The Complex Role of Mast Cells in Head and Neck Squamous Cell Carcinoma: A Systematic Review
Abstract
1. Introduction
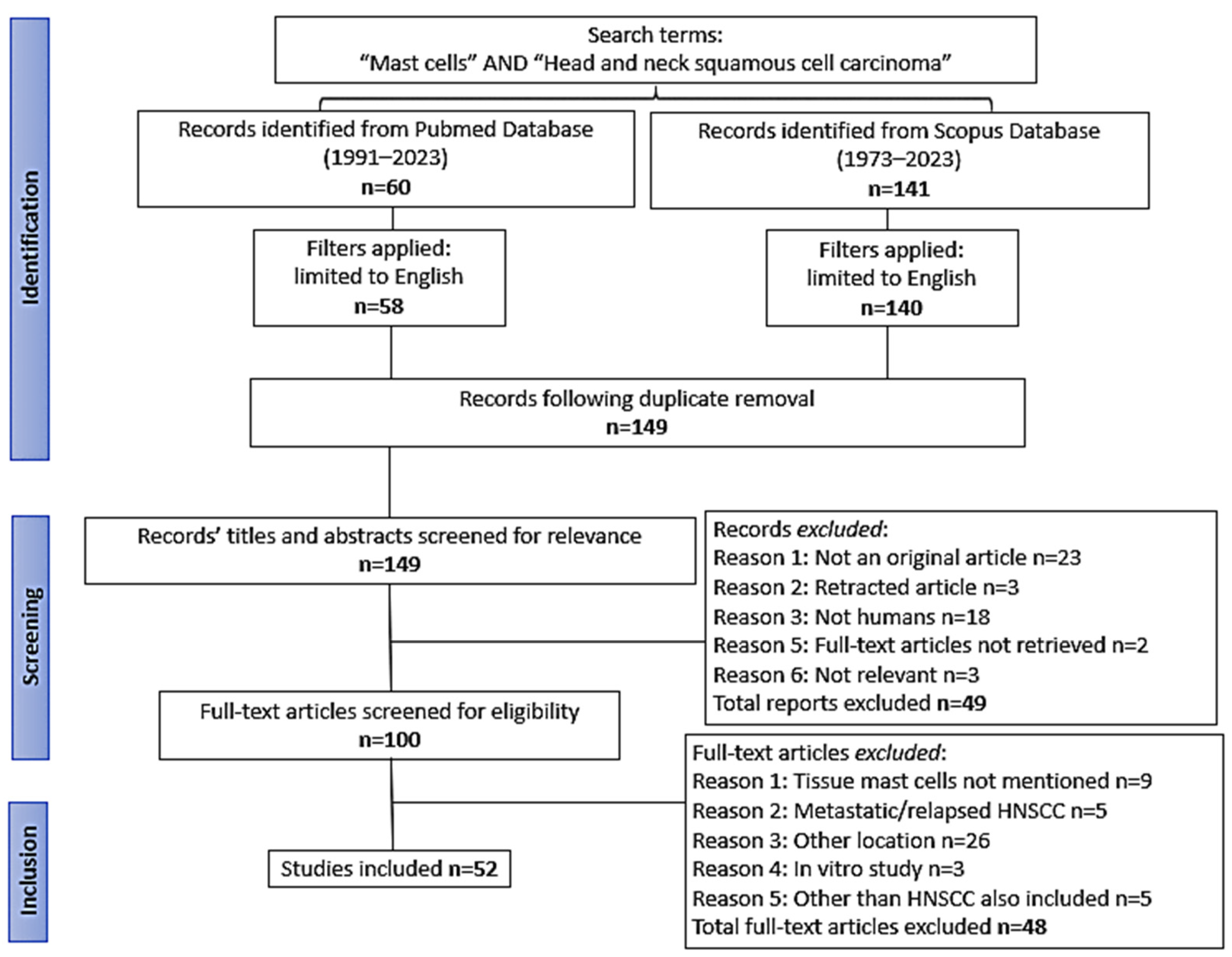
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection and Data Extraction
3. Results
3.1. General Data of the Studies
| A/A | First Author, Year [Ref. No.] | HNSCC Anatomical Site | Method | Reported Gene Alteration or Signature | Main Result Regarding Mast Cells |
|---|---|---|---|---|---|
| 1 | Zhao C, 2022 [24] | HNSCC | MOL | MRGBP | High MRGBP was associated with MCs and adverse prognosis. |
| 2 | Zhang X, 2022 [25] | HNSCC | MOL/database repository | CBX3 | CBX3 expression was negatively correlated with MCs. |
| 3 | Zhang S, 2022 [26] | HNSCC | MOL/database repository | Risk model established by the incorporation of 8 genes related to CD8+ T cell infiltration | High-risk group was characterized by a lower immune score and abundant aMCs and presented with adverse prognosis. |
| 4 | Han S, 2021 [27] | HNSCC | MOL/database repository | Risk model established by the incorporation of 191 genes related to SUVmax on 18F-FDG PET/CT | High-SUV group was characterized by a lower immune score and weak MC density and presented with adverse prognosis. |
| 5 | Li J, 2023 [28] | HNSCC | MOL/database repository | HOXB9 | Low HOXB9 expression was significantly associated with an increase in the proportion of MCs. |
| 6 | Peng C, 2023 [29] | HNSCC | MOL/database repository | GAPDH | Elevated GAPDH expression hindered communication between pDC and MCs. |
| 7 | Sawatsubashi M, 2000 [30] | Larynx | IHC | ND | VEGF staining in squamous cell carcinomas was associated with MC count. Laryngeal cancer cells and MCs may control the angiogenic response by releasing VEGF. |
| 8 | Featherston T, 2017 [31] | Oral | IF | ND | Cathepsin G was localized to the tryptase+ phenotypic MCs within the peri-tumoral stroma. |
| 9 | Chang SR, 2023 [32] | HNSCC | MOL/database repository | Immune checkpoint-related genes | Decreased aMCs were common in tumors compared to normal tissues and found in advanced oral squamous cell carcinoma. |
| 10 | Wang P, 2023 [33] | HNSCC | MOL/database repository | PGK1 | PGK1 was negatively correlated with the level of MC infiltration. |
| 11 | Hess AK, 2017 [34] | Oropharynx, hypopharynx | MOL | miRNA array and immune expression panel | There was no correlation between miR-146a/miR-155 expression and MC infiltration. |
| 12 | Rhee JK, 2023 [35] | Oral | MOL/database repository | CIBERSORTx gene expression profile which quantified the composition of 10 cell subtypes | MC density did not show obvious statistical differences between the tumor tissues and the normal surgical margins. |
| 13 | Barth PJ, 2004 [36] | Oral cavity, pharynx, larynx | IHC | ND | The number of tissue MCs was significantly increased in carcinomas compared to tumor-free mucosa. |
| 14 | Cosoroabă RM, 2022 [37] | HNSCC | IHC | ND | MC density was significantly higher in carcinoma compared to dysplastic lesions and control cases. |
| 15 | Jonsson EL, 2012 [38] | Oral, oropharynx, hypopharynx | IHC | ND | Pre- and post-RT tumors showed an abundance of MCs compared to the corresponding normal tissues. |
| 16 | Zhou D, 2021 [39] | HNSCC | MOL/database repository | Immune-related genes | HPV (+) tumors were richer in resting and aMCs and HPV (-) tumors showed higher rMCs and lower aMCs compared to normal tissues. |
| 17 | Liang B, 2020 [40] | HNSCC | MOL/database repository | A leukocyte gene signature matrix (LM22) | rMCs were significantly lower in advanced T stage. The proportion of aMCs in HNSCC tissues was significantly higher than in adjacent non-cancer tissues. |
| 18 | Liu Z, 2020 [41] | Oral | MOL/database repository | FOXD2-AS1 | FOXD2-AS1 was involved in tumor progression via epithelial-to-mesenchymal transition and cell cycle regulation and was negatively associated with MCs. |
| 19 | Sun Y, 2022 [42] | Oral | MOL/database repository | DDX59-AS1 | The expression of DDX59-AS1 was negatively correlated with MCs. |
| 20 | Han Y, 2021 [43] | Oral | MOL/database repository | MFAP4 | MFAP4 gene expression showed positive correlation with MC infiltration. Higher MC infiltration correlated with better survival. |
| 21 | Chen C, 2022 [44] | Oral | MOL/database repository | SFRP1 | SFRP1 displayed a positive performance in tumor immune infiltration, especially in MCs. |
| 22 | Sobocińska J, 2022 [45] | HNSCC | MOL/database repository | ZNF418 and ZNF540 | Patients with a higher expression of ZNF418 and ZNF540 genes displayed lower levels of MCs. |
| 23 | Li C, 2023 [46] | HNSCC | MOL/database repository | MYL1 | MYL1 expression was positively correlated with MCs. |
