Attentional Bias for Opioids in Taiwanese Heavy Smokers with Chronic Noncancer Pain
Abstract
:1. Introduction
1.1. Smoking and Opioid Misuse in Patients with Pain
1.2. Attentional Bias for Opioids in Chronic Pain
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic Questionnaire
2.2.2. Center for Epidemiologic Studies Depression Scale
2.2.3. Beck Anxiety Inventory
2.2.4. Pain Score
2.2.5. Fagerström Test for Nicotine Dependence
2.2.6. Evaluation of Opioid-Use Behaviors
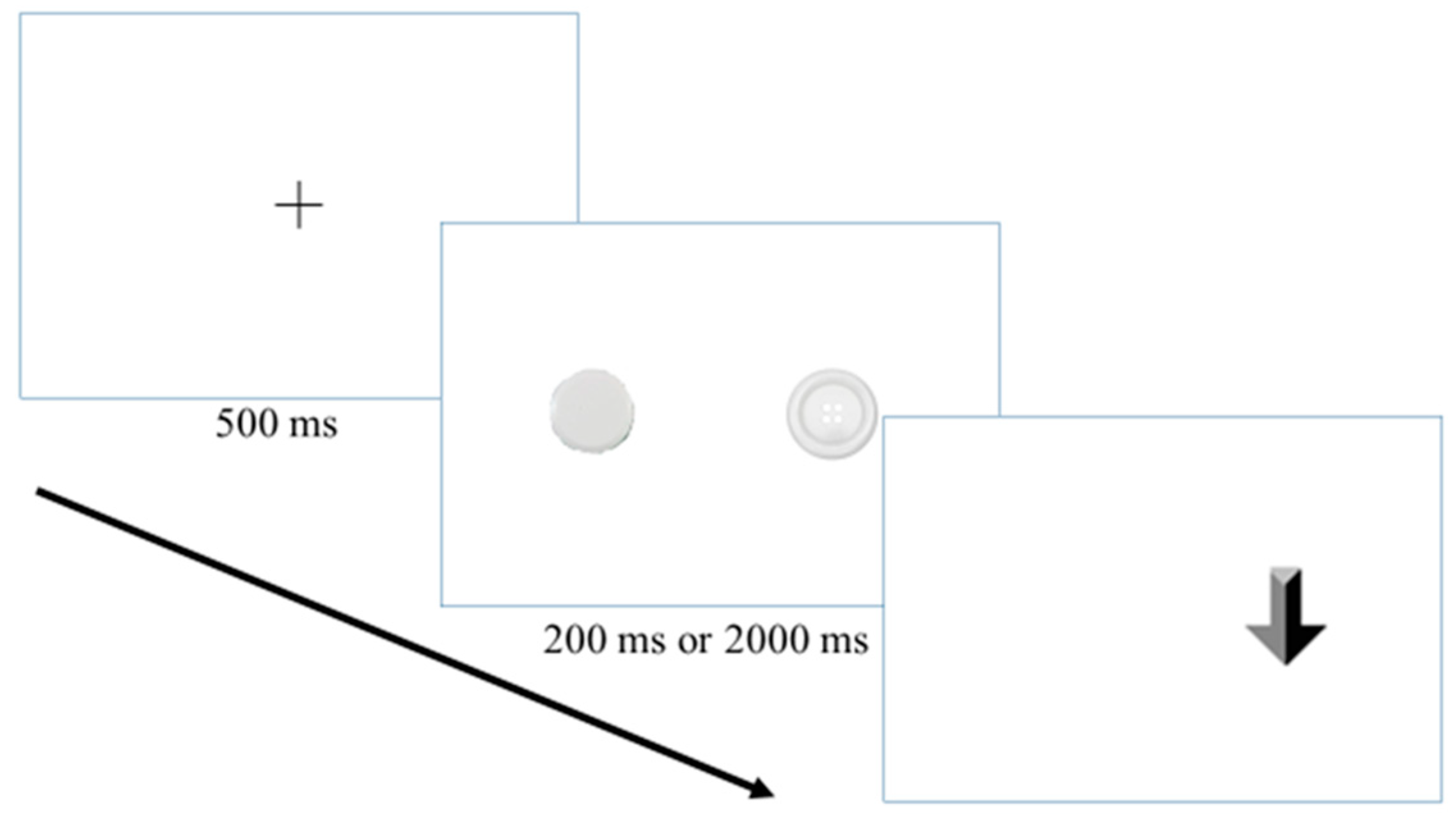
2.2.7. Visual Probe Task
2.2.8. Visual Probe Task Apparatus
2.3. Procedures
2.4. Data Analysis
2.5. Data Availability
3. Results
3.1. Demographic Characteristics
3.2. Attentional Bias and Reaction Time
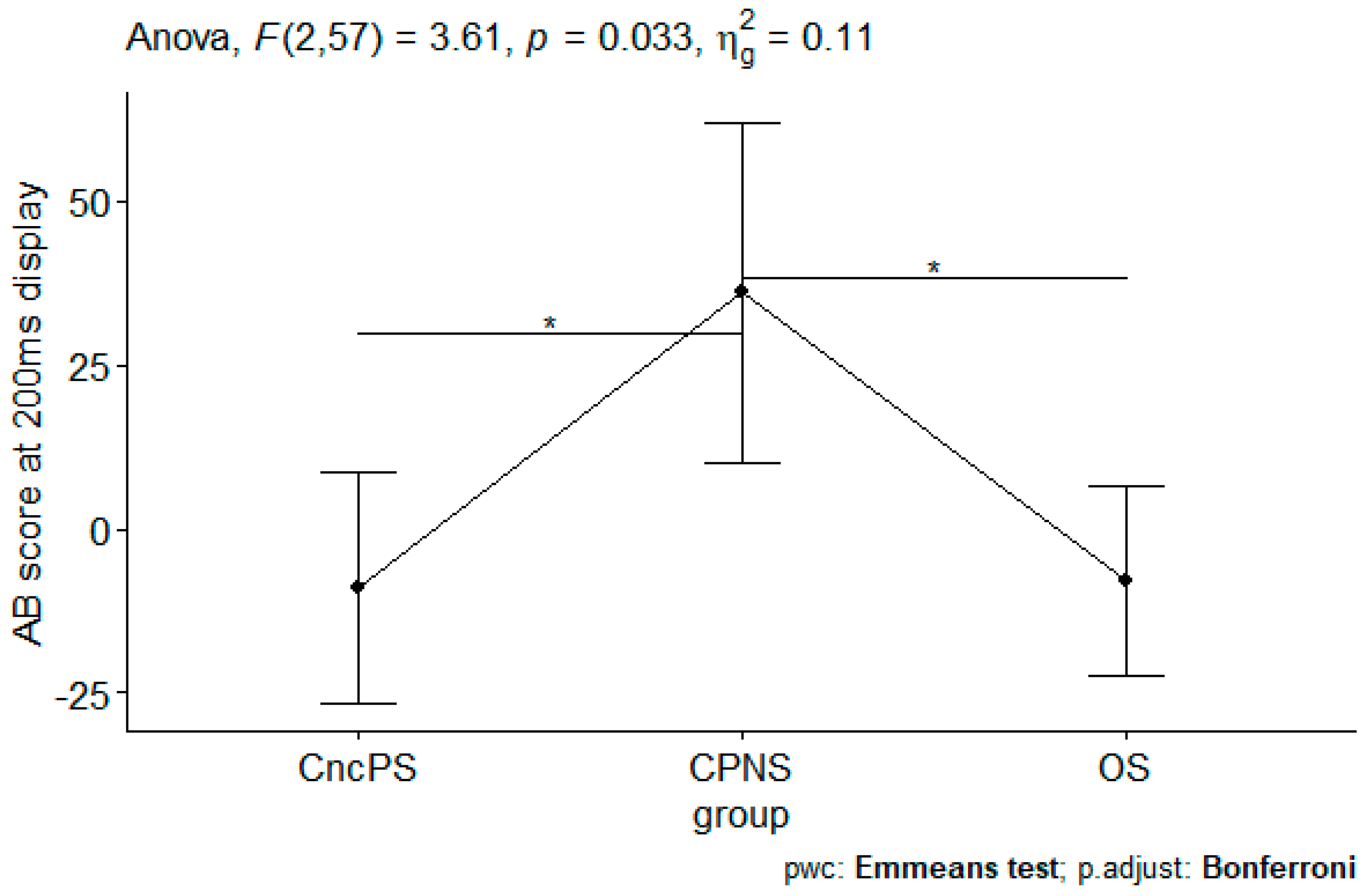
3.2.1. Analysis on AB Scores
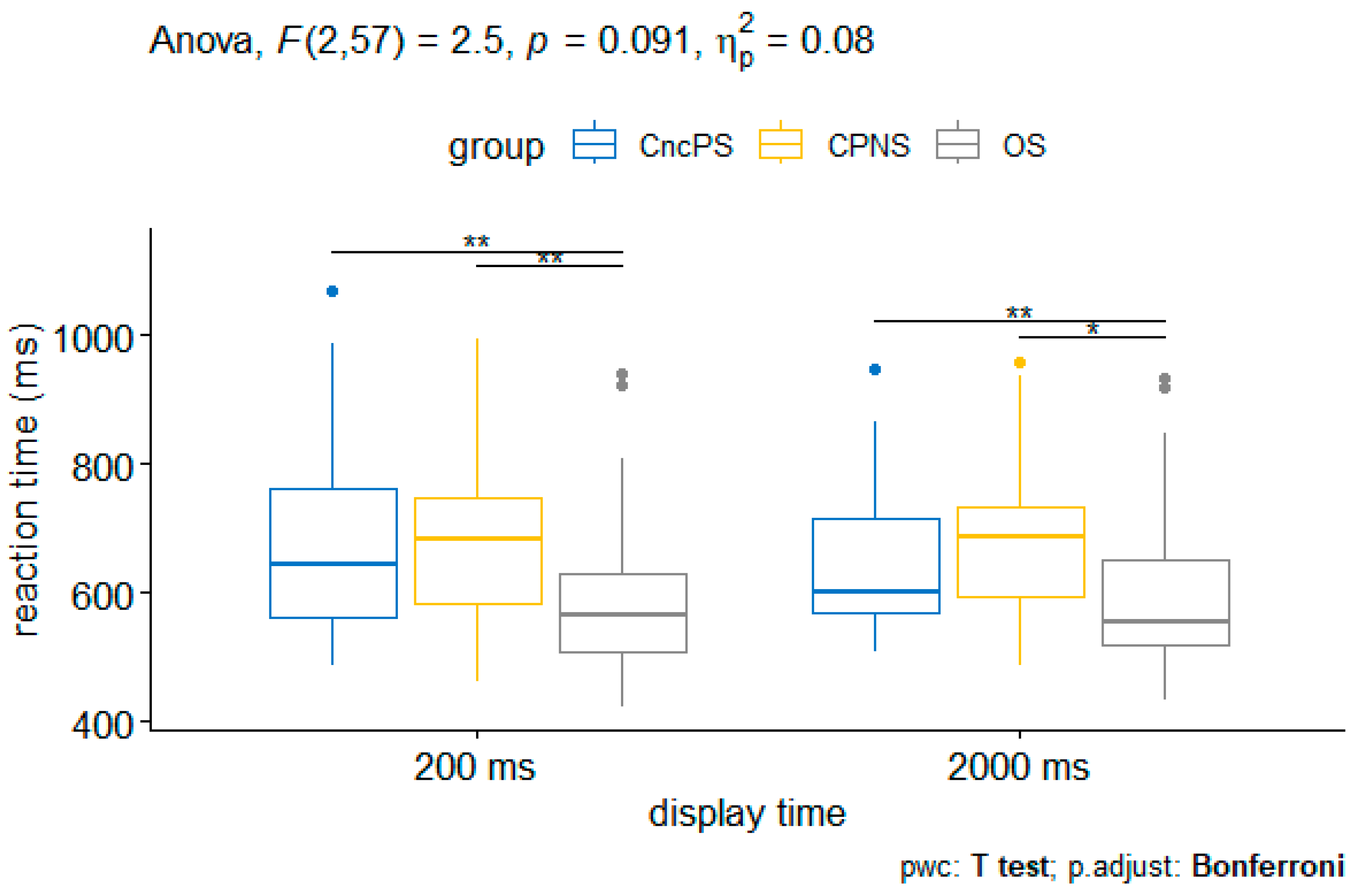
3.2.2. Analysis on Reaction Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deyo, R.A.; Von Korff, M.; Duhrkoop, D. Opioids for low back pain. BMJ 2015, 350, g6380. [Google Scholar] [CrossRef] [PubMed]
- Young-Wolff, K.C.; Klebaner, D.; Weisner, C.; Von Korff, M.; Campbell, C.I. Smoking status and opioid-related problems and concerns among men and women on chronic opioid therapy. Clin. J. Pain 2017, 33, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Zale, E.L.; Dorfman, M.L.; Hooten, W.M.; Warner, D.O.; Zvolensky, M.J.; Ditre, J.W. Tobacco smoking, nicotine dependence, and patterns of prescription opioid misuse: Results from a nationally representative sample. Nicotine Tob. Res. 2015, 17, 1096–1103. [Google Scholar] [CrossRef]
- Montbriand, J.J.; Weinrib, A.Z.; Azam, M.A.; Ladak, S.S.J.; Shah, B.R.; Jiang, J.; McRae, K.; Tamir, D.; Lyn, S.; Katznelson, R.; et al. Smoking, pain intensity, and opioid consumption 1–3 months after major surgery: A retrospective study in a hospital-based transitional pain service. Nicotine Tob. Res. 2018, 20, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Bastian, L.A.; Driscoll, M.A.; Heapy, A.A.; Becker, W.C.; Goulet, J.L.; Kerns, R.D.; DeRycke, E.C.; Perez, E.; Lynch, S.M.; Mattocks, K.; et al. Cigarette smoking status and receipt of an opioid prescription among veterans of recent wars. Pain Med. 2017, 18, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.I.; Erlandsson, M.C.; Bjersing, J.; Dehlin, M.; Mannerkorpi, K. Smoking is associated with reduced leptin and neuropeptide y levels and higher pain experience in patients with fibromyalgia. Mediat. Inflamm. 2014, 2014, 627041. [Google Scholar] [CrossRef] [PubMed]
- Ditre, J.W.; Brandon, T.H.; Zale, E.L.; Meagher, M.M. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychol. Bull. 2011, 137, 1065–1093. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L.; Gritzner, S.; Resnick, M.P.; Dobscha, S.K.; Turk, D.C.; Morasco, B.J. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. J. Pain 2012, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.L.; Wu, L.T. Associations between cigarette smoking and pain among veterans. Epidemiol. Rev. 2015, 37, 86–102. [Google Scholar] [CrossRef]
- Orhurhu, V.J.; Pittelkow, T.P.; Hooten, W.M. Prevalence of smoking in adults with chronic pain. Tob. Induc. Dis. 2015, 13, 17. [Google Scholar] [CrossRef]
- Ekholm, O.; Gronbaek, M.; Peuckmann, V.; Sjogren, P. Alcohol and smoking behavior in chronic pain patients: The role of opioids. Eur. J. Pain 2009, 13, 606–612. [Google Scholar] [CrossRef]
- Hojsted, J.; Ekholm, O.; Kurita, G.P.; Juel, K.; Sjogren, P. Addictive behaviors related to opioid use for chronic pain: A population-based study. Pain 2013, 154, 2677–2683. [Google Scholar] [CrossRef]
- Richardson, E.J.; Ness, T.J.; Redden, D.T.; Stewart, C.C.; Richards, J.S. Effects of nicotine on spinal cord injury pain vary among subtypes of pain and smoking status: Results from a randomized, controlled experiment. J. Pain 2012, 13, 1206–1214. [Google Scholar] [CrossRef]
