Effects of Rehabilitative Exercise and Neuromuscular Electrical Stimulation on Muscle Morphology and Dynamic Balance in Individuals with Chronic Ankle Instability
Abstract
:1. Introduction
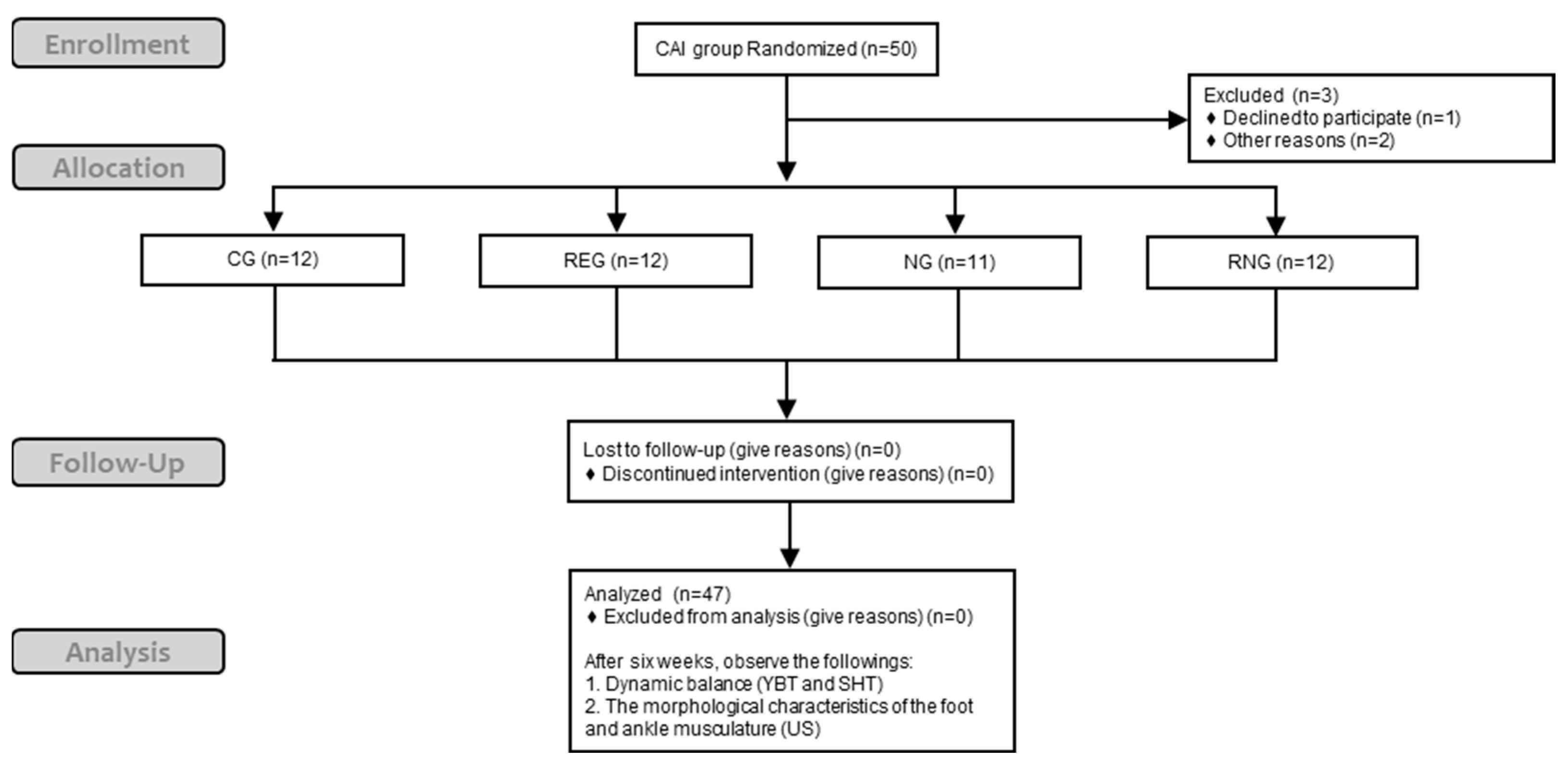
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.4. Instruments
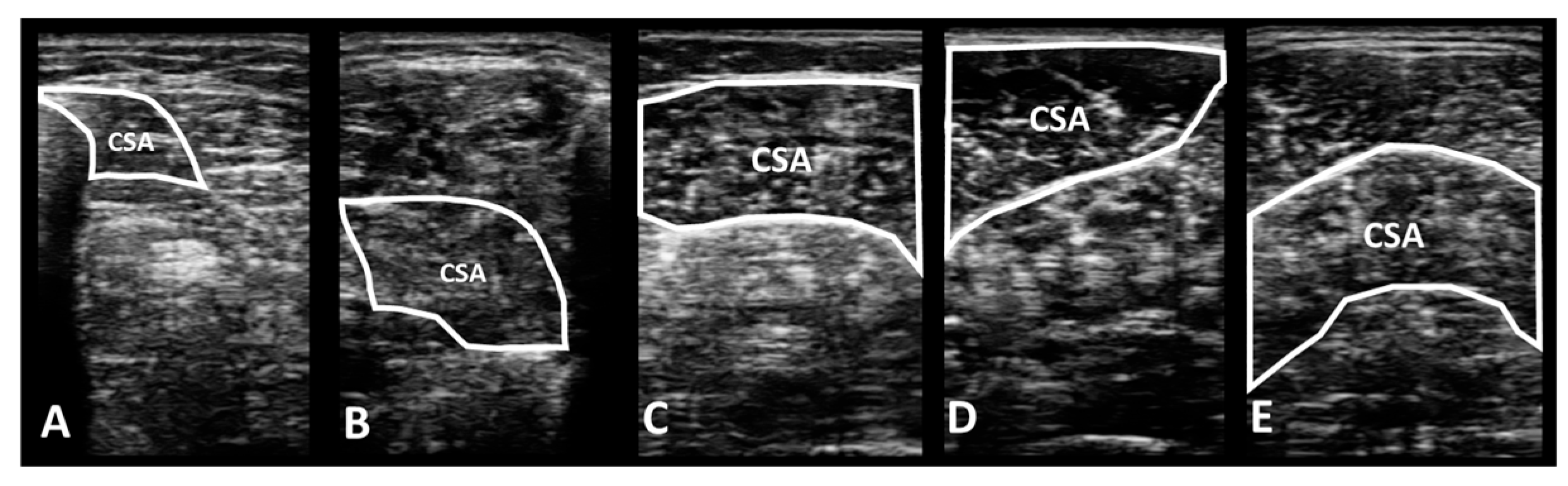
2.4.1. Portable Wireless Diagnostic Ultrasound Device
2.4.2. Dynamic Balance Test
2.5. Interventions
2.5.1. Rehabilitative Exercise
2.5.2. Neuromuscular Electrical Stimulation (NMES)
2.6. Data Processing
2.7. Statistical Analysis
3. Results
3.1. Changes in the Morphological Characteristics of the Foot and Ankle Muscle According to Each Intervention Methods
3.1.1. Deep Muscles in the Lower Leg
- Flexor digitorum longus
- Flexor hallucis longus
3.1.2. Superficial Muscles in the Lower Leg
- Gastrocnemius medial head
- Gastrocnemius lateral head
- Soleus
3.2. Changes in Dynamic Balance on Y-Balance Test and Square Hop Test between Groups According to Each Intervention Method
3.2.1. Y-Balance Test
- Anterior reach direction
- Posteromedial reach direction
- Posterolateral reach direction
- Composite score
3.2.2. Square Hop Test
4. Discussion
4.1. Changes in Muscle Morphology and Dynamic Balance According to Rehabilitative Exercise
4.2. Changes in Muscle Morphology and Dynamic Balance According to NMES
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waterman, B.R.; Owens, B.D.; Davey, S.; Zacchilli, M.A.; Belmont, P.J., Jr. The epidemiology of ankle sprains in the United States. J. Bone Jt. Surg. Am. 2010, 92, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Corbett, R.O. An updated model of chronic ankle instability. J. Athl. Train. 2019, 54, 572–588. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.M.; Kerr, Z.Y.; Marshall, S.W.; Wikstrom, E.A. Epidemiology of ankle sprains and chronic ankle instability. J. Athl. Train. 2019, 54, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Bleakley, C.; Hertel, J.; Caulfield, B.; Ryan, J.; Delahunt, E. Recovery from a first-time lateral ankle sprain and the predictors of chronic ankle instability: A prospective cohort analysis. Am. J. Sports Med. 2016, 44, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Feger, M.A.; Snell, S.; Handsfield, G.G.; Blemker, S.S.; Wombacher, E.; Fry, R.; Hart, J.M.; Saliba, S.A.; Park, J.S.; Hertel, J. Diminished foot and ankle muscle volumes in young adults with chronic ankle instability. Orthop. J. Sports Med. 2016, 4, 2325967116653719. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, E.; Docherty, C.L.; Schrader, J.; Klossner, J. The ability of 4 single-limb hopping tests to detect functional performance deficits in individuals with functional ankle instability. J. Orthop. Sports Phys. Ther. 2009, 39, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Brockett, C.L.; Chapman, G.J.J.O. Biomechanics of the ankle. Orthop. Trauma 2016, 30, 232–238. [Google Scholar] [CrossRef]