| 24 | Jin Y, 2020 [47] | HNSCC | MOL/database repository | ND | rMCs were closely correlated with HNSCC progression. |
| 25 | Jin Y, 2023 [48] | HNSCC | MOL/database repository | Risk model established by the incorporation of circadian genes | High-risk group was characterized by abundant aMCs and was correlated with adverse prognosis, whereas abundant rMCs were associated with favorable overall survival. |
| 26 | Stasikowska-Kanicka O, 2017 [49] | Oral | IHC | ND | Oral squamous cell carcinoma with adverse prognosis exhibited significantly lower mean number of MCs. |
| 27 | Attramadal CG, 2016 [50] | Oral | MOL/database repository/IHC | c-KIT, SCF and genes encoding for MC tryptases | High-budding tumors had lower MC density and relapsed more frequently. High expression of c-KIT- and SCF-mRNA associated with better 5-year survival. |
| 28 | Almahmoudi R, 2018 [51] | Oral | IHC | ND | The extracellular MC-derived IL-17F at the tumor invasion front of oral tongue squamous cell carcinoma was associated with better disease-specific survival. |
| 29 | Ishikawa K, 2014 [52] | Oral | IHC | ND | MC density was associated with adverse prognosis and correlated significantly with IL-33 expression. |
| 30 | Yang C, 2022 [53] | HNSCC | MOL/database repository | Risk model established by the incorporation of 9 hypoxia-related lncRNAs | High-risk group was characterized by abundant activated and few rMCs and presented with adverse prognosis. |
| 31 | Chen Q, 2021 [54] | HNSCC | MOL/database repository | Risk model established by the incorporation of 6 fibrosis–hypoxia–glycolysis-related genes | High-risk group was characterized by abundant MCs and presented with adverse prognosis. |
| 32 | Li Q, 2022 [55] | HNSCC | MOL/database repository | Risk model established by the incorporation of 35 differentially expressed immune-related (Deir) lncRNAs | High-risk group was correlated with high aMCs infiltration and low infiltration of rMCs and presented with adverse prognosis. |
| 33 | Chen F, 2022 [56] | HNSCC | MOL/database repository | Risk model established by the incorporation of 4 aging-related genes | High-risk group had lower immune score than low-risk group, was characterized by abundant resting and few aMCs and presented with adverse prognosis. |
| 34 | Sun Q, 2021 [57] | HNSCC | MOL/database repository | Risk model established by the incorporation of 17 T-reg-related lncRNAs | High-risk group was characterized by abundant aMCs and presented with adverse prognosis. |
| 35 | Fan X, 2021 [58] | HNSCC | MOL/database repository | Risk model established by the incorporation of 17 ferroptosis-related genes (PR-DE-FRGs) | High-risk group was characterized by a lower immune score and weak MC density and presented with adverse prognosis. |
| 36 | Ding Z, 2021 [59] | HNSCC | MOL/database repository | Risk model established by the incorporation of 24 hypoxia-related genes | High-risk group was characterized by abundant activated and few rMCs and presented with adverse prognosis. |
| 37 | Fan X, 2023 [60] | HNSCC | MOL/database repository | Risk model established by the incorporation of 6 endoplasmic reticulum stress-related genes | High-risk group was characterized by abundant aMCs and presented with adverse prognosis. |
| 38 | Ding Y, 2023 [61] | HNSCC | MOL/database repository | Risk model established by the incorporation of 9 immune infiltration-related genes | High-risk group was characterized by increased aMC infiltration and presented with adverse prognosis. |
| 39 | Cai Z, 2022 [62] | HNSCC | MOL/database repository/FISH | Prognostic MC signature comprising 9 genes | High-risk group was characterized by low immune infiltration including MCs and presented with adverse prognosis. |
| 40 | Dong L, 2023 [63] | Oral | MOL/database repository | Risk model established by the incorporation of 8 CAF-related genes | High-risk group was characterized by high infiltration of aMCs and low infiltration of rMCs and presented with adverse prognosis. |
| 41 | Yuan Z, 2022 [64] | HNSCC | MOL/database repository | Risk model established by the incorporation of 22 fatty acid metabolism (FAM) genes | High-risk group was characterized by high infiltration of aMCs and presented with adverse prognosis. |
| 42 | Wang E, 2021 [65] | HNSCC | MOL/database repository | Risk model based on m6A/m5C/m1A-related long non-coding RNAs (lncRNAs) | High-risk group was correlated with high aMCs infiltration and presented with adverse prognosis. |
| 43 | Sun Q, 2023 [66] | HNSCC | MOL | Risk model established by the incorporation of 39 cuproptosis-related lncRNAs | High-risk group was characterized by lower MCs and presented with adverse prognosis. |