- Anderson, K.L.; Pinkerton, K.E.; Uyeminami, D.; Simons, C.T.; Carstens, M.I.; Carstens, E. Antinociception induced by chronic exposure of rats to cigarette smoke. Neurosci. Lett. 2004, 366, 86–91. [Google Scholar] [CrossRef]
- Ditre, J.W.; Heckman, B.W.; Zale, E.L.; Kosiba, J.D.; Maisto, S.A. Acute analgesic effects of nicotine and tobacco in humans: A meta-analysis. Pain 2016, 157, 1373–1381. [Google Scholar] [CrossRef]
- Slawecki, C.J.; Gilder, A.; Roth, J.; Ehlers, C.L. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol. Biochem. Behav. 2003, 75, 355–361. [Google Scholar] [CrossRef]
- Etter, J.F. A self-administered questionnaire to measure cigarette withdrawal symptoms: The cigarette withdrawal scale. Nicotine Tob. Res. 2005, 7, 47–57. [Google Scholar] [CrossRef]
- Dhingra, L.K.; Homel, P.; Grossman, B.; Chen, J.; Scharaga, E.; Calamita, S.; Shin, J.; Portenoy, R. Ecological momentary assessment of smoking behavior in persistent pain patients. Clin. J. Pain 2014, 30, 205–213. [Google Scholar] [CrossRef]
- Kosiba, J.D.; Zale, E.L.; Ditre, J.W. Associations between pain intensity and urge to smoke: Testing the role of negative affect and pain catastrophizing. Drug Alcohol. Depend. 2018, 187, 100–108. [Google Scholar] [CrossRef]
- LaRowe, L.R.; Zvolensky, M.J.; Ditre, J.W. The role of anxiety-relevant transdiagnostic factors in comorbid chronic pain and tobacco cigarette smoking. Cogn. Ther. Res. 2019, 43, 102–113. [Google Scholar] [CrossRef]
- Goesling, J.; Brummett, C.M.; Meraj, T.S.; Moser, S.E.; Hassett, A.L.; Ditre, J.W. Associations between pain, current tobacco smoking, depression, and fibromyalgia status among treatment-seeking chronic pain patients. Pain Med. 2015, 16, 1433–1442. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Mannino, D.M.; Steinke, D.T.; Kryscio, R.J.; Bush, H.M.; Crofford, L.J. Association of smoking and chronic pain syndromes in Kentucky women. J. Pain 2011, 12, 892–899. [Google Scholar] [CrossRef]