- Son, S.J.; Kim, H.; Seeley, M.K.; Hopkins, J.T. Altered walking neuromechanics in patients with chronic ankle instability. J. Athl. Train. 2019, 54, 684–697. [Google Scholar] [CrossRef]
- Chinn, L.; Dicharry, J.; Hart, J.M.; Saliba, S.; Wilder, R.; Hertel, J. Gait kinematics after taping in participants with chronic ankle instability. J. Athl. Train. 2014, 49, 322–330. [Google Scholar] [CrossRef]
- Cruz-Diaz, D.; Lomas-Vega, R.; Osuna-Pérez, M.; Contreras, F.; Martínez-Amat, A. Effects of 6 weeks of balance training on chronic ankle instability in athletes: A randomized controlled trial. Int. J. Sports Med. 2015, 36, 754–760. [Google Scholar] [CrossRef]
- Yang, Y.-R.; Mi, P.-L.; Huang, S.-F.; Chiu, S.-L.; Liu, Y.-C.; Wang, R.-Y. Effects of neuromuscular electrical stimulation on gait performance in chronic stroke with inadequate ankle control-A randomized controlled trial. PLoS ONE 2018, 13, e0208609. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, K.A.; Kakaraparthi, V.N.; Reddy, R.S.; Silvian, P.; Tedla, J.S.; Rengaramanujam, K.; Ahmad, I. Combined effects of strengthening and proprioceptive training on stability, balance, and proprioception among subjects with chronic ankle instability in different age groups: Evaluation of clinical outcome measures. Indian J. Orthop. 2021, 55, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.W.; Micheli, L.J. Training the child athlete: Physical fitness, health and injury. Br. J. Sports Med. 2011, 45, 880–885. [Google Scholar] [CrossRef]
- Cloak, R.; Nevill, A.; Day, S.; Wyon, M. Six-week combined vibration and wobble board training on balance and stability in footballers with functional ankle instability. Clin. J. Sport Med. 2013, 23, 384–391. [Google Scholar] [CrossRef]
- Linens, S.W.; Ross, S.E.; Arnold, B.L. Wobble board rehabilitation for improving balance in ankles with chronic instability. Clin. J. Sport Med. 2016, 26, 76–82. [Google Scholar] [CrossRef]
- Lee, E.; Cho, J.; Lee, S. Short-foot exercise promotes quantitative somatosensory function in ankle instability: A randomized controlled trial. Med. Sci. Monit. 2019, 25, 618. [Google Scholar] [CrossRef]
- Bok, S.-K.; Lee, T.H.; Lee, S.S. The effects of changes of ankle strength and range of motion according to aging on balance. Ann. Rehabil. Med. 2013, 37, 10. [Google Scholar] [CrossRef] [PubMed]
