Prognostic Factors in Idiopathic Sudden Sensorineural Hearing Loss: The Experience of Two Audiology Tertiary Referral Centres
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Diagnostic and Therapeutic Work-Up
- Complete recovery (CR): Final hearing level ≤ 25 dB;
- Partial recovery (PR): More than 15 dB hearing gain and final hearing level 26–45 dB;
- Slight improvement (SI): More than 15 dB hearing gain and final hearing level 46–75 dB;
- No improvement (NI): Less than 15 dB hearing gain or final hearing level 76–90 dB;
- Non-serviceable ear (NS): Final hearing level > 90 dB. For the statistical analysis, the outcomes were dichotomized as CR vs. non-complete recovery (including PR, SI, NI, and NS).
2.3. Statistical Analysis
3. Results
3.1. General Clinical Features and Outcomes
3.2. Clinical Prognostic Factors
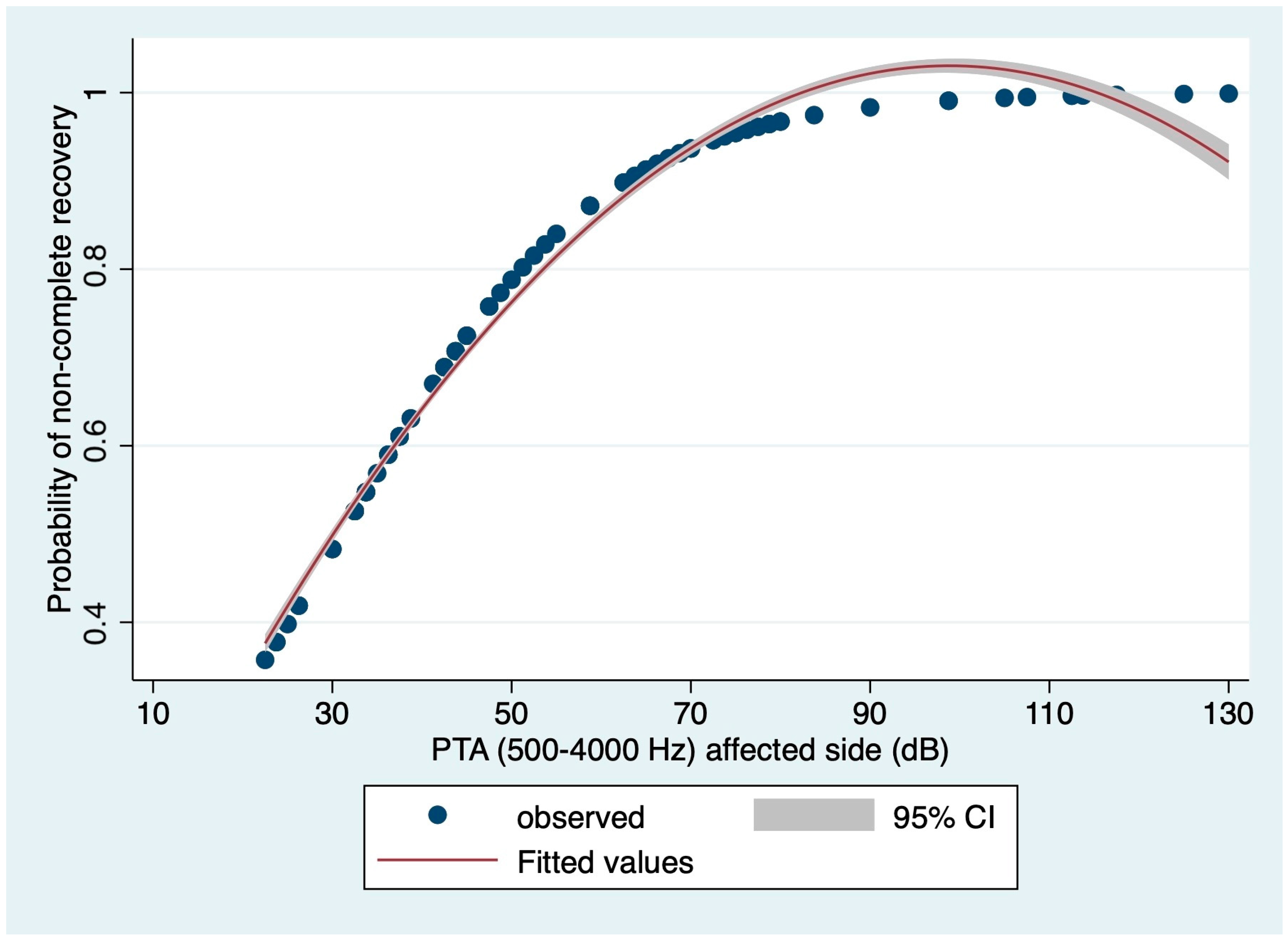
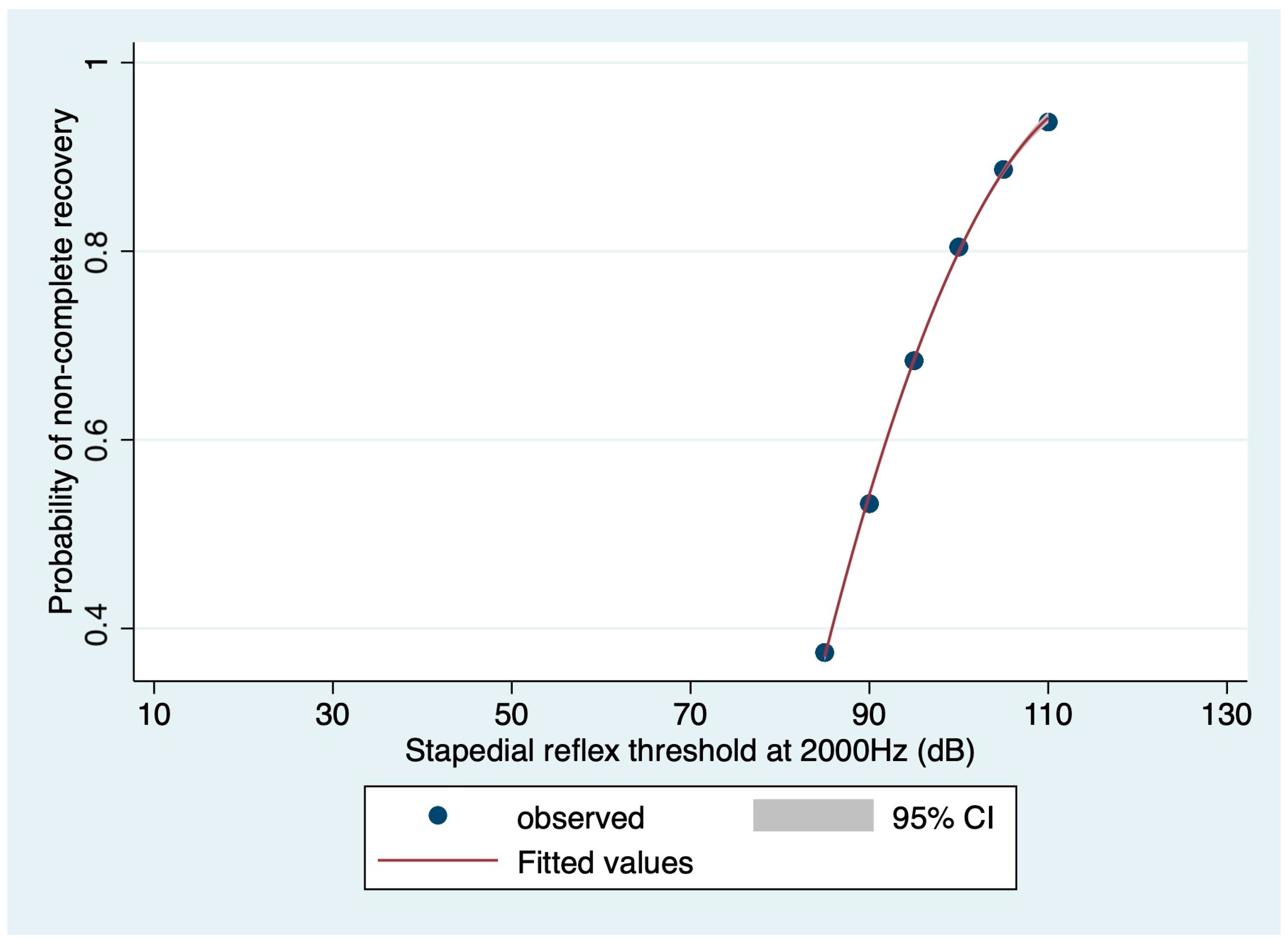
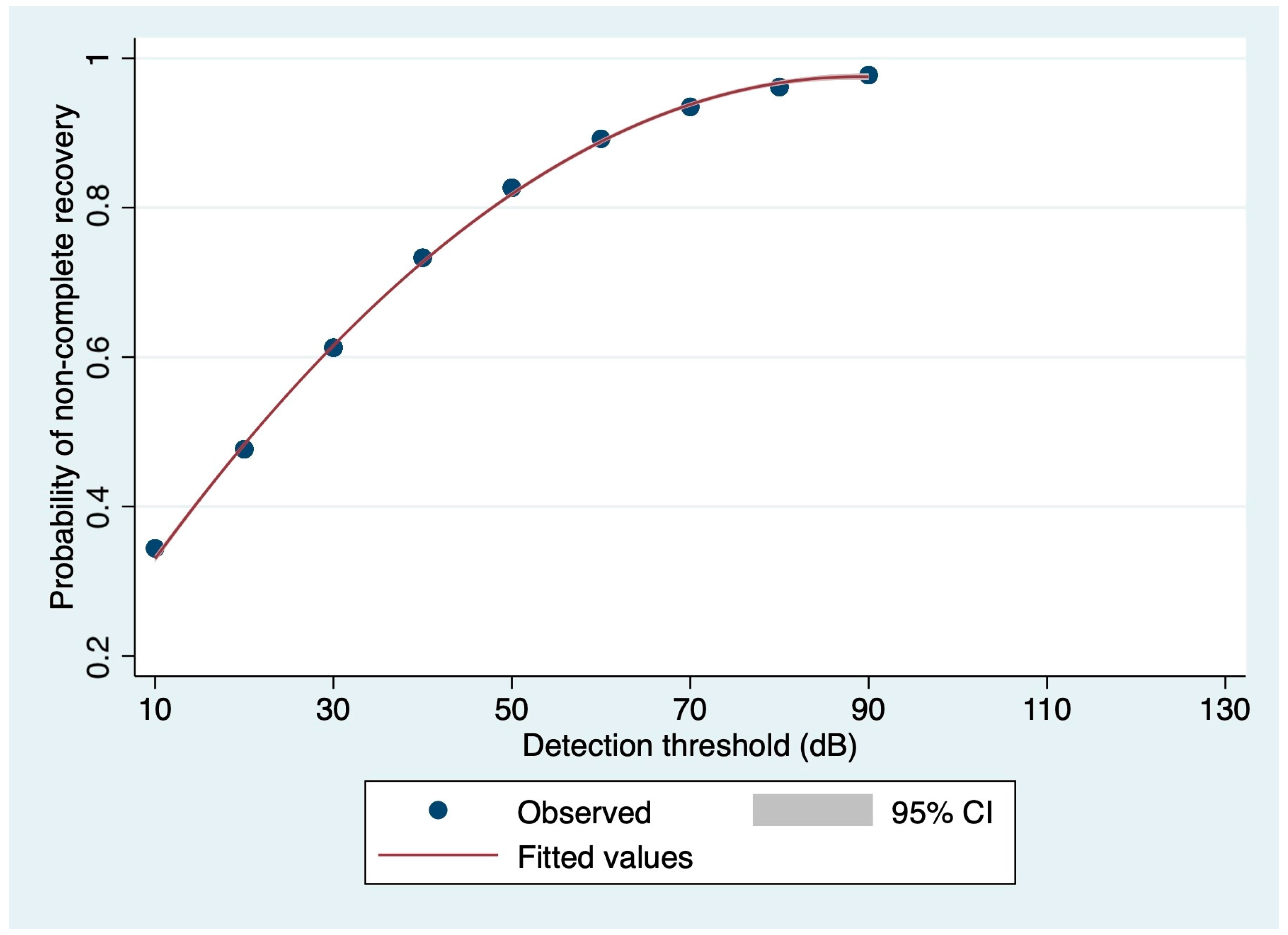
3.3. Prognostic Value of Audiometric Findings at Diagnosis: Logistic Regression-Based Estimations
4. Discussion
4.1. Demographics, Comorbidities and Prognosis
4.2. Pre-Treatment Audiological Variables and Prognosis