- Ho, M.C.; Chang, C.F.; Li, R.H.; Tang, T.C. Attentional biases for betel nut cues in heavy and light chewers. Psychol. Addict. Behav. 2013, 27, 1044–1049. [Google Scholar] [CrossRef]
- Garland, E.L.; Froeliger, B.E.; Passik, S.D.; Howard, M.O. Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. J. Behav. Med. 2013, 36, 611–620. [Google Scholar] [CrossRef]
- Drobes, D.J.; Oliver, J.A.; Correa, J.B.; Evans, D.E. Attentional bias and smoking. In Neuroscience of Nicotine: Mechanisms and Treatment; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Chanon, V.W.; Sours, C.R.; Boettiger, C.A. Attentional bias toward cigarette cues in active smokers. Psychopharmacology 2010, 212, 309–320. [Google Scholar] [CrossRef]
- Zhang, M.; Ying, J.; Wing, T.; Song, G.; Fung, D.S.S.; Smith, H. A systematic review of attention biases in opioid, cannabis, stimulant use disorders. Int. J. Environ. Res. Public Health 2018, 15, 1138. [Google Scholar] [CrossRef]
- Starzomska, M. Applications of the dot probe task in attentional bias research in eating disorders: A review. Psicológica 2017, 38, 283–346. [Google Scholar]
- MacLeod, C.; Mathews, A.; Tata, P. Attentional bias in emotional disorders. J. Abnorm. Psychol. 1986, 95, 15–20. [Google Scholar] [CrossRef]
- Posner, M.I.; Inhoff, A.W.; Friedrich, F.J.; Cohen, A. Isolating attentional systems: A cognitive-anatomical analysis. Psychobiology 1987, 15, 107–121. [Google Scholar] [CrossRef]
- Koster, E.H.W.; Crombez, G.; Verschuere, B.; De Houwer, J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behav. Res. Ther. 2004, 42, 1183–1192. [Google Scholar] [CrossRef]
- Field, M.; Cox, W.M. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol. Depend. 2008, 97, 1–20. [Google Scholar] [CrossRef]
- Frankland, L.; Bradley, B.P.; Mogg, K. Time course of attentional bias to drug cues in opioid dependence. Psychol. Addict. Behav. 2016, 30, 601–606. [Google Scholar] [CrossRef]
