Adapted Training to Boost Upper Body Sensorimotor Control and Daily Living Functionality in Visually Impaired Baseball Players
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Participant Evaluations
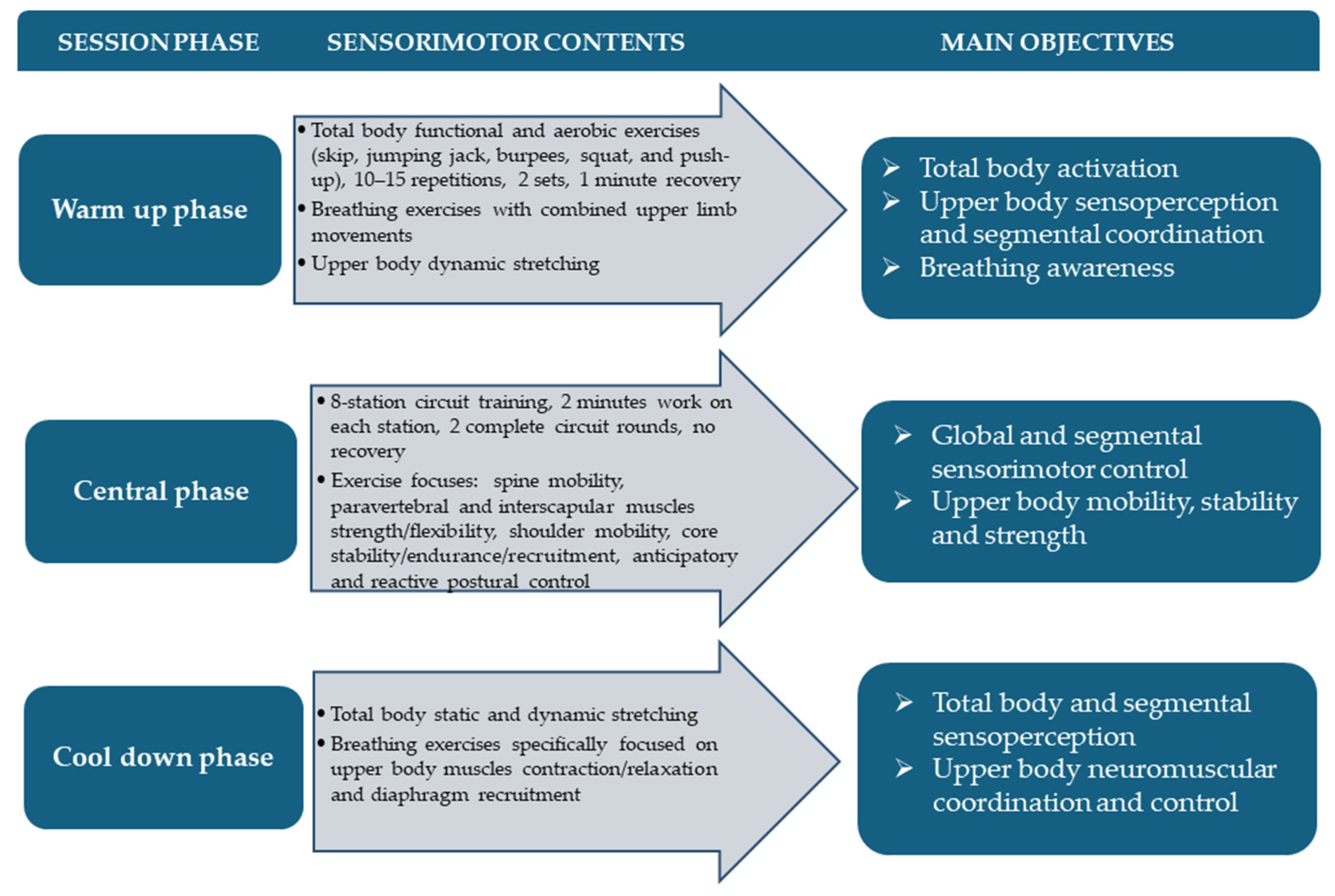
2.3. Adapted Sensorimotor Training
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, A.M. Multisensory Integration in Balance Control. Handb. Clin. Neurol. 2016, 137, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J. Sensory Integration for Human Balance Control. Handb. Clin. Neurol. 2018, 159, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.P.; Villeneuve, P. Interaction between Feet and Gaze in Postural Control. Brain Sci. 2022, 12, 1459. [Google Scholar] [CrossRef] [PubMed]
- Sarlegna, F.R.; Sainburg, R.L. The Roles of Vision and Proprioception in the Planning of Reaching Movements. Adv. Exp. Med. Biol. 2009, 629, 317–335. [Google Scholar] [CrossRef]
- Job, X.; Arnold, G.; Kirsch, L.P.; Auvray, M. Vision Shapes Tactile Spatial Perspective Taking. J. Exp. Psychol. Gen. 2021, 150, 1918–1925. [Google Scholar] [CrossRef]
- Pasqualotto, A.; Proulx, M.J. The Role of Visual Experience for the Neural Basis of Spatial Cognition. Neurosci. Biobehav. Rev. 2012, 36, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Trouilloud, A.; Kauffmann, L.; Roux-Sibilon, A.; Rossel, P.; Boucart, M.; Mermillod, M.; Peyrin, C. Rapid Scene Categorization: From Coarse Peripheral Vision to Fine Central Vision. Vis. Res. 2020, 170, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Bollini, A.; Gori, M. Early Blindness Limits the Head-Trunk Coordination Development for Horizontal Reorientation. Front. Hum. Neurosci. 2021, 15, 699312. [Google Scholar] [CrossRef]
- Land, M.F. Eye Movements and the Control of Actions in Everyday Life. Prog. Retin. Eye Res. 2006, 25, 296–324. [Google Scholar] [CrossRef]
- Voss, P. Auditory Spatial Perception without Vision. Front. Psychol. 2016, 7, 1960. [Google Scholar] [CrossRef] [PubMed]
- Schott, N.; Haibach-Beach, P.; Knöpfle, I.; Neuberger, V. The Effects of Visual Impairment on Motor Imagery in Children and Adolescents. Res. Dev. Disabil. 2021, 109, 103835. [Google Scholar] [CrossRef] [PubMed]
- Haibach, P.S.; Wagner, M.O.; Lieberman, L.J. Determinants of Gross Motor Skill Performance in Children with Visual Impairments. Res. Dev. Disabil. 2014, 35, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Rogge, A.-K.; Hamacher, D.; Cappagli, G.; Kuhne, L.; Hötting, K.; Zech, A.; Gori, M.; Röder, B. Balance, Gait, and Navigation Performance Are Related to Physical Exercise in Blind and Visually Impaired Children and Adolescents. Exp. Brain Res. 2021, 239, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.O.; Haibach, P.S.; Lieberman, L.J. Gross Motor Skill Performance in Children with and without Visual Impairments--Research to Practice. Res. Dev. Disabil. 2013, 34, 3246–3252. [Google Scholar] [CrossRef]