4.3. Weaknesses and Strengths of the Investigation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Overall N = 86 | Complete Recovery N = 21 | Non-Complete Recovery N = 65 | p-Value |
|---|---|---|---|---|
| Age (years) Median (IQR) | 58.00 (47.00–69.00) | 54.00 (50.00–60.00) | 60.00 (46.00–63.00) | 0.165 * |
| Age < 65 years N (%) | 54 (62.79) | 17 (80.95) | 37 (56.92) | 0.040 ** |
| Age ≥ 65 years N (%) | 32 (37.21) | 4 (19.05) | 28 (43.08) | |
| Female N (%) | 38 (44.19) | 11 (52.38) | 27 (41.54) | 0.268 ** |
| Male N (%) | 48 (55.81) | 10 (47.62) | 38 (58.46) | |
| Time-to-diagnosis (days) Median (IQR) | 7.00 (1.50–20.00) | 5.00 (1.00–7.00) | 7.00 (2.00–20.00) | 0.102 * |
| Time-to-diagnosis ≤ 7 days N (%) | 49 (56.98) | 16 (76.19) | 33 (50.77) | 0.035 ** |
| Time-to-diagnosis > 7 days N (%) | 37 (43.02) | 5 (23.81) | 32 (49.23) | |
| No hypertension N (%) | 43 (53.09) | 13 (65.00) | 30 (49.18) | 0.166 ** |
| Hypertension N (%) | 38 (46.91) | 7 (35.00) | 31 (50.82) | |
| No diabetes N (%) | 71 (87.65) | 20 (100.00) | 51 (83.61) | 0.048 ** |
| Diabetes N (%) | 10 (12.39) | 0 (0.00) | 10 (16.39) | |
| No vascular disease N (%) | 70 (86.42) | 16 (80.00) | 54 (88.52) | 0.268 ** |
| Vascular disease N (%) | 11 (13.58) | 4 (20.00) | 7 (11.48) | |
