, 22, 8214. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Group 1 (n) | Group 2 (n) | Group 3 (n) | p † Value | P1–2 | P1–3 | P2–3 |
|---|---|---|---|---|---|---|---|
| TAS | 1.19 ± 0.15 (7) | 1.38 ± 0.09 (7) | 1.45 ± 0.08 (7) | 0.001 ** | 0.011 * | 0.001 ** | 0.458 |
| TOS | 15.21 ± 1.35 (7) | 11.81 ± 0.65 (7) | 11.11 ± 0.88 (7) | <0.001 ** | <0.001 ** | <0.001 ** | 0.410 |
| OSI | 1.30 ± 0.20 (7) | 0.87 ± 0.05 (7) | 0.77 ± 0.07 (7) | <0.001 ** | <0.001 ** | <0.001 ** | 0.302 |
| Groups | Control (M ± SS) | Capsaicin 25 (M ± SS) | Capsaicin 50 (M ± SS) | p * Value |
|---|---|---|---|---|
| Capsaicin | 31 ± 2.16 | 33 ± 2.94 | 34.29 ± 3.50 | 0.134 |
| Group | Kruskal–Wallis H Test | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | Sd | Mean Rank | H | p | ||
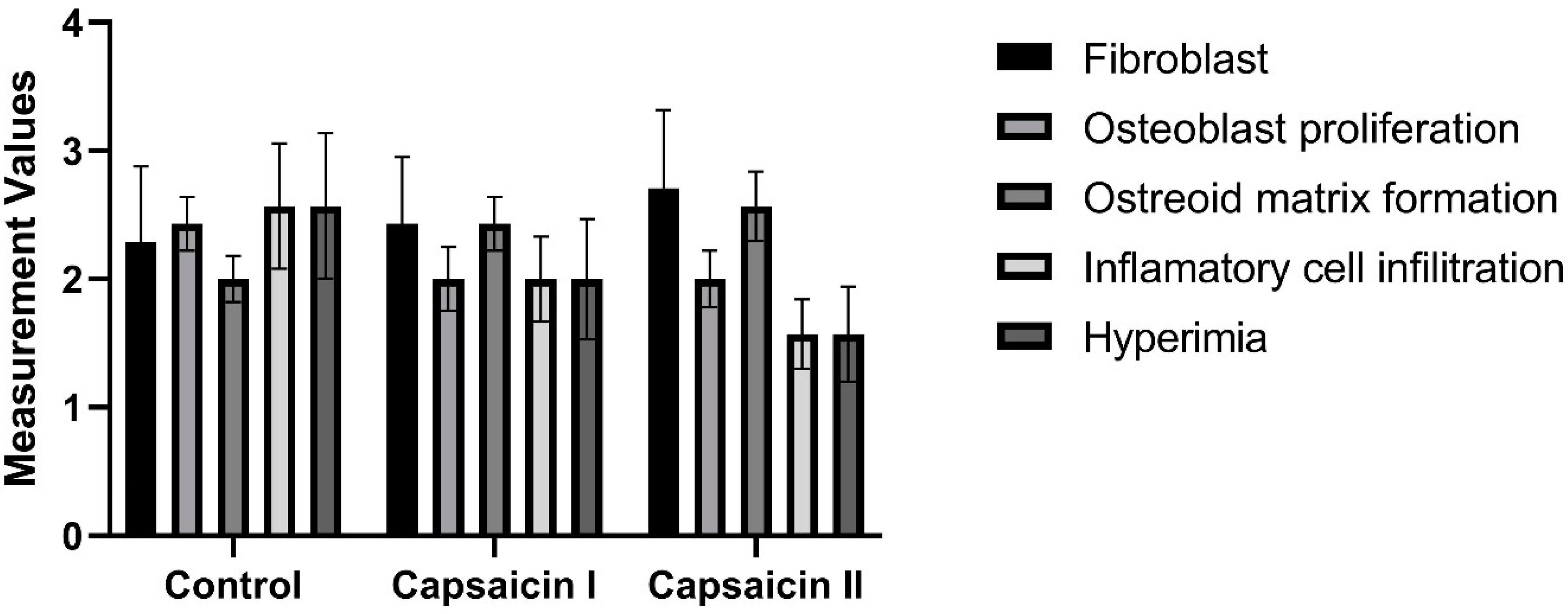
| Fibroblast | Control | 7 | 2.29 | 2 | 0.76 | 9.57 | 1.667 | 0.435 |
| Capsaisin I | 7 | 2.43 | 2 | 0.53 | 10.29 | |||
| Capsaisin II | 7 | 2.71 | 3 | 0.49 | 13.14 | |||
| Total | 21 | 2.48 | 3 | 0.6 | ||||
| Osteoblast proliferation | Control | 7 | 2.43 | 3 | 0.79 | 13.36 | 1.788 | 0.409 |
| Capsaisin I | 7 | 2 | 2 | 0.82 | 9.93 | |||
| Capsaisin II | 7 | 2 | 2 | 0.58 | 9.71 | |||
| Total | 21 | 2.14 | 2 | 0.73 | ||||
| Osteoid matrix formation | Control | 7 | 2 | 2 | 0.58 | 8 | 3.475 | 0.176 |
| Capsaisin I | 7 | 2.43 | 2 | 0.53 | 11.79 | |||
| Capsaisin II | 7 | 2.57 | 3 | 0.53 | 13.21 | |||
| Total | 21 | 2.33 | 2 | 0.58 | ||||
| Inflammatory cell infiltration | Control | 7 | 2.57 | 3 | 0.53 | 14.79 | 5.469 | 0.065 |
| Capsaisin I | 7 | 2 | 2 | 1 | 10.71 | |||
| Capsaisin II | 7 | 1.57 | 2 | 0.53 | 7.5 | |||
| Total | 21 | 2.05 | 2 | 0.8 | ||||
| Hyperemia | Control | 7 | 2.57 | 3 | 0.79 | 14.86 | 5.591 | 0.061 |
| Capsaisin I | 7 | 2 | 2 | 0.82 | 10.64 | |||
| Capsaisin II | 7 | 1.57 | 2 | 0.53 | 7.5 | |||
| Total | 21 | 2.05 | 2 | 0.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingül, M.B.; Gul, M.; Dündar, S.; Sökmen, K.; Artas, G.; Polat, M.E.; Tanrisever, M.; Ozcan, E.C. Effect of Different Administered Doses of Capsaicin and Titanium Implant Osseointegration. Medicina 2024, 60, 1094. https://doi.org/10.3390/medicina60071094
Bingül MB, Gul M, Dündar S, Sökmen K, Artas G, Polat ME, Tanrisever M, Ozcan EC. Effect of Different Administered Doses of Capsaicin and Titanium Implant Osseointegration. Medicina. 2024; 60(7):1094. https://doi.org/10.3390/medicina60071094
Chicago/Turabian StyleBingül, Muhammet Bahattin, Mehmet Gul, Serkan Dündar, Kevser Sökmen, Gökhan Artas, Mehmet Emrah Polat, Murat Tanrisever, and Erhan Cahit Ozcan. 2024. "Effect of Different Administered Doses of Capsaicin and Titanium Implant Osseointegration" Medicina 60, no. 7: 1094. https://doi.org/10.3390/medicina60071094