| 44 | Zhou S, 2022 [67] | Larynx | MOL | Risk model established by the incorporation of 12 microRNA pairs | High-risk group was characterized by higher aMCs proportion and presented with adverse prognosis. |
| 45 | Tao ZY, 2024 [68] * | Oral, pharynx | MOL/database repository | Risk model established by the incorporation of 4 neural-related genes | High-risk group was characterized by high infiltration of rMCs and weak infiltration of aMCs and presented with adverse prognosis. |
| 46 | Li YJ, 2022 [69] | HNSCC | MOL/database repository | Risk model established by the incorporation of 10 cuproptosis-related lncRNAs | High-risk group had lower immune score than low-risk group, was characterized by abundant activated and few rMCs and presented with adverse prognosis. |
| 47 | Wu L, 2023 [70] | Oral | MOL/database repository | CAF-related risk model based on TISCH2-single-cell RNA sequencing analysis | MCs were closely correlated with the CAF-based periodontal disease-related risk model. |
| 48 | Huang J, 2021 [71] | Oral, Larynx | MOL/database repository | FOXD1 | The FOXD1 high-expression group was significantly associated with the proportion of aMCs and with adverse prognosis. |
| 49 | Cui L, 2020 [72] | HNSCC | MOL/database repository | MTHFD2 | Increased MTHFD2 expression was positively correlated with MC activation. |
| 50 | Liu Y, 2021 [73] | Oral | MOL/database repository | SQLE | aMCs were negatively correlated with SQLE mRNA expression. |
| 51 | Chu M, 2022 [74] | HNSCC | MOL/database repository | SLC2A3 | High SLC2A3 expression was associated with MC infiltration and adverse prognosis. |
| 52 | Lin YH, 2022 [75] | HNSCC | MOL/database repository | FCGBP | The FCGBP mRNA level positively correlated with MC infiltration rates. |
3.2. Site-Specific MC Infiltration Among Normal and Malignant Tissues
3.3. Gene Alterations Associated With MCs in HNSCC
3.4. Prognostic Role of MCs in HNSCC
3.5. Prognostic Gene Signatures Associated With MC Density
4. Discussion
4.1. MCs in TME
4.2. MCs in Normal Tissues and HNSCC
4.3. Genomic Alterations and MC Recruitment
4.4. Prognostic Impact of MCs
4.5. Therapeutic Implications of MCs in HNSCC
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Farah, C.S. Molecular landscape of head and neck cancer and implications for therapy. Ann. Transl. Med. 2021, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Aponte-López, A.; Fuentes-Pananá, E.M.; Cortes-Muñoz, D.; Muñoz-Cruz, S. Mast Cell, the Neglected Member of the Tumor Microenvironment: Role in Breast Cancer. J. Immunol. Res. 2018, 2018, 2584243. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; O’Dowd, G.; Woodford, P. Wheater’s Functional Histology—Inkling Enhanced E-Book: Wheater’s Functional Histology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mescher, A. Junqueira’s Basic Histology: Text and Atlas, 15th ed.; McGraw Hill LLC: New York, NY, USA, 2018. [Google Scholar]
- Delves, P.J.; Roitt, I.M. Encyclopedia of Immunology; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Cruse, J.M.; Lewis, R.E. Atlas of Immunology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Tete, S.; Tripodi, D.; Rosati, M.; Conti, F.; Maccauro, G.; Saggini, A.; Salini, V.; Cianchetti, E.; Caraffa, A.; Antinolfi, P.; et al. Role of mast cells in innate and adaptive immunity. J. Biol. Regul. Homeost. Agents 2012, 26, 193–201. [Google Scholar] [PubMed]
- Gilfillan, A.M.; Austin, S.J.; Metcalfe, D.D. Mast cell biology: Introduction and overview. Adv. Exp. Med. Biol. 2011, 716, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Marieb, E.N.; Hoehn, K.N. Human Anatomy & Physiology; Pearson Education: London, UK, 2015. [Google Scholar]
- Saluja, R.; Metz, M.; Maurer, M. Role and relevance of mast cells in fungal infections. Front. Immunol. 2012, 3, 146. [Google Scholar] [CrossRef] [PubMed]
- Piliponsky, A.M.; Acharya, M.; Shubin, N.J. Mast Cells in Viral, Bacterial, and Fungal Infection Immunity. Int. J. Mol. Sci. 2019, 20, 2851. [Google Scholar] [CrossRef] [PubMed]
- Frossi, B.; Mion, F.; Tripodo, C.; Colombo, M.P.; Pucillo, C.E. Rheostatic Functions of Mast Cells in the Control of Innate and Adaptive Immune Responses. Trends Immunol. 2017, 38, 648–656. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast Cell Function: A New Vision of an Old Cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef] [PubMed]