| No dyslipidemia N (%) | 61 (75.31) | 17 (85.00) | 44 (72.13) | 0.197 ** |
| Dyslipidemia N (%) | 20 (24.69) | 3 (15.00) | 17 (27.87) | |
| No kidney failure N (%) | 79 (97.53) | 20 (100.00) | 59 (96.72) | 0.565 ** |
| Kidney failure N (%) | 2 (2.47) | 0 (0.00) | 2 83.28) | |
| No autoimmune disorders N (%) | 74 (92.50) | 10 (90.00) | 56 (93.33) | 0.470 ** |
| Autoimmune disorders N (%) | 4 (7.50) | 2 (10.00) | 4 (6.67) | |
| No subjective hypoacusis N (%) | 2 (2.38) | 0 (0.00) | 2 (3.17) | 0.560 ** |
| Subjective hypoacusis N (%) | 82 (97.62) | 21 (100.00) | 61 (96.83) | |
| No fullness N (%) | 46 (55.42) | 13 (61.90) | 33 (53.23) | 0.333 ** |
| Fullness N (%) | 37 (44.58) | 8 (38.10) | 29 (46.77) | |
| No tinnitus N (%) | 26 (32.10) | 6 (28.57) | 20 (33.33) | 0.454 ** |
| Tinnitus N (%) | 55 (67.90) | 15 (71.43) | 40 (66.67) | |
| No vestibular symptoms N (%) | 57 (68.67) | 18 (85.71) | 39 (62.90) | 0.043 ** |
| Vestibular symptoms N (%) | 26 (31.33) | 3 (14.29) | 23 (37.10) | |
| No spontaneous nystagmus N (%) | 50 (89.29) | 14 (93.33) | 36 (87.80) | 0.485 ** |
| Spontaneous nystagmus N (%) | 6 (10.71) | 1 (6.67) | 5 (12.20) | |
| No positional nystagmus N (%) | 44 (86.27) | 13 (92.86) | 31 (83.78) | 0.370 ** |