- Spink, M.J.; Fotoohabadi, M.R.; Wee, E.; Hill, K.D.; Lord, S.R.; Menz, H.B. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Arch. Phys. Med. Rehabil. 2011, 92, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.P.; Song, J.-E.; Wang, M.-Y.; Greendale, G.A.; Azen, S.P.; Salem, G.J. Biomechanics of the heel-raise exercise. J. Aging Phys. Act. 2005, 13, 160–171. [Google Scholar] [CrossRef]
- Fujiwara, K.; Toyama, H.; Asai, H.; Maeda, K.; Yaguchi, C. Regular heel-raise training focused on the soleus for the elderly: Evaluation of muscle thickness by ultrasound. J. Physiol. Anthropol. 2010, 29, 23–28. [Google Scholar] [CrossRef]
- Lee, G.; Kim, B.; Kim, J.; Nam, I.; Park, Y.; Shin, W.; Woo, S.; Cha, S. The effect of calf-raise exercise on gastrocnemius muscle based on other type of supports. J. Korean Soc. Integr. Med. 2014, 2, 109–116. [Google Scholar] [CrossRef]
- Kudo, S.; Sato, T.; Miyashita, T. Effect of plyometric training on the fascicle length of the gastrocnemius medialis muscle. J. Phys. Ther. Sci. 2020, 32, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Lake, D.A. Neuromuscular electrical stimulation. Sports Med. 1992, 13, 320–336. [Google Scholar] [CrossRef]
- Asakawa, Y.; Jung, J.-H.; Koh, S.-E. Neuromuscular electrical stimulation improves strength, pain and weight distribution on patients with knee instability post surgery. Phys. Ther. Rehabil. Sci. 2014, 3, 112–118. [Google Scholar] [CrossRef]
- Devrimsel, G.; Metin, Y.; Serdaroglu Beyazal, M. Short-term effects of neuromuscular electrical stimulation and ultrasound therapies on muscle architecture and functional capacity in knee osteoarthritis: A randomized study. Clin. Rehabil. 2019, 33, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-M.; Croy, T.; Hertel, J.; Saliba, S. Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: A systematic review. J. Orthop. Sports Phys. Ther. 2010, 40, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Karabay, I.; Öztürk, G.T.; Malas, F.Ü.; Kara, M.; Tiftik, T.; Ersöz, M.; Özçakar, L. Short-term effects of neuromuscular electrical stimulation on muscle architecture of the tibialis anterior and gastrocnemius in children with cerebral palsy: Preliminary results of a prospective controlled study. Am. J. Phys. Med. Rehabil. 2015, 94, 728–733. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Gribble, P.A.; Delahunt, E.; Bleakley, C.; Caulfield, B.; Docherty, C.; Fourchet, F.; Fong, D.; Hertel, J.; Hiller, C.; Kaminski, T.; et al. Selection criteria for patients with chronic ankle instability in controlled research: A position statement of the International Ankle Consortium. J. Orthop. Sports Phys. Ther. 2013, 43, 585–591. [Google Scholar] [CrossRef]
- Chang, W.-D.; Chen, S.; Tsou, Y.-A. Effects of whole-body vibration and balance training on female athletes with chronic ankle instability. J. Clin. Med. 2021, 10, 2380. [Google Scholar] [CrossRef]