- Garland, E.L.; Howard, M.O. Opioid attentional bias and cue-elicited craving predict future risk of prescription opioid misuse among chronic pain patients. Drug Alcohol. Depend. 2014, 144, 283–287. [Google Scholar] [CrossRef]
- Morris, C.D.; Garver-Apgar, C.E. Nicotine and opioids: A call for co-treatment as the standard of care. J. Behav. Health Ser. R 2020, 47, 601–613. [Google Scholar] [CrossRef]
- Moeller, S.J.; Hanley, A.W.; Garland, E.L. Behavioral preference for viewing drug versus pleasant images predicts current and future opioid misuse among chronic pain patients. Psychol. Med. 2020, 50, 644–652. [Google Scholar] [CrossRef]
- Zhang, M.W.B.; Ying, J.; Wing, T.; Song, G.; Fung, D.S.S.; Smith, H.E. Cognitive biases in cannabis, opioid, and stimulant disorders: A systematic review. Front. Psychiatry 2018, 9, 376. [Google Scholar] [CrossRef]
- Onishi, E.; Kobayashi, T.; Dexter, E.; Marino, M.; Maeno, T.; Deyo, R.A. Comparison of opioid prescribing patterns in the united states and japan: Primary care physicians’ attitudes and perceptions. J. Am. Board Fam. Med. 2017, 30, 248–254. [Google Scholar] [CrossRef]
- Cleary, J.; Radbruch, L.; Torode, J.; Cherny, N.I. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in asia: A report from the global opioid policy initiative (gopi). Ann. Oncol. 2013, 24, xi24–xi32. [Google Scholar] [CrossRef]
- Wang, J.J.; Teng, S.F.; Chu, Y.R.; Chu, C.C.; Ho, C.H.; Chu, L.L. Evaluation of opioid consumption trends for pain in taiwan and comparison with neighboring asian countries. J. Food Drug Anal. 2022, 30, 104–110. [Google Scholar] [CrossRef]
- van Rooijen, R.; Ploeger, A.; Kret, M.E. The dot-probe task to measure emotional attention: A suitable measure in comparative studies? Psychon. Bull. Rev. 2017, 24, 1686–1717. [Google Scholar] [CrossRef]