- Bell, L.; Wagels, L.; Neuschaefer-Rube, C.; Fels, J.; Gur, R.E.; Konrad, K. The Cross-Modal Effects of Sensory Deprivation on Spatial and Temporal Processes in Vision and Audition: A Systematic Review on Behavioral and Neuroimaging Research since 2000. Neural Plast. 2019, 2019, e9603469. [Google Scholar] [CrossRef] [PubMed]
- Heinze, N.; Davies, F.; Jones, L.; Castle, C.L.; Gomes, R.S.M. Conceptualizations of Well-Being in Adults with Visual Impairment: A Scoping Review. Front. Psychol. 2022, 13, 964537. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Lee, M.J.; Lee, S.-M. Visual Impairment and Risk of Depression: A Longitudinal Follow-up Study Using a National Sample Cohort. Sci. Rep. 2018, 8, 2083. [Google Scholar] [CrossRef]
- West, S.K.; Rubin, G.S.; Broman, A.T.; Muñoz, B.; Bandeen-Roche, K.; Turano, K. How Does Visual Impairment Affect Performance on Tasks of Everyday Life? The SEE Project. Salisbury Eye Evaluation. Arch. Ophthalmol. 2002, 120, 774–780. [Google Scholar] [CrossRef]
- Carretti, G.; Manetti, M.; Marini, M. Physical Activity and Sport Practice to Improve Balance Control of Visually Impaired Individuals: A Narrative Review with Future Perspectives. Front. Sports Act. Living 2023, 5, 1260942. [Google Scholar] [CrossRef]
- Carretti, G.; Bianco, R.; Sgambati, E.; Manetti, M.; Marini, M. Reactive Agility and Pitching Performance Improvement in Visually Impaired Competitive Italian Baseball Players: An Innovative Training and Evaluation Proposal. Int. J. Environ. Res. Public Health 2023, 20, 6166. [Google Scholar] [CrossRef]
- Urbaniak-Olejnik, M.; Loba, W.; Stieler, O.; Komar, D.; Majewska, A.; Marcinkowska-Gapińska, A.; Hojan-Jezierska, D. Body Balance Analysis in the Visually Impaired Individuals Aged 18-24 Years. Int. J. Environ. Res. Public Health 2022, 19, 14383. [Google Scholar] [CrossRef]
- Shiota, K.; Tokui, A. Audiospatial Cognitive Ability of Visually Impaired Athletes in Static and Dynamic Spatial Cognitive Tasks. J. Phys. Ther. Sci. 2017, 29, 1981–1986. [Google Scholar] [CrossRef]
- Lewald, J. Opposing Effects of Head Position on Sound Localization in Blind and Sighted Human Subjects. Eur. J. Neurosci. 2002, 15, 1219–1224. [Google Scholar] [CrossRef]
- Ozdemir, R.A.; Pourmoghaddam, A.; Paloski, W.H. Sensorimotor Posture Control in the Blind: Superior Ankle Proprioceptive Acuity Does Not Compensate for Vision Loss. Gait Posture 2013, 38, 603–608. [Google Scholar] [CrossRef]
- Voss, P.; Tabry, V.; Zatorre, R.J. Trade-off in the Sound Localization Abilities of Early Blind Individuals between the Horizontal and Vertical Planes. J. Neurosci. 2015, 35, 6051–6056. [Google Scholar] [CrossRef]
- Tunik, E.; Poizner, H.; Levin, M.F.; Adamovich, S.V.; Messier, J.; Lamarre, Y.; Feldman, A.G. Arm-Trunk Coordination in the Absence of Proprioception. Exp. Brain Res. 2003, 153, 343–355. [Google Scholar] [CrossRef]
- Seroyer, S.T.; Nho, S.J.; Bach, B.R.; Bush-Joseph, C.A.; Nicholson, G.P.; Romeo, A.A. The Kinetic Chain in Overhand Pitching: Its Potential Role for Performance Enhancement and Injury Prevention. Sports Health 2010, 2, 135–146. [Google Scholar] [CrossRef]