- Cheon, S.; Chang, E. Inter-rater reliability of a portable ultrasound for the quadriceps and hamstrings thickness measurement in healthy adults. Exerc. Sci. 2020, 29, 71–76. [Google Scholar] [CrossRef]
- Mickle, K.J.; Nester, C.J.; Crofts, G.; Steele, J.R. Reliability of ultrasound to measure morphology of the toe flexor muscles. J. Foot Ankle Res. 2013, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Crofts, G.; Angin, S.; Mickle, K.J.; Hill, S.; Nester, C. Reliability of ultrasound for measurement of selected foot structures. Gait Posture 2014, 39, 35–39. [Google Scholar] [CrossRef]
- Bandholm, T.; Sonne-Holm, S.; Thomsen, C.; Bencke, J.; Pedersen, S.A.; Jensen, B.R. Calf muscle volume estimates: Implications for botulinum toxin treatment? Pediatr. Neurol. 2007, 37, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Y.; Tian, G. Ultrasound measurements of gastrocnemius muscle thickness in older people with sarcopenia. Clin. Interv. Aging 2018, 13, 2193. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, A.; Cromwell, R. Kinetic chain rehabilitation: A theoretical framework. Rehabil. Res. Pract. 2012, 2012, 853037. [Google Scholar] [CrossRef]
- Plisky, P.J.; Gorman, P.P.; Butler, R.J.; Kiesel, K.B.; Underwood, F.B.; Elkins, B. The reliability of an instrumented device for measuring components of the star excursion balance test. N. Am. J. Sports Phys. Ther. 2009, 4, 92. [Google Scholar]
- Gribble, P.A.; Hertel, J. Considerations for normalizing measures of the Star Excursion Balance Test. Meas. Phys. Educ. Exerc. 2003, 7, 89–100. [Google Scholar] [CrossRef]
- Brasileiro, J.S.; Pinto, O.M.; Ávila, M.A.; Salvini, T.F. Functional and morphological changes in the quadriceps muscle induced by eccentric training after ACL reconstruction. Braz. J. Phys. Ther. 2011, 15, 284–290. [Google Scholar] [CrossRef]
- Landin, D.; Thompson, M.; Reid, M. Actions of two bi-articular muscles of the lower extremity: A review. J. Clin. Med. Res. 2016, 8, 489. [Google Scholar] [CrossRef]
- Lepley, L.K.; Lepley, A.S.; Onate, J.A.; Grooms, D.R. Eccentric exercise to enhance neuromuscular control. Sports Health 2017, 9, 333–340. [Google Scholar] [CrossRef]
- Geremia, J.M.; Baroni, B.M.; Bini, R.R.; Lanferdini, F.J.; de Lima, A.R.; Herzog, W.; Vaz, M.A. Triceps surae muscle architecture adaptations to eccentric training. Front. Physiol. 2019, 10, 1456. [Google Scholar] [CrossRef] [PubMed]
- Boonyarom, O.; Inui, K. Atrophy and hypertrophy of skeletal muscles: Structural and functional aspects. Acta Physiol. 2006, 188, 77–89. [Google Scholar] [CrossRef] [PubMed]