- Eaton, W.W.; Smith, C.; Ybarra, M.; Muntaner, C.; Tien, A. Center for Epidemiologic Studies Depression Scale: Review and revision (CESD and CSED-R). Use Psychol. Test. Treat. Plan. Outcomes Assess. 2004, 3, 363–377. [Google Scholar]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Myers, J.K.; Weissman, M.M. Use of a self-report symptom scale to detect depression in a community sample. Am. J. Psychiatry 1980, 137, 1081–1084. [Google Scholar]
- Beck, A.T.; Steer, R.A. BAI: Beck Anxiety Inventory; Psychological Corporation: San Antonio, TX, USA, 1993. [Google Scholar]
- Che, H.-H.; Lu, M.-L.; Chen, H.-C.; Chang, S.-W.; Lee, Y.-J. Validation of the Chinese version of the Beck Anxiety Inventory. J. Formos Med. Assoc. 2006, 10, 447–454. [Google Scholar]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerström, K.O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Meneses-Gaya, I.C.d.; Zuardi, A.W.; Loureiro, S.R.; Crippa, J.A.d.S. Psychometric properties of the Fagerström Test for Nicotine Dependence. J. Bras. Pneumol. 2009, 35, 73–82. [Google Scholar] [CrossRef]
- Schneider, W.; Eschman, A.; Zuccolotto, A. E-prime User’s Guide; Psychology Software Tool, Inc. Learning Research and Development Center, University of Pittsburgh: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Shen, B.; Chiu, M.C.; Li, S.H.; Huang, G.J.; Liu, L.J.; Ho, M.C. Attentional bias to betel quid cues: An eye tracking study. Psychol. Addict. Behav. 2016, 30, 705–711. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*power 3.1.7: A flexible statistical power analysis program for the social, behavioral and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Kazak, A. Editorial: Journal article reporting standards. Am. Psychol. 2018, 73, 1–2. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 12 January 2024).
- Wickham, H.; Bryan, J. Readxl: Read Excel Files. 2023. Available online: https://CRAN.R-project.org/package=readxl (accessed on 12 January 2024).