- Middel, P.; Reich, K.; Polzien, F.; Blaschke, V.; Hemmerlein, B.; Herms, J.; Korabiowska, M.; Radzun, H.J. Interleukin 16 expression and phenotype of interleukin 16 producing cells in Crohn’s disease. Gut 2001, 49, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Erbagci, Z.; Erkiliç, S. Can smoking and/or occupational UV exposure have any role in the development of the morpheaform basal cell carcinoma? A critical role for peritumoral mast cells. Int. J. Dermatol. 2002, 41, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Pesci, A.; Rossi, G.A.; Bertorelli, G.; Aufiero, A.; Zanon, P.; Olivieri, D. Mast cells in the airway lumen and bronchial mucosa of patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 1994, 149, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wei, C.; Chen, X.; Li, P. MRGBP: A New Factor for Diagnosis and Prediction of Head and Neck Squamous Cell Carcinoma. BioMed Res. Int. 2022, 2022, 7281120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, W.; Zhang, Y.; Liu, Z. CBX3 is a Prognostic Biomarker Correlated with ATR Activation and Immune Infiltration in Head and Neck Squamous Cell Carcinoma. Int. J. Gen. Med. 2022, 15, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Zhang, J. 8-Gene signature related to CD8(+) T cell infiltration by integrating single-cell and bulk RNA-sequencing in head and neck squamous cell carcinoma. Front. Genet. 2022, 13, 938611. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Oh, J.S.; Kim, J.S. Immune microenvironment of the gene signature reflecting the standardised uptake value on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in head and neck squamous cell carcinoma. Ann. Nucl. Med. 2021, 35, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ran, H.; Zeng, X.; Yang, D.; Zeng, X.; Zhang, P. Identification of HOXB9 based on comprehensive bioinformatics analysis for predicting prognosis of head and neck squamous cell carcinoma. Medicine 2023, 102, e35035. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ye, H.; Yi, Z. GAPDH: Unveiling its impact as a key hypoxia-related player in head and neck squamous cell carcinoma tumor progression, prognosis, and therapeutic potential. Am. J. Cancer Res. 2023, 13, 5846–5867. [Google Scholar] [PubMed]
- Sawatsubashi, M.; Yamada, T.; Fukushima, N.; Mizokami, H.; Tokunaga, O.; Shin, T. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch. 2000, 436, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Featherston, T.; Marsh, R.W.; van Schaijik, B.; Brasch, H.D.; Tan, S.T.; Itinteang, T. Expression and Localization of Cathepsins B, D, and G in Two Cancer Stem Cell Subpopulations in Moderately Differentiated Oral Tongue Squamous Cell Carcinoma. Front. Med. 2017, 4, 100. [Google Scholar] [CrossRef]
- Chang, S.-R.; Chou, C.-H.; Liu, C.-J.; Lin, Y.-C.; Tu, H.-F.; Chang, K.-W.; Lin, S.-C. The Concordant Disruption of B7/CD28 Immune Regulators Predicts the Prognosis of Oral Carcinomas. Int. J. Mol. Sci. 2023, 24, 5931. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.Y.; Xu, Y.L.; Zhang, C.Y.; Wang, K.; Wang, Q. Phosphoglycerate-kinase-1 Is a Potential Prognostic Biomarker in HNSCC and Correlates With Immune Cell Infiltration. Cancer Genom. Proteom. 2023, 20, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.K.; Müer, A.; Mairinger, F.D.; Weichert, W.; Stenzinger, A.; Hummel, M.; Budach, V.; Tinhofer, I. MiR-200b and miR-155 as predictive biomarkers for the efficacy of chemoradiation in locally advanced head and neck squamous cell carcinoma. Eur. J. Cancer 2017, 77, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.K. Distinct cellular composition between normal surgical margins and tumor tissues in oral squamous cell carcinoma. Genes Genom. 2023, 45, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.J.; Schenck zu Schweinsberg, T.; Ramaswamy, A.; Moll, R. CD34+ fibrocytes, alpha-smooth muscle antigen-positive myofibroblasts, and CD117 expression in the stroma of invasive squamous cell carcinomas of the oral cavity, pharynx, and larynx. Virchows Arch. Int. J. Pathol. 2004, 444, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Cosoroabă, R.M.; Gaje, N.P.; Ceauşu, A.R.; Dumitru, C.; Todor, L.; Popovici, R.A.; Porumb, A.; Domocoş, D.; Miron, M.I. The mast cell reaction in premalignant and malignant lesions of the head and neck. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2022, 63, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, E.L.; Nylander, K.; Hallén, L.; Laurell, G. Effect of radiotherapy on expression of hyaluronan and EGFR and presence of mast cells in squamous cell carcinoma of the head and neck. Oncol. Lett. 2012, 4, 1177–1182. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, J.; Wang, J.; Liu, X. Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of HNSCC with or without HPV infection. Am. J. Transl. Res. 2021, 13, 2163–2180. [Google Scholar] [PubMed]