- LaStayo, P.C.; Woolf, J.M.; Lewek, M.D.; Snyder-Mackler, L.; Reich, T.; Lindstedt, S.L. Eccentric muscle contractions: Their contribution to injury, prevention, rehabilitation, and sport. J. Orthop. Sports Phys. Ther. 2003, 33, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.P.; Marcus, R.L.; Dibble, L.E.; Greis, P.E.; Burks, R.T.; LaStayo, P.C. Effects of early progressive eccentric exercise on muscle structure after anterior cruciate ligament reconstruction. J. Bone Jt. Surg. Am. 2007, 89, 559–570. [Google Scholar] [CrossRef]
- Nardone, A.; Romano, C.; Schieppati, M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J. Physiol. 1989, 409, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Hedayatpour, N.; Falla, D. Physiological and neural adaptations to eccentric exercise: Mechanisms and considerations for training. Biomed. Res. Int. 2015, 2015, 193741. [Google Scholar] [CrossRef] [PubMed]
- McKeon, P.O.; Ingersoll, C.D.; Kerrigan, D.C.; Saliba, E.; Bennett, B.C.; Hertel, J. Balance training improves function and postural control in those with chronic ankle instability. Med. Sci. Sports Exerc. 2008, 40, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.M.; Burden, A.M. A 4-week wobble board exercise programme improved muscle onset latency and perceived stability in individuals with a functionally unstable ankle. Phys. Ther. Sport 2005, 6, 181–187. [Google Scholar] [CrossRef]
- Strøm, M.; Thorborg, K.; Bandholm, T.; Tang, L.; Zebis, M.; Nielsen, K.; Bencke, J. Ankle joint control during single-legged balance using common balance training devices–implications for rehabilitation strategies. Int. J. Sports Phys. Ther. 2016, 11, 388. [Google Scholar]
- De Brito Silva, P.; Oliveira, A.S.; Mrachacz-Kersting, N.; Laessoe, U.; Kersting, U.G. Strategies for equilibrium maintenance during single leg standing on a wobble board. Gait Posture 2016, 44, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.A.; Chomistek, A.K.; Kingma, J.J.; Docherty, C.L. Balance-and strength-training protocols to improve chronic ankle instability deficits, part I: Assessing clinical outcome measures. J. Athl. Train. 2018, 53, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, V.; Alizadeh, M.; Gaieni, A. The effects of six weeks strength exercises on static and dynamic balance of young male athletes. Procedia Soc. Behav. Sci. 2012, 31, 247–250. [Google Scholar] [CrossRef]
- Jung, K.-S.; Jung, J.-H.; In, T.-S.; Cho, H.-Y. Effectiveness of heel-raise-lower exercise after transcutaneous electrical nerve stimulation in patients with stroke: A randomized controlled study. J. Clin. Med. 2020, 9, 3532. [Google Scholar] [CrossRef]