- Cetisli Korkmaz, N.; Can Akman, T.; Kilavuz Oren, G.; Bir, L.S. Trunk Control: The Essence for Upper Limb Functionality in Patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2018, 24, 101–106. [Google Scholar] [CrossRef]
- Granacher, U.; Gollhofer, A.; Hortobágyi, T.; Kressig, R.W.; Muehlbauer, T. The Importance of Trunk Muscle Strength for Balance, Functional Performance, and Fall Prevention in Seniors: A Systematic Review. Sports Med. 2013, 43, 627–641. [Google Scholar] [CrossRef]
- Willson, J.D.; Dougherty, C.P.; Ireland, M.L.; Davis, I.M. Core Stability and Its Relationship to Lower Extremity Function and Injury. J. Am. Acad. Orthop. Surg. 2005, 13, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Huxel Bliven, K.C.; Anderson, B.E. Core Stability Training for Injury Prevention. Sports Health 2013, 5, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Brian, A.; Pennell, A.; Haibach-Beach, P.; Foley, J.; Taunton, S.; Lieberman, L.J. Correlates of Physical Activity among Children with Visual Impairments. Disabil. Health J. 2019, 12, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Haegele, J.A.; Zhu, X. Physical Activity, Self-Efficacy and Health-Related Quality of Life among Adults with Visual Impairments. Disabil. Rehabil. 2021, 43, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, J.; Merom, D.; Astuti, P.A.S.; Antoun, M.; Edwards, K.; Ding, D. Physical Activity Interventions for Adults Who Are Visually Impaired: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e034036. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, E.L.; Hassell, J.B.; Keeffe, J.E. The Determinants of Participation in Activities of Daily Living in People with Impaired Vision. Am. J. Ophthalmol. 2004, 137, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Schrack, J.A.; Wang, H.; E, J.-Y.; Wanigatunga, A.A.; Agrawal, Y.; Urbanek, J.K.; Simonsick, E.M.; Ferrucci, L.; Swenor, B.K. Visual Impairment and Objectively Measured Physical Activity in Middle-Aged and Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Rodríguez, V.; Medina-Rebollo, D.; Muñoz-Llerena, A.; Fernández-Gavira, J. Influence of Physical Activity and Sport on the Inclusion of People with Visual Impairment: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 19, 443. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Sarchielli, E.; Portas, M.F.; Ranieri, V.; Meli, A.; Piazza, M.; Sgambati, E.; Monaci, M. Can Baseball Improve Balance in Blind Subjects? J. Sports Med. Phys. Fit. 2011, 51, 227–232. [Google Scholar]
- Mirandola, D.; Monaci, M.; Miccinesi, G.; Vannuzzi, A.; Sgambati, E.; Manetti, M.; Marini, M. Psychological Well-Being and Quality of Life in Visually Impaired Baseball Players: An Italian National Survey. PLoS ONE 2019, 14, e0218124. [Google Scholar] [CrossRef]
- Agency, L.W. Il Gioco e Le Regole—Baseball x Ciechi—AIBXC. Available online: https://www.aibxc.it/giocoregole/index.php?l=en (accessed on 9 June 2024).