| Positional nystagmus N (%) | 7 (13.73) | 1 (7.14) | 6 (16.22) | |
| No asymmetric vestibular reflectivity N (%) | 38 (86.36) | 12 (92.31) | 26 (83.87) | 0.417 ** |
| Asymmetric vestibular reflectivity N (%) | 6 (13.64) | 1 (7.69) | 5 816.13) | |
| Oral Steroid dose (mg) Median (IQR) | 30 (25-50) | 40 (25-50) | 25 (25-37.5) | 0.0249 * |
| PTA affected side (dB) Median (IQR) | 48.13 (36.25–73.75) | 36.25 (30.00–37.50) | 58.75 (42.50–76.25) | <0.0001 * |
| Threshold 250 Hz affected side (dB) Median (IQR) | 45.00 (30.00–65.00) | 35.00 (15.00–45.00) | 50.00 (35.00–70.00) | 0.0038 * |
| Threshold 500 Hz affected side (dB) Median (IQR) | 50.00 (35.00–70.00) | 40.00 (25.00–45.0) | 55.00 (40.00–75.00) | 0.0012 * |
| Threshold 1000 Hz affected side (dB) Median (IQR) | 50.00 (35.00–75.00) | 35.00 (20.00–40.00) | 60.00 (35.00–80.00) | 0.0010 * |
| Threshold 2000 Hz affected side (dB) Median (IQR) | 50.00 (30.00–75.00) | 30.00 (20.00–35.00) | 60.00 (40.00–75.00) | 0.0001 * |
| Threshold 4000 Hz affected side (dB) Median (IQR) | 60.00 (40–00–80.00) | 45.00 (35.00–50.00) | 70.00 (50.00–90.00) | 0.0003 * |
| Threshold 8000 Hz affected side (dB) Median (IQR) | 7.00 (50.00–90.00) | 50.00 (40.00–65.00) | 80.00 (55.00–95.00) | 0.0006 * |