- Liang, B.; Tao, Y.; Wang, T. Profiles of immune cell infiltration in head and neck squamous carcinoma. Biosci. Rep. 2020, 40, BSR20192724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, W.; Lin, C.; Wang, X.; Zhang, X.; Zhang, Y.; Yang, R.; Chen, W.; Cao, W. Dysregulation of FOXD2-AS1 promotes cell proliferation and migration and predicts poor prognosis in oral squamous cell carcinoma: A study based on TCGA data. Aging 2020, 13, 2379–2396. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.; Sun, J.; Bi, W.; Li, R.; Wu, X.; Li, N.; Song, L.; Yang, F.; Yu, Y. DDX59-AS1 is a prognostic biomarker and correlated with immune infiltrates in OSCC. Front. Genet. 2022, 13, 892727. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xia, K.; Su, T. Exploration of the Important Role of Microfibril-Associated Protein 4 Gene in Oral Squamous Cell Carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e931238. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Liu, Y.; Hang, L.; Yang, J. Expression of Tumor Suppressor SFRP1 Predicts Biological Behaviors and Prognosis: A Potential Target for Oral Squamous Cell Carcinoma. Biomolecules 2022, 12, 1034. [Google Scholar] [CrossRef] [PubMed]
- Sobocińska, J.; Nowakowska, J.; Molenda, S.; Olechnowicz, A.; Kozłowska-Masłoń, J.; Kazimierczak, U.; Machnik, M.; Oleksiewicz, U.; Teresiak, A.; Lamperska, K.; et al. Zinc Finger Proteins in Head and Neck Squamous Cell Carcinomas: ZNF540 May Serve as a Biomarker. Curr. Oncol. 2022, 29, 9896–9915. [Google Scholar] [CrossRef]
- Li, C.; Guan, R.; Li, W.; Wei, D.; Cao, S.; Chang, F.; Wei, Q.; Wei, R.; Chen, L.; Xu, C.; et al. Analysis of myosin genes in HNSCC and identify MYL1 as a specific poor prognostic biomarker, promotes tumor metastasis and correlates with tumor immune infiltration in HNSCC. BMC Cancer 2023, 23, 840. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qin, X. Profiles of immune cell infiltration and their clinical significance in head and neck squamous cell carcinoma. Int. Immunopharmacol. 2020, 82, 106364. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Z.; Huang, S.; Liu, C.; Wu, X.; Wang, H. Identify and validate circadian regulators as potential prognostic markers and immune infiltrates in head and neck squamous cell carcinoma. Sci. Rep. 2023, 13, 19939. [Google Scholar] [CrossRef] [PubMed]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. Association of infiltrating cells with microvessel density in oral squamous cell carcinoma. Pol. J. Pathol. Off. J. Pol. Soc. Pathol. 2017, 68, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Attramadal, C.G.; Kumar, S.; Gao, J.; Boysen, M.E.; Halstensen, T.S.; Bryne, M. Low Mast Cell Density Predicts Poor Prognosis in Oral Squamous Cell Carcinoma and Reduces Survival in Head and Neck Squamous Cell Carcinoma. Anticancer. Res. 2016, 36, 5499–5506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almahmoudi, R.; Salem, A.; Sieviläinen, M.; Sundquist, E.; Almangush, A.; Toppila-Salmi, S.; Paavonen, T.; Salo, T.; Al-Samadi, A. Extracellular interleukin-17F has a protective effect in oral tongue squamous cell carcinoma. Head Neck 2018, 40, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Yagi-Nakanishi, S.; Nakanishi, Y.; Kondo, S.; Tsuji, A.; Endo, K.; Wakisaka, N.; Murono, S.; Yoshizaki, T. Expression of interleukin-33 is correlated with poor prognosis of patients with squamous cell carcinoma of the tongue. Auris Nasus Larynx 2014, 41, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zheng, X. Identification of a Hypoxia-Related lncRNA Biomarker Signature for Head and Neck Squamous Cell Carcinoma. J. Oncol. 2022, 2022, 6775496. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chu, L.; Li, X.; Li, H.; Zhang, Y.; Cao, Q.; Zhuang, Q. Investigation of an FGFR-Signaling-Related Prognostic Model and Immune Landscape in Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 801715. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, Z.; Shen, Y.; Deng, H.; Shen, Y.; Wang, J.; Zhan, G.; Zhou, C. Identification of immune-related lncRNA panel for predicting immune checkpoint blockade and prognosis in head and neck squamous cell carcinoma. J. Clin. Lab. Anal. 2022, 36, e24484. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gong, X.; Xia, M.; Yu, F.; Wu, J.; Yu, C.; Li, J. The Aging-Related Prognostic Signature Reveals the Landscape of the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 857994. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Y.; Yang, X.; Wu, X.; Liu, Z.; Mou, Y.; Song, X. Identification and Validation of 17-lncRNA Related to Regulatory T Cell Heterogeneity as a Prognostic Signature for Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 782216. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ou, Y.; Liu, H.; Zhan, L.; Zhu, X.; Cheng, M.; Li, Q.; Yin, D.; Liao, L. A Ferroptosis-Related Prognostic Signature Based on Antitumor Immunity and Tumor Protein p53 Mutation Exploration for Guiding Treatment in Patients With Head and Neck Squamous Cell Carcinoma. Front. Genet. 2021, 12, 732211. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, H.; Yu, D. Development and validation of a hypoxia-related gene pair signature to predict overall survival in head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3973–3983. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, X.; Guo, N.; Gao, X.; Zhao, Y. Development of an endoplasmic reticulum stress-related signature with potential implications in prognosis and immunotherapy in head and neck squamous cell carcinoma. Diagn. Pathol. 2023, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chu, L.; Cao, Q.; Lei, H.; Li, X.; Zhuang, Q. A meta-validated immune infiltration-related gene model predicts prognosis and immunotherapy sensitivity in HNSCC. BMC Cancer 2023, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Tang, B.; Chen, L.; Lei, W. Mast cell marker gene signature in head and neck squamous cell carcinoma. BMC Cancer 2022, 22, 577. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Sun, Q.; Song, F.; Song, X.; Lu, C.; Li, Y.; Song, X. Identification and verification of eight cancer-associated fibroblasts related genes as a prognostic signature for head and neck squamous cell carcinoma. Heliyon 2023, 9, e14003. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Huang, J.; Teh, B.M.; Hu, S.; Hu, Y.; Shen, Y. Exploration of a predictive model based on genes associated with fatty acid metabolism and clinical treatment for head and neck squamous cell carcinoma. J. Clin. Lab. Anal. 2022, 36, e24722. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Li, Y.; Ming, R.; Wei, J.; Du, P.; Zhou, P.; Zong, S.; Xiao, H. The Prognostic Value and Immune Landscapes of a m(6)A/m(5)C/m(1)A-Related LncRNAs Signature in Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 718974. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Qin, X.; Zhao, J.; Gao, T.; Xu, Y.; Chen, G.; Bai, G.; Guo, Z.; Liu, J. Cuproptosis-related LncRNA signatures as a prognostic model for head and neck squamous cell carcinoma. Apoptosis 2023, 28, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Meng, Q.; Wang, Z. Prognostic value of a microRNA-pair signature in laryngeal squamous cell carcinoma patients. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 4451–4460. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.Y.; Yang, W.F.; Zhu, W.Y.; Wang, L.L.; Li, K.Y.; Guan, X.Y.; Su, Y.X. A neural-related gene risk score for head and neck squamous cell carcinoma. Oral Dis. 2024, 30, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Li, H.Y.; Zhang, Q.; Wei, S.L. The prognostic value and immune landscape of a cuproptosis-related lncRNA signature in head and neck squamous cell carcinoma. Front. Genet. 2022, 13, 942785. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, J.; She, P.; Kong, F.; Mao, Z.; Wang, S. Single-cell RNA sequencing and traditional RNA sequencing reveals the role of cancer-associated fibroblasts in oral squamous cell carcinoma cohort. Front. Oncol. 2023, 13, 1195520. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, B.; Wang, T. FOXD1 expression in head and neck squamous carcinoma: A study based on TCGA, GEO and meta-analysis. Biosci. Rep. 2021, 41, BSR20210158. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Chen, H.; Zhao, X. The Prognostic Significance of Immune-Related Metabolic Enzyme MTHFD2 in Head and Neck Squamous Cell Carcinoma. Diagnostics 2020, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, L.; Liu, W. High SQLE Expression and Gene Amplification Correlates with Poor Prognosis in Head and Neck Squamous Cell Carcinoma. Cancer Manag. Res. 2021, 13, 4709–4723. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Zheng, K.; Li, X.; Luo, Z.; Yang, X.; Wei, C. Comprehensive Analysis of the Role of SLC2A3 on Prognosis and Immune Infiltration in Head and Neck Squamous Cell Carcinoma. Anal. Cell. Pathol. 2022, 2022, 2371057. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Yang, Y.F.; Shiue, Y.L. Multi-Omics Analyses to Identify FCGBP as a Potential Predictor in Head and Neck Squamous Cell Carcinoma. Diagnostics 2022, 12, 1178. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. New insights into the role of mast cells as a therapeutic target in cancer through the blockade of immune checkpoint inhibitors. Front. Med. 2024, 11, 1373230. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef] [PubMed]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Et. Biophys. Acta 2009, 1796, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, T.R.; Monteiro, M.R.; Murphy, G.F. Mast cells and dendritic cells in basal cell carcinoma stroma. Dermatol. Surg. 2000, 26, 200–203, discussion 203–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhou, P.; Tang, T.; Si, R.; Ji, Y.; Hu, X.; Li, A.; Jiang, Y. Levels of circulating mast cell progenitors and tumour-infiltrating mast cells in patients with colorectal cancer. Oncol. Rep. 2022, 47, 89. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Wei, J.; Meng, L.; Zhang, Q.; Qu, C.; Xin, Y.; Jiang, X. Molecular mechanisms underlying increased radiosensitivity in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Segura-Villalobos, D.; Ramírez-Moreno, I.G.; Martínez-Aguilar, M.; Ibarra-Sánchez, A.; Muñoz-Bello, J.O.; Anaya-Rubio, I.; Padilla, A.; Macías-Silva, M.; Lizano, M.; González-Espinosa, C. Mast Cell-Tumor Interactions: Molecular Mechanisms of Recruitment, Intratumoral Communication and Potential Therapeutic Targets for Tumor Growth. Cells 2022, 11, 349. [Google Scholar] [CrossRef]
- Ammendola, M.; Leporini, C.; Marech, I.; Gadaleta, C.D.; Scognamillo, G.; Sacco, R.; Sammarco, G.; De Sarro, G.; Russo, E.; Ranieri, G. Targeting mast cells tryptase in tumor microenvironment: A potential antiangiogenetic strategy. Biomed. Res. Int. 2014, 2014, 154702. [Google Scholar] [CrossRef] [PubMed]
- Piconese, S.; Gri, G.; Tripodo, C.; Musio, S.; Gorzanelli, A.; Frossi, B.; Pedotti, R.; Pucillo, C.E.; Colombo, M.P. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 2009, 114, 2639–2648. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, Y.; Wang, X.; Chen, N.; Mao, F.; Teng, Y.; Wang, T.; Peng, L.; Zhang, J.; Cheng, P.; et al. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway. J. Immunother. Cancer 2019, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, P.; Kling, A.; Schulz, X.; Perske, C.; Schliephake, H.; Hemmerlein, B. High mast cell density indicates a longer overall survival in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 14677. [Google Scholar] [CrossRef] [PubMed]
- Carroll-Portillo, A.; Cannon, J.L.; te Riet, J.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Birbrair, A. Tumor Microenvironment: Molecular Players—Part B; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef] [PubMed]
- Kabiraj, A.; Jaiswal, R.; Singh, A.; Gupta, J.; Singh, A.; Samadi, F.M. Immunohistochemical evaluation of tumor angiogenesis and the role of mast cells in oral squamous cell carcinoma. J. Cancer Res. Ther. 2018, 14, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Singh, A.; Singh, P. Tumor associated mast cells: Biological roles and therapeutic applications. Anat. Cell Biol. 2020, 53, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Lichterman, J.N.; Reddy, S.M. Mast Cells: A New Frontier for Cancer Immunotherapy. Cells 2021, 10, 1270. [Google Scholar] [CrossRef] [PubMed]
- Majorini, M.T.; Colombo, M.P.; Lecis, D. Few, but Efficient: The Role of Mast Cells in Breast Cancer and Other Solid Tumors. Cancer Res. 2022, 82, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A.; Finlay-Jones, J.J.; Hart, P.H. Mast cells in photodamaged skin: What is their role in skin cancer? Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2006, 5, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Parizi, A.C.; Barbosa, R.L.; Parizi, J.L.; Nai, G.A. A comparison between the concentration of mast cells in squamous cell carcinomas of the skin and oral cavity. An. Bras. Dermatol. 2010, 85, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Spector, M.E.; Bellile, E.; Amlani, L.; Zarins, K.; Smith, J.; Brenner, J.C.; Rozek, L.; Nguyen, A.; Thomas, D.; McHugh, J.B.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Balermpas, P.; Rödel, F.; Rödel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int. J. Cancer 2016, 138, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zenga, J.; Awan, M.J.; Frei, A.; Petrie, E.; Sharma, G.P.; Shreenivas, A.; Shukla, M.; Himburg, H.A. Chronic stress promotes an immunologic inflammatory state and head and neck cancer growth in a humanized murine model. Head Neck 2022, 44, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Ngan, H.L.; Liu, Y.; Fong, A.Y.; Poon, P.H.Y.; Yeung, C.K.; Chan, S.S.M.; Lau, A.; Piao, W.; Li, H.; Tse, J.S.W.; et al. MAPK pathway mutations in head and neck cancer affect immune microenvironments and ErbB3 signaling. Life Sci. Alliance 2020, 3, e201900545. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, J.; Shi, Y.; Fang, X.; Tang, Z. MAPK Signaling Pathway in Oral Squamous Cell Carcinoma: Biological Function and Targeted Therapy. Cancers 2022, 14, 4625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Goto, Y.; Allevato, M.M.; Wu, V.H.; Saddawi-Konefka, R.; Gilardi, M.; Alvarado, D.; Yung, B.S.; O’Farrell, A.; Molinolo, A.A.; et al. Disruption of the HER3-PI3K-mTOR oncogenic signaling axis and PD-1 blockade as a multimodal precision immunotherapy in head and neck cancer. Nat. Commun. 2021, 12, 2383. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.; Vu, L.; Spanos, W.; Pyeon, D. The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers 2021, 13, 5206. [Google Scholar] [CrossRef] [PubMed]