- Lee, S.-M.; Cynn, H.-S.; Yoon, T.-L.; Lee, J.-H. Effects of different heel-raise-lower exercise interventions on the strength of plantarflexion, balance, and gait parameters in stroke survivors. Physiother. Theory Pract. 2017, 33, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Man, W.D.C.; Gao, W.; Higginson, I.J.; Wilcock, A.; Maddocks, M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Blazevich, A.J.; Collins, D.F.; Millet, G.Y.; Vaz, M.A.; Maffiuletti, N.A. Enhancing adaptations to neuromuscular electrical stimulation training interventions. Exerc. Sport Sci. Rev. 2021, 49, 244. [Google Scholar] [CrossRef]
- Wright, P.A.; Durham, S.; Ewins, D.J.; Swain, I.D. Neuromuscular electrical stimulation for children with cerebral palsy: A review. Arch. Dis. Child. 2012, 97, 364–371. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; Snijders, T.; Ottenbros, C.L.; Verdijk, L.B.; Van Loon, L.J. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. 2014, 210, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Functional significance of cell size in spinal motoneurons. J. Neurophysiol. 1965, 28, 560–580. [Google Scholar] [CrossRef]
- Bickel, C.S.; Gregory, C.M.; Dean, J.C. Motor unit recruitment during neuromuscular electrical stimulation: A critical appraisal. Eur. J. Appl. Physiol. 2011, 111, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Smith, K.; Rennie, M. Prevention of disuse muscle atrophy by means of electrical stimulation: Maintenance of protein synthesis. Lancet 1988, 332, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Dirks, M.L.; Verdijk, L.B.; Snijders, T.; Hansen, D.; Vranckx, P.; Burd, N.A.; Dendale, P.; Van Loon, L.J. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic men. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E614–E623. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Lapsley, J.E.; Balter, J.E.; Wolfe, P.; Eckhoff, D.G.; Kohrt, W.M. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: A randomized controlled trial. Phys. Ther. 2012, 92, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Pool, D.; Elliott, C.; Bear, N.; Donnelly, C.J.; Davis, C.; Stannage, K.; Valentine, J. Neuromuscular electrical stimulation-assisted gait increases muscle strength and volume in children with unilateral spastic cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Vanderthommen, M.; Duchateau, J. Electrical stimulation as a modality to improve performance of the neuromuscular system. Exerc. Sport Sci. Rev. 2007, 35, 180–185. [Google Scholar] [CrossRef]
- DeJong, A.F.; Mangum, L.C.; Hertel, J. Ultrasound imaging of the gluteal muscles during the Y-balance test in individuals with or without chronic ankle instability. J. Athl. Train. 2020, 55, 49–57. [Google Scholar] [CrossRef]



| Sub-Group | p Value | ||||
|---|---|---|---|---|---|
| CG | REG | NG | RNG | ||
| n | 12 | 12 | 11 | 12 | N/A |
| Sex (M/F) | 8:4 | 6:6 | 6:5 | 6:6 | N/A |