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots, 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 12 January 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Voorhis, C.; Morgan, B. Understanding power and rules of thumb for determining sample size. Tutor Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Etcheson, J.I.; Gwam, C.U.; George, N.E.; Walia, N.; Jerjian, C.; Han, G.-R.; Virani, S.; Miller, S.J.; Delanois, R.E. Opiate pain medication consumption in cigarette smokers following total hip arthroplasty. Joints 2018, 06, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, P.; Thomsen, A.B.; Olsen, A.K. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long-term oral opioid therapy. J. Pain Symptom. Manag. 2000, 19, 100–108. [Google Scholar] [CrossRef]
- Cameron-Burr, K.T.; Conicella, A.; Neavyn, M.J. Opioid use and driving performance. J. Med. Toxicol. 2021, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Franken, I.H.A.; Marhe, R. The clinical relevance of attentional bias in substance use disorders. CNS Spectr. 2014, 19, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Ehrman, R.N.; Robbins, S.J.; Bromwell, M.A.; Lankford, M.E.; Monterosso, J.R.; O’Brien, C.P. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol. Depend. 2002, 67, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Snelleman, M.; Schoenmakers, T.M.; van de Mheen, D. Attentional bias and approach/avoidance tendencies do not predict relapse or time to relapse in alcohol dependency. Alcohol. Clin. Exp. Res. 2015, 39, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, E.; Harmon, D. Chronic pain patients’ perceptions of prescription opioids: A systematic review. SN Compr. Clin. Med. 2020, 2, 2816–2824. [Google Scholar] [CrossRef]
- Jalali, M.S.; Botticelli, M.; Hwang, R.C.; Koh, H.K.; McHugh, R.K. The opioid crisis: A contextual, social-ecological framework. Health Res. Policy Syst. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Jantarada, C.; Silva, C.; Guimarães-Pereira, L. Prevalence of problematic use of opioids in patients with chronic noncancer pain: A systematic review with meta-analysis. Pain Prac. 2021, 21, 715–729. [Google Scholar] [CrossRef]
- Ger, L.-P.; Ho, S.-T.; Wang, J.-J. Physicians’ knowledge and attitudes toward the use of analgesics for cancer pain management: A survey of two medical centers in Taiwan. J. Pain Symptom. Manag. 2000, 20, 335–344. [Google Scholar]
- Hashemi, M.; Akbari, M.E.; Razavi, S.S.; Saadat-Niaki, A.; Hoseini Khameneh, S.M. Evaluating resident physicians’ knowledge, attitude, and practice regarding the pain control in cancer patients. Iran. J. Cancer Prev. 2015, 8, 1–10. [Google Scholar] [PubMed]
- CDC National Center for Health Statistics Wide-Ranging Online Data for Epidemiologic Research (Wonder). Available online: http://wonder.cdc.gov (accessed on 12 January 2024).
- Blanco, C.; Wiley, T.R.A.; Lloyd, J.J.; Lopez, M.F.; Volkow, N.D. America’s opioid crisis: The need for an integrated public health approach. Transl. Psychiatry 2020, 10, 167. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.L.; Hong, S.J.; Lim, Y.H.; Jeong, J.H.; Moon, H.S.; Choi, H.R.; Park, S.K.; Kim, J.E.; You, H.; Kim, J.H. Patients’ perception about opioids and addiction in South Korea. Korean J. Pain 2020, 33, 234–244. [Google Scholar] [CrossRef]