- Gray, R. Changes in Movement Coordination Associated With Skill Acquisition in Baseball Batting: Freezing/Freeing Degrees of Freedom and Functional Variability. Front. Psychol. 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.; Maemichi, T.; Torii, S. Identification of Physical Characteristics Associated with Swing Velocity of Batting in Youth Baseball Players. J. Sports Med. Phys. Fit. 2022, 62, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- McCrary, J.M.; Ackermann, B.J.; Halaki, M. A Systematic Review of the Effects of Upper Body Warm-up on Performance and Injury. Br. J. Sports Med. 2015, 49, 935–942. [Google Scholar] [CrossRef]
- Toole, A.J.; Fogt, N. Review: Head and Eye Movements and Gaze Tracking in Baseball Batting. Optom. Vis. Sci. 2021, 98, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Kishita, Y.; Ueda, H.; Kashino, M. Eye and Head Movements of Elite Baseball Players in Real Batting. Front. Sports Act. Living 2020, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Meron, A.; Saint-Phard, D. Track and Field Throwing Sports: Injuries and Prevention. Curr. Sports Med. Rep. 2017, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.J.; Winkelmann, Z.K.; Powden, C.J.; Games, K.E. Proprioceptive Training for the Prevention of Ankle Sprains: An Evidence-Based Review. J. Athl. Train. 2017, 52, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, F.; Perilli, R.; Piccioni, M. Blindness and social security in Italy: Critical issues and proposals. Recent. Prog. Med. 2018, 109, 371–373. [Google Scholar] [CrossRef]
- WMA–The World Medical Association. Declaration of Helsinki. Available online: https://www.wma.net/what-we-do/medicalethics/declaration-of-helsinki/ (accessed on 27 May 2024).
- Kendall, F.P.; Kendall McCreary, E.; Geise Provance, P.; McIntyre Rodgers, M.; Romani, W.A. I muscoli. Funzioni e test con postura e dolore; Verduci Editore: Rome, Italy, 2005. [Google Scholar]
- EasyAngle Clinical Studies|Evidence Based Goniometry. Available online: https://meloqdevices.com/pages/completed-easyangle-clinical-studies (accessed on 10 July 2024).
- Majewska, J.; Kołodziej-Lackorzyńska, G.; Cyran-Grzebyk, B.; Szymczyk, D.; Kołodziej, K.; Wądołkowski, P. Effects of Core Stability Training on Functional Movement Patterns in Tennis Players. Int. J. Environ. Res. Public Health 2022, 19, 16033. [Google Scholar] [CrossRef]
- Minthorn, L.M.; Fayson, S.D.; Stobierski, L.M.; Welch, C.E.; Anderson, B.E. The Functional Movement Screen’s Ability to Detect Changes in Movement Patterns After a Training Intervention. J. Sport. Rehabil. 2015, 24, 322–326. [Google Scholar] [CrossRef]
- Gnacinski, S.L.; Cornell, D.J.; Meyer, B.B.; Arvinen-Barrow, M.; Earl-Boehm, J.E. Functional Movement Screen Factorial Validity and Measurement Invariance Across Sex Among Collegiate Student-Athletes. J. Strength Cond. Res. 2016, 30, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Carretti, G.; Dabraio, A.; Manetti, M.; Marini, M. Biofeedback-Based Proprioceptive Training to Improve Functional Prerequisites of Dragon Boating in Breast Cancer Survivors. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Cady, K.; Powis, M.; Hopgood, K. Intrarater and Interrater Reliability of the Modified Thomas Test. J. Bodyw. Mov. Ther. 2022, 29, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Otoshi, K.-I.; Tominaga, R.; Kaga, T.; Igari, T.; Sato, R.; Konno, S.-I. Influences of Limited Flexibility of the Lower Extremities and Occurrence of Low Back Pain in Adolescent Baseball Players: A Prospective Cohort Study. J. Orthop. Sci. 2022, 27, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Vega, D.; Merino-Marban, R.; Viciana, J. Criterion-Related Validity of Sit-and-Reach Tests for Estimating Hamstring and Lumbar Extensibility: A Meta-Analysis. J. Sports Sci. Med. 2014, 13, 1–14. [Google Scholar] [PubMed]
- Plisky, P.; Schwartkopf-Phifer, K.; Huebner, B.; Garner, M.B.; Bullock, G. Systematic Review and Meta-Analysis of the Y-Balance Test Lower Quarter: Reliability, Discriminant Validity, and Predictive Validity. Int. J. Sports Phys. Ther. 2021, 16, 1190–1209. [Google Scholar] [CrossRef] [PubMed]
- Gribble, P.A.; Hertel, J.; Plisky, P. Using the Star Excursion Balance Test to Assess Dynamic Postural-Control Deficits and Outcomes in Lower Extremity Injury: A Literature and Systematic Review. J. Athl. Train. 2012, 47, 339–357. [Google Scholar] [CrossRef]
- LIBRA–Dispositivo Per Rieducazione Propriocettiva. Easytech. Available online: https://easytechitalia.com/libra/ (accessed on 27 May 2024).