| Age (years) | 23.08 ± 1.83 | 22.58 ± 1.08 | 22.27 ± 1.19 | 24.17 ± 3.83 | 0.22 |
| Height (cm) | 173.53 ± 8.97 | 169.98 ± 8.41 | 173.95 ± 7.40 | 171.05 ± 8.64 | 0.61 |
| Weight (kg) | 73.63 ± 16.51 | 68.45 ± 16.13 | 69.19 ± 11.93 | 69.98 ± 19.58 | 0.87 |
| CAIT | 16.42 ± 2.54 | 15.75 ± 6.72 | 15.82 ± 3.76 | 13.92 ± 3.60 | 0.55 |
| IdFAI | 22.42 ± 5.50 | 23.58 ± 8.59 | 25.91 ± 4.85 | 23.50 ± 6.24 | 0.63 |
| Exercise | Sessions per Week | Volume | Rest in between Trials |
|---|---|---|---|
| HRE | 3 sessions | 15 rep × 3 sets | 30 s |
| WBE | 3 sessions | 40 sec × 3 sets | 1 min |
| Muscle | Morphology | Group | Baseline | Post-Intervention | Cohen’s d ES with 95% CI |
|---|---|---|---|---|---|
| FDL | CSA (cm2) | CG | 0.84 ± 0.17 | 0.82 ± 0.18 | −0.16 [−0.96, 0.64] |
| REG | 0.86 ± 0.10 | 1.21 ± 0.21 | 2.07 [1.08, 3.06] | ||
| NG | 0.87 ± 0.07 | 1.19 ± 0.15 | 2.67 [1.52, 3.82] | ||
| RNG | 0.86 ± 0.08 | 1.25 ± 0.27 | 2.02 [1.04, 3.00] | ||
| Thickness (cm) | CG | 0.80 ± 0.09 | 0.79 ± 0.09 | −0.14 [−0.94, 0.66] | |
| REG | 0.83 ± 0.07 | 0.99 ± 0.13 | 1.54 [0.63, 2.45] | ||
| NG | 0.84 ± 0.08 | 0.98 ± 0.13 | 1.21 [0.30, 2.12] | ||
| RNG | 0.81 ± 0.07 | 1.04 ± 0.12 | 2.43 [1.38, 3.48] | ||
| FHL | CSA (cm2) | CG | 4.95 ± 0.32 | 4.98 ± 0.30 | 0.08 [−0.72, 0.88] |
| REG | 4.97 ± 0.28 | 4.97 ± 0.27 | 0.02 [−0.78, 0.82] | ||
| NG | 4.96 ± 0.41 | 4.97 ± 0.37 | 0.03 [−0.81, 0.87] | ||
| RNG | 4.97 ± 0.26 | 4.98 ± 0.27 | 0.04 [−0.76, 0.84] | ||
| Thickness (cm) | CG | 1.36 ± 0.08 | 1.37 ± 0.09 | 0.14 [−0.66, 0.94] | |
| REG | 1.36 ± 0.05 | 1.37 ± 0.06 | 0.15 [−0.66, 0.95] | ||
| NG | 1.36 ± 0.06 | 1.37 ± 0.04 | 0.24 [−0.60, 1.08] | ||
| RNG | 1.36 ± 0.07 | 1.37 ± 0.09 | 0.10 [−0.70, 0.90] | ||
| GA-M | CSA (cm2) | CG | 6.81 ± 1.13 | 6.80 ± 0.94 | 0.00 [−0.80, 0.80] |
| REG | 6.78 ± 0.57 | 7.47 ± 0.83 | 0.96 [0.12, 1.81] | ||
| NG | 6.77 ± 0.71 | 7.37 ± 0.65 | 0.89 [0.02, 1.77] | ||
| RNG | 6.78 ± 1.05 | 7.57 ± 0.76 | 0.87 [0.03, 1.70] | ||
| Thickness (cm) | CG | 1.65 ± 0.12 | 1.66 ± 0.07 | 0.06 [−0.74, 0.86] | |
| REG | 1.64 ± 0.12 | 1.90 ± 0.17 | 1.83 [0.88, 2.78] | ||
| NG | 1.64 ± 0.11 | 1.82 ± 0.15 | 1.37 [0.45, 2.30] | ||
| RNG | 1.64 ± 0.23 | 1.96 ± 0.21 | 1.48 [0.58, 2.38] | ||
| GA-L | CSA (cm2) | CG | 5.08 ± 0.59 | 5.11 ± 0.50 | 0.05 [−0.75, 0.86] |
| REG | 5.02 ± 0.92 | 6.16 ± 1.39 | 0.97 [0.12, 1.81] | ||
| NG | 5.06 ± 0.56 | 5.97 ± 0.74 | 1.39 [0.12, 1.81] | ||
| RNG | 5.03 ± 0.57 | 6.32 ± 0.45 | 2.50 [1.43, 3.57] | ||
| Thickness (cm) | CG | 1.35 ± 0.17 | 1.36 ± 0.12 | 0.09 [−0.71, 0.89] | |
| REG | 1.36 ± 0.25 | 1.63 ± 0.24 | 1.12 [0.26, 1.98] | ||
| NG | 1.37 ± 0.21 | 1.59 ± 0.12 | 1.36 [0.43, 2.28] | ||
| RNG | 1.36 ± 0.11 | 1.66 ± 0.15 | 2.26 [1.24, 3.28] | ||
| SOL | CSA (cm2) | CG | 6.06 ± 0.60 | 6.09 ± 0.36 | 0.07 [−0.74, 0.87] |