| Stapedial reflex threshold 500 Hz affected side (dB) Median (IQR) | 95.00 (90.00–100.00) | 95.00 (85.00–97.50) | 97.50 (90.00–100.00) | 0.0808 * |
| Stapedial reflex threshold 1000 Hz affected side (dB) Median (IQR) | 95.00 (90.00–100.00) | 95.00 (85.00–100.00) | 100.00 (90.00–100.00) | 0.1150 * |
| Stapedial reflex threshold 2000 Hz affected side (dB) Median (IQR) | 95.00 (90.00–100.00) | 87.50 (85.00–100.00) | 100.00 (95.00–105.00) | 0.0301 * |
| Stapedial reflex threshold 4000 Hz affected side (dB) Median (IQR) | 95.00 (90.00–100.00) | 90.00 (85.00–100.00) | 100.00 (90.00–105.00) | 0.1787 * |
| Detection threshold affected side (dB) Median (IQR) | 30.00 (30.00–70.00) | 30.00 (20.00–30.00) | 45.00 (30.00–70.00) | 0.0017 * |
| SDS50 affected side (dB) Median (IQR) | 40.00 (30.00–50.00) | 40.00 (30.00–40.00) | 45.00 (40.00–55.00) | 0.0323 * |
| SDS100 affected side (dB) Median (IQR) | 50.00 (40.00–60.00) | 50.00 (40.00–60.00) | 50.00 (50.00–70.00) | 0.2816 * |
References
- National Institute on Deafness and Other Communication Disorders. NIDCD Fact Sheet: Sudden Deafness; US Department of Health and Human Services: Washington, DC, USA, 2018.