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Benvenuti, F. Physiology of Human Balance. Adv. Neurol. 2001, 87, 41–51. [Google Scholar]
- Cheung, T.C.K.; Schmuckler, M.A. Multisensory Postural Control in Adults: Variation in Visual, Haptic, and Proprioceptive Inputs. Hum. Mov. Sci. 2021, 79, 102845. [Google Scholar] [CrossRef]
- Forbes, P.A.; Chen, A.; Blouin, J.-S. Sensorimotor Control of Standing Balance. Handb. Clin. Neurol. 2018, 159, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Goodworth, A.D.; Mellodge, P.; Peterka, R.J. Stance Width Changes How Sensory Feedback Is Used for Multisegmental Balance Control. J. Neurophysiol. 2014, 112, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Mouchnino, L.; Aurenty, R.; Massion, J.; Pedotti, A. Coordination between Equilibrium and Head-Trunk Orientation during Leg Movement: A New Strategy Build up by Training. J. Neurophysiol. 1992, 67, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Noamani, A.; Lemay, J.-F.; Musselman, K.E.; Rouhani, H. Postural Control Strategy after Incomplete Spinal Cord Injury: Effect of Sensory Inputs on Trunk-Leg Movement Coordination. J. Neuroeng. Rehabil. 2020, 17, 141. [Google Scholar] [CrossRef]
- Maaswinkel, E.; van Drunen, P.; Veeger, D.-J.H.E.J.; van Dieën, J.H. Effects of Vision and Lumbar Posture on Trunk Neuromuscular Control. J. Biomech. 2015, 48, 298–303. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Clark, V.L.; Furlanetto, K.C.; Gibson, P.G.; McDonald, V.M. Core Function in Adults With Severe Asthma and Its Relationship With Breathing Symptoms. J. Allergy Clin. Immunol. Pr. 2024, 12, 1254–1262.e1. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Kimura, Y.; Fujita, Y.; Ishibashi, Y. Core-Muscle Training and Neuromuscular Control of the Lower Limb and Trunk. J. Athl. Train. 2019, 54, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Truijen, S.; Schröder, J.; Maebe, Z.; Blanckaert, K.; van der Waal, C.; Vink, M.; Saeys, W. The Effectiveness of Trunk Training on Trunk Control, Sitting and Standing Balance and Mobility Post-Stroke: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2019, 33, 992–1002. [Google Scholar] [CrossRef]
- Dello Iacono, A.; Padulo, J.; Ayalon, M. Core Stability Training on Lower Limb Balance Strength. J. Sports Sci. 2016, 34, 671–678. [Google Scholar] [CrossRef]
- Lupowitz, L.G. Comprehensive Approach to Core Training in Sports Physical Therapy: Optimizing Performance and Minimizing Injuries. Int. J. Sports Phys. Ther. 2023, 18, 800–806. [Google Scholar] [CrossRef]