- Hooten, W.M.; Shi, Y.; Gazelka, H.M.; Warner, D.O. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain 2011, 152, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Burma, N.E.; Kwok, C.H.T.; Trang, T. Therapies and mechanisms of opioid withdrawal. Pain Manag. 2017, 7, 455–459. [Google Scholar] [CrossRef]
- Aitkin, M. The analysis of unbalanced cross-classifications. J. R. Stat. Soc. Ser. A 1978, 141, 195–223. [Google Scholar] [CrossRef]
| Pain Smokers (n = 17) | Pain Nonsmokers (n = 16) | Smokers without Pain (n = 28) | F(2, 58) | p | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |||
| Daily cigarette consumption | 20.88 | (7.12) | 0 | 0 | 21.07 | (8.32) | t = −0.08 | 0.936 |
| Years of smoking | 28.24 | (6.86) | 1.44 | (5.01) | 24.22 | (10.28) | t = 1.75 | 0.076 |
| FTND a | 6.65 | (2.23) | 6.11 | (1.37) | t = 0.90 | 0.378 | ||
| Age | 47.06 | (7.71) | 46.44 | (8.10) | 41.18 | (10.82) | 2.70 | 0.075 |
| Education | 11.82 | (2.63) | 13.75 | (2.05) | 12.11 | (2.42) | 3.23 | 0.047 |
| CESD b | 25.59 | (12.63) | 24.50 | (10.80) | 10.89 | (7.39) | 15.32 | *** |
| BAI c | 14.29 | (10.78) | 16.75 | (8.87) | 4.64 | (4.72) | 14.59 | *** |
| Pain scores | 5.35 | (1.21) | 5.36 | (1.57) | 0.96 | (1.42) | 74.10 | *** |
| MME d | 253.71 | (132.24) | 208.61 | (178.17) | t = 0.82 | 0.418 | ||
| AB scores e | F(2, 57) | |||||||
| 200 ms | −9.03 | (8.75) | 36.09 | (12.92) | −7.94 | (7.21) | 3.61 | 0.033 |
| 2000 ms | −1.07 | (9.09) | 6.24 | (13.42) | 8.65 | (7.49) | 0.43 | 0.656 |
| Display Time | AB Scores for Opioid | |||
|---|---|---|---|---|
| 200 ms | 2000 ms | 200 ms | 2000 ms | |
| CncPs a | −9.03 (8.75) | −1.07 (9.09) | ||
| Incongruent | 675.88 (155.12) | 652.40 (124.12) | ||
| Congruent | 678.68 (142.00) | 652.99 (110.85) | ||
| CPNS b | 36.09 (12.92) | 6.24 (13.42) | ||
| Incongruent | 693.24 (120.82) | 680.40 (119.05) | ||
| Congruent | 675.05 (136.40) | 675.54 (113.51) | ||
| OS c | −7.94 (7.21) | 8.65 (7.49) | ||
| Incongruent | 582.51 (116.61) | 593.63 (121.95) | ||
| Congruent | 584.00 (112.90) | 584.48 (116.01) | ||
| Source of Variance | F | df | p | ηp2 | |
|---|---|---|---|---|---|
| Main effects | |||||
| Daily cigarette (co-variate) | 2.75 | (1, 57) | 0.102 | 0.046 | |
| Group | 5.31 | (2, 57) | 0.008 | 0.157 | |
| Display time | 2.15 | (1, 57) | 0.148 | 0.036 | |
| Probe location | 0.26 | (1, 57) | 0.612 | 0.005 | |
| Interactions | |||||
| Daily cigarette × Probe location | 1.42 | (1, 57) | 0.239 | 0.024 | |
| Display time × Group | 3.39 | (2, 57) | 0.041 | 0.106 | |
| Probe location × Group | 1.66 | (2, 57) | 0.200 | 0.055 | |
| Daily cigarette × Display time | 0.71 | (1, 57) | 0.402 | 0.012 | |
| Display time × Probe location | 1.47 | (1, 57) | 0.231 | 0.025 | |
| Daily cigarette × Probe location × Display time | 1.85 | (1, 57) | 0.179 | 0.023 | |
| Display time × Probe location × Group | 2.50 | (2, 57) | 0.091 | 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-J.; Lin, E.M.-H.; Tsao, S.-L.; Wang, H.-Y.; Ho, M.-C. Attentional Bias for Opioids in Taiwanese Heavy Smokers with Chronic Noncancer Pain. Medicina 2024, 60, 1107. https://doi.org/10.3390/medicina60071107
Liu L-J, Lin EM-H, Tsao S-L, Wang H-Y, Ho M-C. Attentional Bias for Opioids in Taiwanese Heavy Smokers with Chronic Noncancer Pain. Medicina. 2024; 60(7):1107. https://doi.org/10.3390/medicina60071107
Chicago/Turabian StyleLiu, Ling-Jun, Edward Meng-Hua Lin, Shao-Lun Tsao, Hsin-Yu Wang, and Ming-Chou Ho. 2024. "Attentional Bias for Opioids in Taiwanese Heavy Smokers with Chronic Noncancer Pain" Medicina 60, no. 7: 1107. https://doi.org/10.3390/medicina60071107