- Zenga, J.; Awan, M.J.; Frei, A.; Massey, B.; Bruening, J.; Shukla, M.; Sharma, G.P.; Shreenivas, A.; Wong, S.J.; Zimmermann, M.T.; et al. Type I interferon signaling promotes radioresistance in head and neck cancer. Transl. Cancer Res. 2024, 13, 2535–2543. [Google Scholar] [CrossRef]
- Fakhry, C.; Zhang, Q.; Nguyen-Tan, P.F.; Rosenthal, D.; El-Naggar, A.; Garden, A.S.; Soulieres, D.; Trotti, A.; Avizonis, V.; Ridge, J.A.; et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A Review of HPV-Related Head and Neck Cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Massari, N.A.; Nicoud, M.B.; Medina, V.A. Histamine receptors and cancer pharmacology: An update. Br. J. Pharmacol. 2020, 177, 516–538. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Sobiepanek, A.; Gryciuk, A.; Kuryk, L.; Mazurkiewicz-Pisarek, A.; Rogalska, M.; Abraham, S.N.; Staniszewska, M. Mast Cells as a Target-A Comprehensive Review of Recent Therapeutic Approaches. Cells 2023, 12, 1187. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Wargo, J.A.; Wang, F.; Xiao, M.; Brafford, P.; Yang, X.; et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef] [PubMed]
| A/A | First Author, Year | HNSCC Anatomical Site | Genomic Alteration | Prognostic Impact of Genomic Alteration | Correlation with Mast Cells |
|---|---|---|---|---|---|
| High expression of transcription factors | |||||
| 1 | Huang, 2021 [71] | HNSCC | FOXD1 | Adverse prognosis | Positive correlation with aMC infiltration |
| 2 | Zhao, 2022 [24] | HNSCC | MRGBP | Adverse prognosis | Positive correlation with MC infiltration |
| 3 | Sobocińska, 2022 [45] | HNSCC | ZNF418 and ZNF540 | Favorable prognosis | Negative correlation with MC infiltration |
| High expression of genes involved in cellular metabolic processes | |||||
| 4 | Cui, 2020 [72] | HNSCC | MTHFD2 | Adverse prognosis | Positive correlation with aMC infiltration |
| 5 | Liu, 2021 [73] | Oral | SQLE | Adverse prognosis | Positive correlation with rMC infiltration |
| 6 | Chu, 2022 [74] | HNSCC | SLC2A3 | Adverse prognosis | Positive correlation with MC infiltration |
| 7 | Lin, 2022 [75] | HNSCC | FCGBP | Adverse prognosis | Positive correlation with rMC infiltration |
| 8 | Wang, 2023 [33] | HNSCC | PGK1 | Adverse prognosis | Negative correlation with MC infiltration |
| 9 | Peng, 2023 [29] | HNSCC | GAPDH | NM | Hindering communication between pDC and MCs |
| High expression of genes involved in ageing | |||||
| 10 | Zhang, 2022 [25] | HNSCC | CBX3 | Adverse prognosis | Negative correlation with MC infiltration |
| 11 | Li, 2023 [28] | HNSCC | HOXB9 | Adverse prognosis | Negative correlation with MC infiltration |
| High expression of other genes | |||||
| 12 | Chen, 2022 [44] | Oral | SFRP1 | Favorable prognosis | Positive correlation with MC infiltration |
| 13 | Li, 2023 [46] | HNSCC | MYL1 | Adverse prognosis | Positive correlation with MC infiltration |
| 14 | Han, 2021 [43] | Oral | MFAP4 | Favorable prognosis | Positive correlation with MC infiltration |
| High expression of lncRNA | |||||
| 15 | Liu, 2020 [41] | Oral | FOXD2-AS1 | Adverse prognosis | Negative correlation with MC infiltration |
| 16 | Sun, 2022 [42] | Oral | DDX59-AS1 | Adverse prognosis | Negative correlation with MC infiltration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzorakoleftheraki, S.-E.; Koletsa, T. The Complex Role of Mast Cells in Head and Neck Squamous Cell Carcinoma: A Systematic Review. Medicina 2024, 60, 1173. https://doi.org/10.3390/medicina60071173
Tzorakoleftheraki S-E, Koletsa T. The Complex Role of Mast Cells in Head and Neck Squamous Cell Carcinoma: A Systematic Review. Medicina. 2024; 60(7):1173. https://doi.org/10.3390/medicina60071173
Chicago/Turabian StyleTzorakoleftheraki, Sofia-Eleni, and Triantafyllia Koletsa. 2024. "The Complex Role of Mast Cells in Head and Neck Squamous Cell Carcinoma: A Systematic Review" Medicina 60, no. 7: 1173. https://doi.org/10.3390/medicina60071173
APA StyleTzorakoleftheraki, S.-E., & Koletsa, T. (2024). The Complex Role of Mast Cells in Head and Neck Squamous Cell Carcinoma: A Systematic Review. Medicina, 60(7), 1173. https://doi.org/10.3390/medicina60071173