- Anderson, K.; Behm, D.G. The Impact of Instability Resistance Training on Balance and Stability. Sports Med. 2005, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Andreopoulou, G.; Maaswinkel, E.; Cofré Lizama, L.E.; van Dieën, J.H. Effects of Support Surface Stability on Feedback Control of Trunk Posture. Exp. Brain Res. 2015, 233, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Muehlbauer, T.; Kibele, A.; Granacher, U. Effects of Strength Training Using Unstable Surfaces on Strength, Power and Balance Performance Across the Lifespan: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1645–1669. [Google Scholar] [CrossRef] [PubMed]
- Mayes, M.; Salesky, M.; Lansdown, D.A. Throwing Injury Prevention Strategies with a Whole Kinetic Chain-Focused Approach. Curr. Rev. Musculoskelet. Med. 2022, 15, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Winters, E.; Doty, S.; Lott, M.; Baker, J. Neuromechanical Integration of Pelvic-Thoracic Rotation among Youth Baseball Throwers. Sports Med. Int. Open 2022, 6, E47–E52. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.; McConnell, A.K.; Pijnenburg, M.; Claeys, K.; Goossens, N.; Lysens, R.; Troosters, T.; Brumagne, S. Inspiratory Muscle Training Affects Proprioceptive Use and Low Back Pain. Med. Sci. Sports Exerc. 2015, 47, 12–19. [Google Scholar] [CrossRef]
- Surakka, A.; Kivelä, T. THE EFFECT OF A PHYSICAL TRAINING PROGRAMME ON FLEXIBILITY OF UPPER BODY AND TRUNK IN VISUALLY IMPAIRED AND DEAF-BLIND PERSONS. Eur. J. Adapt. Phys. Act. 2011, 4, 7–21. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Huang, J.-C.; Tseng, H.-Y.; Yang, Y.-C.; Lin, S.-I. Effects of Trunk Exercise on Unstable Surfaces in Persons with Stroke: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 9135. [Google Scholar] [CrossRef]
- Borghuis, J.; Hof, A.L.; Lemmink, K.A.P.M. The Importance of Sensory-Motor Control in Providing Core Stability: Implications for Measurement and Training. Sports Med. 2008, 38, 893–916. [Google Scholar] [CrossRef]
- Munoz-Martel, V.; Santuz, A.; Bohm, S.; Arampatzis, A. Neuromechanics of Dynamic Balance Tasks in the Presence of Perturbations. Front. Hum. Neurosci. 2021, 14, 560630. [Google Scholar] [CrossRef]
- Riva, D.; Fani, M.; Benedetti, M.G.; Scarsini, A.; Rocca, F.; Mamo, C. Effects of High-Frequency Proprioceptive Training on Single Stance Stability in Older Adults: Implications for Fall Prevention. Biomed. Res. Int. 2019, 2019, 2382747. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of Dual Tasks and Dual-Task Training on Postural Stability: A Systematic Review and Meta-Analysis. Clin. Interv. Aging 2017, 12, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Schmitz, G.; Hwang, T.-H.; Effenberg, A.O. Training Proprioception with Sound: Effects of Real-Time Auditory Feedback on Intermodal Learning. Ann. N. Y Acad. Sci. 2019, 1438, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, J.B.; Lappin, J.S.; Zhang, R.; Tadin, D. Perceptual Training Yields Rapid Improvements in Visually Impaired Youth. Sci. Rep. 2016, 6, 37431. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, J.