- Aldè, M.; Ambrosetti, U.; Piatti, G.; Romanini, C.; Filipponi, E.; Di Berardino, F.; Zanetti, D.; Pignataro, L.; Cantarella, G.; Barozzi, S. Sudden Sensorineural Hearing Loss in Patients Aged from 15 to 40 Years. J. Clin. Med. 2024, 13, 3303. [Google Scholar] [CrossRef] [PubMed]
- Yélamos Lorente, M.Á.; Perez-Carpena, P.; Lopez-Escamez, J.A. A Systematic Review on Heritability of Sudden Sensorineural Hearing Loss. Laryngoscope, 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical practice guideline: Sudden hearing loss (Update). Otolaryngol. Head Neck Surg. 2019, 161 (Suppl. 1), S1–S45. [Google Scholar] [CrossRef]
- Lionello, M.; Staffieri, C.; Breda, S.; Turato, C.; Giacomelli, L.; Magnavita, P.; de Filippis, C.; Staffieri, A.; Marioni, G. Uni- and multivariate models for investigating potential prognostic factors in idiopathic sudden sensorineural hearing loss. Eur. Arch. Otorhinolaryngol. 2015, 272, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Frosolini, A.; Franz, L.; Daloiso, A.; Lovato, A.; de Filippis, C.; Marioni, G. Digging into the role of inflammatory biomarkers in sudden sensorineural hearing loss diagnosis and prognosis: A systematic review and meta-analysis. Medicina 2022, 58, 963. [Google Scholar] [CrossRef]
- Boesen, E.I.; Kakalij, R.M. Autoimmune-mediated renal disease and hypertension. Clin. Sci. 2021, 135, 2165–2196. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, S.H.; Kim, I.; Kim, H.; Kim, J.H.; Lee, J.B. Does metabolic syndrome affect the incidence and prognosis of sudden sensorineural hearing loss? Life 2022, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Shen, Y.; Xia, L.; Xiao, L.; Sun, Y.; Wang, H.; Chen, Z.; Wu, Y.; Shi, H.; He, J.; et al. Thyroid-related hormone levels in clinical patients with moderately severe-to-profound sudden sensorineural hearing loss: A prospective study. Front. Neurol. 2021, 12, 753270. [Google Scholar] [CrossRef] [PubMed]
- Cvorović, L.; Deric, D.; Probst, R.; Hegemann, S. Prognostic model for predicting hearing recovery in idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 2008, 29, 464–469. [Google Scholar] [CrossRef]
- Edizer, D.T.; Çelebi, Ö.; Hamit, B.; Baki, A.; Yiğit, Ö. Recovery of idiopathic sudden sensorineural hearing loss. J. Int. Adv. Otol. 2015, 11, 122–126. [Google Scholar] [CrossRef]
- Stanton, V.A.; Hsieh, Y.H.; Camargo, C.A., Jr.; Edlow, J.A.; Lovett, P.B.; Goldstein, J.N.; Abbuhl, S.; Lin, M.; Chanmugam, A.; Rothman, R.E.; et al. Overreliance on symptom quality in diagnosing dizziness: Results of a multicenter survey of emergency physicians. Mayo Clin. Proc. 2007, 82, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Aldè, M.; Barozzi, S.; Di Berardino, F.; Zuccotti, G.; Consonni, D.; Ambrosetti, U.; Socci, M.; Bertoli, S.; Battezzati, A.; Foppiani, A.; et al. Prevalence of symptoms in 1512 COVID-19 patients: Have dizziness and vertigo been underestimated thus far? Intern. Emerg. Med. 2022, 17, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Chu, Y.C.; Tu, T.Y.; Shiao, A.S.; Wu, S.L.; Liao, W.H. Modified Siegel’s criteria for sudden sensorineural hearing loss: Reporting recovery outcomes with matched pretreatment hearing grades. J. Chin. Med. Assoc. 2018, 81, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Lionello, M.; Tealdo, G.; Breda, S.; Giacomelli, L.; Staffieri, A.; Marioni, G. Idiopathic sudden sensorineural hearing loss in elderly patients: Univariate and multivariate analysis of potential clinical prognostic factors. Hear. Balance Commun. 2014, 12, 182–188. [Google Scholar] [CrossRef]
- Lee, H.A.; Chung, J.H. Contemporary review of idiopathic sudden sensorineural hearing loss: Management and prognosis. J. Audiol. Otol. 2024, 28, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar Irugu, D.V. Sudden sensorineural hearing loss—A contemporary review of management issues. J. Otol. 2020, 15, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Askar, A.A.; Ghonim, M.R.; Shabana, Y.K. Discriminant analysis of the prognostic factors for hearing outcomes in patients with idiopathic sudden sensorineural hearing loss. J. Int. Adv. Otol. 2023, 19, 162–168. [Google Scholar] [CrossRef]