| REG | 5.82 ± 0.60 | 6.95 ± 0.56 | 1.94 [0.97, 2.91] | ||
| NG | 5.90 ± 0.82 | 6.68 ± 0.80 | 0.96 [0.08, 1.85] | ||
| RNG | 5.93 ± 0.42 | 6.98 ± 0.51 | 2.26 [1.23, 3.28] | ||
| Thickness (cm) | CG | 1.54 ± 0.18 | 1.54 ± 0.17 | 0.02 [−0.78, 0.82] | |
| REG | 1.52 ± 0.14 | 1.80 ± 0.15 | 1.94 [0.97, 2.91] | ||
| NG | 1.53 ± 0.13 | 1.76 ± 0.16 | 1.59 [0.64, 2.55] | ||
| RNG | 1.53 ± 0.16 | 1.83 ± 0.20 | 1.64 [0.72, 2.57] |
| Reach Direction | Group | Baseline | Post-Intervention | Cohen’s d ES with 95%CI |
|---|---|---|---|---|
| ANT | CG | 44.51 ± 3.51 | 44.44 ± 3.22 | −0.02 [−0.82, 0.78] |
| REG | 45.18 ± 3.06 | 55.97 ± 5.03 | 2.59 [1.51, 3.68] | |
| NG | 44.86 ± 3.08 | 53.31 ± 5.76 | 1.83 [0.83, 2.82] | |
| RNG | 46.81 ± 3.52 | 55.23 ± 5.65 | 1.79 [0.84, 2.74] | |
| PM | CG | 86.42 ± 8.41 | 85.47 ± 6.20 | −0.13 [−0.93, 0.67] |
| REG | 86.82 ± 6.18 | 98.77 ± 8.22 | 1.64 [0.72, 2.57] | |
| NG | 87.18 ± 8.45 | 96.32 ± 4.84 | 1.33 [0.40, 2.25] | |
| RNG | 84.24 ± 9.86 | 97.92 ± 7.61 | 1.55 [0.64, 2.47] | |
| PL | CG | 81.53 ± 7.04 | 81.47 ± 6.06 | −0.01 [−0.81. 0.79] |
| REG | 83.90 ± 9.93 | 94.86 ± 7.10 | 1.27 [0.39, 2.15] | |
| NG | 81.75 ± 13.47 | 94.97 ± 3.39 | 1.35 [0.42, 2.27] | |
| RNG | 79.34 ± 13.34 | 95.60 ± 5.70 | 1.59 [0.67, 2.50] | |
| CS, % | CG | 78.69 ± 4.98 | 78.42 ± 5.52 | −0.05 [−0.85, 0.75] |
| REG | 80.96 ± 6.87 | 93.55 ± 5.67 | 2.00 [1.02, 2.98] | |
| NG | 79.40 ± 8.37 | 90.96 ± 6.40 | 1.55 [0.60, 2.50] | |
| RNG | 79.94 ± 8.53 | 94.60 ± 5.70 | 2.02 [1.04, 3.00] |
| Variable | Group | Baseline | Post-Intervention | Cohen’s d ES with 95% CI |
|---|---|---|---|---|
| Square hop test (s) | CG | 25.76 ± 8.05 | 25.99 ± 10.40 | 0.02 [−0.78, 0.82] |
| REG | 24.80 ± 8.11 | 18.06 ± 1.86 | −1.15 [−2.01, −0.28] | |
| NG | 28.07 ± 10.39 | 20.00 ± 5.17 | −0.98 [−1.87, −0.10] | |
| RNG | 29.51 ± 11.49 | 18.44 ± 3.08 | −1.32 [−2.20, −0.43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Jun, H.-p. Effects of Rehabilitative Exercise and Neuromuscular Electrical Stimulation on Muscle Morphology and Dynamic Balance in Individuals with Chronic Ankle Instability. Medicina 2024, 60, 1187. https://doi.org/10.3390/medicina60071187
Choi S, Jun H-p. Effects of Rehabilitative Exercise and Neuromuscular Electrical Stimulation on Muscle Morphology and Dynamic Balance in Individuals with Chronic Ankle Instability. Medicina. 2024; 60(7):1187. https://doi.org/10.3390/medicina60071187
Chicago/Turabian StyleChoi, Sujin, and Hyung-pil Jun. 2024. "Effects of Rehabilitative Exercise and Neuromuscular Electrical Stimulation on Muscle Morphology and Dynamic Balance in Individuals with Chronic Ankle Instability" Medicina 60, no. 7: 1187. https://doi.org/10.3390/medicina60071187