-Y.; Brusson, A.; Rousseau, M.-A. Hip–Spine Relations and Sagittal Balance Clinical Consequences. Eur. Spine J. 2011, 20, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Talis, V.L.; Grishin, A.A.; Solopova, I.A.; Oskanyan, T.L.; Belenky, V.E.; Ivanenko, Y.P. Asymmetric Leg Loading during Sit-to-Stand, Walking and Quiet Standing in Patients after Unilateral Total Hip Replacement Surgery. Clin. Biomech. 2008, 23, 424–433. [Google Scholar] [CrossRef]
- Arabzadeh, S.; Kamali, F.; Bervis, S.; Razeghi, M. The Hip Joint Mobilization with Movement Technique Improves Muscle Activity, Postural Stability, Functional and Dynamic Balance in Hemiplegia Secondary to Chronic Stroke: A Blinded Randomized Controlled Trial. BMC Neurol. 2023, 23, 262. [Google Scholar] [CrossRef]
- Ackley-Holbrook, E.; Kang, M.; Morgan, D.W. Development and Evaluation of the Walk for Health Program: A Physical Activity Intervention for Adults with Visual Impairments. J. Vis. Impair. Blind. 2016, 110, 103–114. [Google Scholar] [CrossRef]
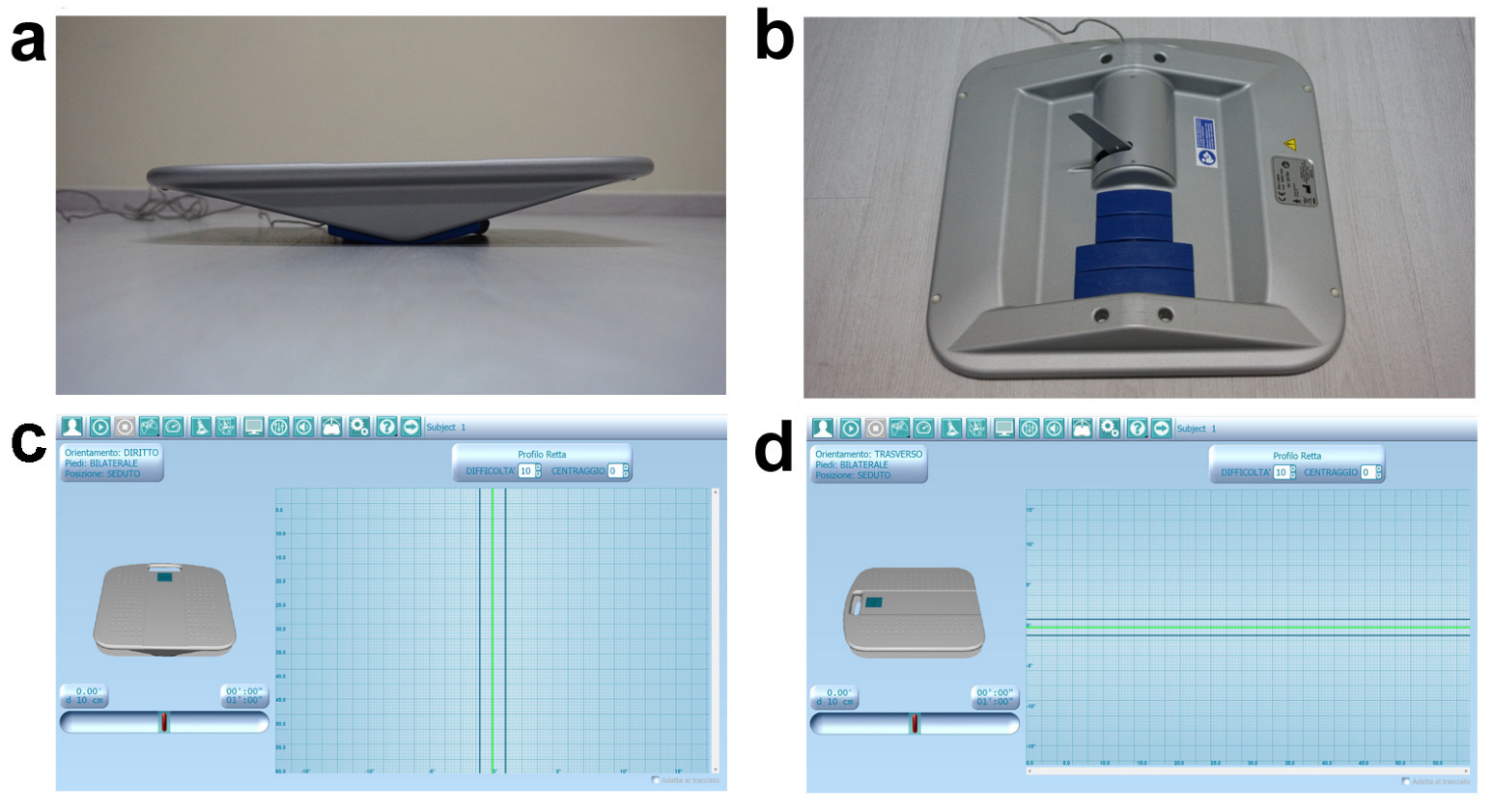
| Variables | Baseline Mean ± SD | Post-AST Mean ± SD | p-Value * | 95% CI Lower Upper | r |
|---|---|---|---|---|---|
| AROM head, degrees | |||||
| Flexion | 37.65 ± 4.98 | 52.25 ± 5.06 | 0.01 | 10.10 19.20 | 14.97 |
| Extension | 43.36 ± 5.29 | 57.16 ± 3.53 | 0.01 | 9.30 18.70 | 13.95 |
| Right inclination | 30.07 ± 6.96 | 41.45 ± 6.39 | 0.01 | 7.05 16.60 | 11.55 |
| Left inclination | 32.36 ± 5.96 | 42.41 ± 6.08 | 0.01 | 7.00 13.05 | 10.25 |
| Right rotation | 46.67 ± 9.36 | 65.56 ± 7.33 | 0.01 | 9.80 28.70 | 18.75 |
| Left rotation | 50.83 ± 9.09 | 66.56 ± 5.79 | 0.01 | 8.35 23.70 | 15.77 |
| AROM right upper limb, degrees | |||||
| Flexion | 147.38 ± 23.22 | 172.08 ± 11.95 | 0.02 | 2.65 41.80 | 22.25 |
| Extension | 39.28 ± 9.39 | 49.21 ± 1.29 | 0.03 | 2.75 17.35 | 10.05 |
| Abduction | 137.61 ± 32.20 | 172.56 ± 16.63 | 0.02 | 16.65 56.85 | 36.72 |
| Adduction | 26.95 ± 8.49 | 45.81 ± 13.55 | 0.01 | 10.75 27.00 | 17.87 |
| External rotation | 76.26 ± 10.10 | 91.91 ± 7.48 | 0.02 | 4.70 26.45 | 14.72 |
| Internal rotation | 72.68 ± 11.64 | 98.18 ± 5.99 | 0.01 | 7.80 28.10 | 17.05 |
| AROM left upper limb, degrees | |||||
| Flexion | 147.53 ± 18.15 | 176.80 ± 9.94 | 0.02 | 8.00 48.80 | 30.12 |
| Extension | 40.92 ± 7.59 | 49.86 ± 0.69 | 0.03 | 3.40 15.40 | 8.27 |
| Abduction | 139.00 ± 31.77 | 172.15 ± 21.52 | 0.03 | 12.25 54.10 | 34.05 |
| Adduction | 28.98 ± 10.65 | 48.83 ± 13.98 | 0.01 | 10.75 29.95 | 19.90 |
| External rotation | 78.86 ± 11.89 | 93.73 ± 10.61 | 0.04 | 0.00 33.40 | 11.10 |
| Internal rotation | 72.32 ± 14.62 | 89.68 ± 5.29 | 0.01 | 4.95 31.60 | 17.87 |
| AROM trunk, degrees | |||||
| Right sitting twist | 28.47 ± 6.82 | 51.08 ± 8.80 | 0.01 | 14.70 30.90 | 23.37 |
| Left sitting twist | 30.92 ± 8.70 | 50.38 ± 6.46 | 0.01 | 9.10 30.60 | 19.27 |