- Weng, S.F.; Chen, Y.S.; Hsu, C.J.; Tseng, F.Y. Clinical features of sudden sensorineural hearing loss in diabetic patients. Laryngoscope 2005, 115, 1676–1680. [Google Scholar] [CrossRef]
- Ryu, O.H.; Choi, M.G.; Park, C.H.; Kim, D.K.; Lee, J.S.; Lee, J.H. Hyperglycemia as a potential prognostic factor of idiopathic sudden sensorineural hearing loss. Otolaryngol. Head Neck Surg. 2014, 150, 853–858. [Google Scholar] [CrossRef]
- Dong, A.; Peng, J.; Lin, R. Predictive model for prognosis of sudden sensorineural hearing loss by nomogram. Ear Nose Throat J. 2024, 23, 1455613241230823. [Google Scholar] [CrossRef]
- Psillas, G.; Rizou, A.; Rachovitsas, D.; Tsiropoulos, G.; Constantinidis, J. Hearing outcome of low-tone compared to high-tone sudden sensorineural hearing loss. Int. Arch. Otorhinolaryngol. 2019, 23, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Choo, O.S.; Yang, S.M.; Park, H.Y.; Lee, J.B.; Jang, J.H.; Choi, S.J.; Choung, Y.H. Differences in clinical characteristics and prognosis of sudden low- and high-frequency hearing loss. Laryngoscope 2017, 127, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.J.; Chang, J.; Im, G.J.; Kwon, S.Y.; Jung, H.; Choi, J. Analysis of frequency loss as a prognostic factor in idiopathic sensorineural hearing loss. Acta Otolaryngol. 2012, 132, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Kitoh, R.; Nishio, S.Y.; Usami, S.I. Speech perception in noise in patients with idiopathic sudden hearing loss. Acta Otolaryngol. 2022, 142, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Diao, T.; Duan, M.; Ma, X.; Liu, J.; Yu, L.; Jing, Y.; Wang, M. The impairment of speech perception in noise following pure tone hearing recovery in patients with sudden sensorineural hearing loss. Sci. Rep. 2022, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.H. Detection of hearing impairment with the acoustic stapedius reflex. Ear Hear. 1993, 14, 3–10. [Google Scholar] [CrossRef]
- Gerwin, J.M.; LaCoste, P. The acoustic stapedial reflex as a prognostic indicator in sudden onset sensorineural hearing loss. Otolaryngol. Head Neck Surg. 1982, 90, 857–861. [Google Scholar] [CrossRef]
| Variable | Odds Ratio (95% C.I.) | Standard Error | p-Value |
|---|---|---|---|
| Age at diagnosis | 1.0174 (0.9687–1.0686) | 0.0255 | 0.490 |
| Time to diagnosis | 1.0400 (0.9835–1.0996) | 0.0296 | 0.168 |
| Oral steroid dose | 1.0214 (0.9646–1.0814) | 0.0298 | 0.469 |
| PTA on the affected side at diagnosis | 1.0615 (1.0185–1.1063) | 0.0224 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caragli, V.; Franz, L.; Incognito, A.; Bitonti, S.; Guarnaccia, M.; Cenedese, R.; Cocimano, D.; Romano, A.; Canova, G.; Zanatta, P.; et al. Prognostic Factors in Idiopathic Sudden Sensorineural Hearing Loss: The Experience of Two Audiology Tertiary Referral Centres. Medicina 2024, 60, 1130. https://doi.org/10.3390/medicina60071130
Caragli V, Franz L, Incognito A, Bitonti S, Guarnaccia M, Cenedese R, Cocimano D, Romano A, Canova G, Zanatta P, et al. Prognostic Factors in Idiopathic Sudden Sensorineural Hearing Loss: The Experience of Two Audiology Tertiary Referral Centres. Medicina. 2024; 60(7):1130. https://doi.org/10.3390/medicina60071130
Chicago/Turabian StyleCaragli, Valeria, Leonardo Franz, Alessandro Incognito, Salvatore Bitonti, Maria Guarnaccia, Roberta Cenedese, Debora Cocimano, Aaron Romano, Giuseppe Canova, Paolo Zanatta, and et al. 2024. "Prognostic Factors in Idiopathic Sudden Sensorineural Hearing Loss: The Experience of Two Audiology Tertiary Referral Centres" Medicina 60, no. 7: 1130. https://doi.org/10.3390/medicina60071130
APA StyleCaragli, V., Franz, L., Incognito, A., Bitonti, S., Guarnaccia, M., Cenedese, R., Cocimano, D., Romano, A., Canova, G., Zanatta, P., Genovese, E., de Filippis, C., & Marioni, G. (2024). Prognostic Factors in Idiopathic Sudden Sensorineural Hearing Loss: The Experience of Two Audiology Tertiary Referral Centres. Medicina, 60(7), 1130. https://doi.org/10.3390/medicina60071130