| Right half-kneeling twist | 31.88 ± 10.05 | 55.72 ± 8.91 | 0.01 | 13.60 34.45 | 23.72 |
| Left half-kneeling twist | 35.28 ± 9.52 | 55.62 ± 7.27 | 0.01 | 11.50 31.40 | 19.97 |
| Libra performance index | |||||
| Frontal plane trunk stability | 23.26 ± 5.47 | 14.15 ± 2.78 | 0.01 | −12.18 −5.61 | −9.94 |
| Sagittal plane trunk stability | 17.92 ± 5.16 | 12.17 ± 3.75 | 0.01 | −9.72 −3.30 | −5.33 |
| Trunk isometric strength, seconds | |||||
| Extensor muscles | 36.50 ± 29.76 | 63.75 ± 32.64 | 0.01 | 12.05 41.50 | 25.75 |
| Abdominal muscles | 33.62 ±17.92 | 55.00 ± 22.44 | 0.01 | 13.00 32.00 | 21.50 |
| Right lateral muscles | 20.37 ± 12.50 | 39.87 ± 12.57 | 0.01 | 6.50 31.00 | 20.75 |
| Left lateral muscles | 25.87 ± 9.70 | 42.62 ± 12.30 | 0.01 | 8.00 28.50 | 14.25 |
| Variables | Baseline Mean ± SD | Post-AST Mean ± SD | p-Value * | 95% CI Lower Upper | r |
|---|---|---|---|---|---|
| Sit-and-reach test, centimeters | 38.28 ± 10.75 | 54.50 ± 9.03 | 0.01 | 8.50 26.40 | 16.00 |
| AROM hip, degrees | |||||
| Right external rotation | 41.48 ± 2.43 | 54.76 ± 4.49 | 0.01 | 9.00 18.00 | 13.17 |
| Right internal rotation | 31.96 ± 2.67 | 42.13 ± 4.38 | 0.01 | 6.60 14.50 | 10.12 |
| Left external rotation | 41.42 ± 3.89 | 52.75 ± 2.87 | 0.01 | 7.45 15.35 | 10.87 |
| Left internal rotation | 31.88 ± 3.40 | 40.81 ± 5.83 | 0.01 | 3.45 14.40 | 9.20 |
| Variables | Baseline Mean ± SD | Post-AST Mean ± SD | p-Value * | 95% CI Lower Upper | r |
|---|---|---|---|---|---|
| Libra SDG test, index | |||||
| No constraint | 15.86 ± 1.20 | 13.88 ± 1.97 | 0.01 | −3.11 −1.03 | −1.74 |
| Straight head | 15.54 ± 1.92 | 12.54 ± 1.98 | 0.01 | −4.93 −1.55 | −3.11 |
| Closed eyes | 15.34 ± 1.68 | 11.94 ± 2.09 | 0.01 | −5.97 −0.95 | −3.39 |
| Y Balance test, centimeters | |||||
| Right | 70.00 ± 11.32 | 96.12 ± 8.28 | 0.01 | 12.50 42.50 | 27.25 |
| Left | 67.25 ± 10.09 | 95.87 ± 7.51 | 0.01 | 17.00 41.50 | 27.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carretti, G.; Spano, F.; Sgambati, E.; Manetti, M.; Marini, M. Adapted Training to Boost Upper Body Sensorimotor Control and Daily Living Functionality in Visually Impaired Baseball Players. Medicina 2024, 60, 1136. https://doi.org/10.3390/medicina60071136
Carretti G, Spano F, Sgambati E, Manetti M, Marini M. Adapted Training to Boost Upper Body Sensorimotor Control and Daily Living Functionality in Visually Impaired Baseball Players. Medicina. 2024; 60(7):1136. https://doi.org/10.3390/medicina60071136
Chicago/Turabian StyleCarretti, Giuditta, Francesca Spano, Eleonora Sgambati, Mirko Manetti, and Mirca Marini. 2024. "Adapted Training to Boost Upper Body Sensorimotor Control and Daily Living Functionality in Visually Impaired Baseball Players" Medicina 60, no. 7: 1136. https://doi.org/10.3390/medicina60071136







