Abstract
Background and Objectives: Systemic-inflammation-based prognostic scores and hematological indices have shown value in predicting outcomes in various clinical settings. However, their effectiveness in predicting outcomes specifically for IgA nephropathy (IgAN) and membranous nephropathy (MN), the most common primary glomerular diseases diagnosed by kidney biopsy, has not been thoroughly investigated. Materials and Methods: We conducted a retrospective, observational study involving 334 adult patients with biopsy-proven IgAN (196 patients) and MN (138 patients) from January 2008 to December 2017 at a tertiary center. We assessed six prognostic scores [Glasgow prognostic score (GPS), modified GPS (mGPS), prognostic nutritional index (PNI), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-C-reactive protein ratio (LCRP)] and two hematological indices [red blood cell distribution width (RDW), platelet distribution width (PDW)] at diagnosis and examined their relationship with kidney and patient survival. Results: End-stage kidney disease (ESKD) occurred more frequently in the IgAN group compared to the MN group (37% vs. 12%, p = 0.001). The mean kidney survival time was 10.7 years in the IgAN cohort and 13.8 years in the MN cohort. After adjusting for eGFR and proteinuria, lower NLR and higher LCRP were significant risk factors for ESKD in IgAN. In the MN cohort, no systemic-inflammation-based scores or hematological indices were associated with kidney survival. There were 38 deaths (19%) in the IgAN group and 29 deaths (21%) in the MN group, showing no significant difference in mortality rates. The mean survival time was 13.4 years for the IgAN group and 12.7 years for the MN group. In the IgAN group, a lower PLR was associated with a higher mortality after adjusting for age, the Charlson comorbidity score, eGFR, and proteinuria. In patients with MN, higher NLR, PLR, and RDW were associated with increased mortality. Conclusions: NLR and LCRP are significant predictors of ESKD in IgAN, while PLR is linked to increased mortality. In MN, NLR, PLR, and RDW are predictors of mortality but not kidney survival. These findings underscore the need for disease-specific biomarkers and indicate that systemic inflammatory responses play varying roles in the progression and outcomes of these glomerular diseases. Future studies on larger cohorts are necessary to validate these markers.
1. Introduction
IgA nephropathy (IgAN) and membranous nephropathy (MN) are the most common primary immune-mediated glomerular diseases diagnosed through kidney biopsy, each having a highly variable prognosis [1].
IgAN is characterized by the deposition of IgA immune complexes in the glomeruli, leading to local inflammation and glomerular injury. This condition often presents with hematuria and varying degrees of proteinuria. The current markers for IgAN include serum creatinine, proteinuria levels, and eGFR, though these are not specific to the disease’s unique immune-mediated mechanisms. MN involves the deposition of immune complexes on the glomerular basement membrane, causing thickening and damage to the glomeruli. Patients typically present with heavy proteinuria and nephrotic syndrome. The established markers include anti-PLA2R antibodies and proteinuria levels, which are used in clinical practice to monitor the disease activity and response to therapy.
The current guidelines recommend tailoring treatment based on the level of immunologic activity and overall prognosis [1]. However, using proteinuria and the glomerular filtration rate as the primary indicators for the prognosis and treatment of primary glomerular diseases can be misleading, as these metrics can be affected by chronic damage and nephron scarring, which are unrelated to immune activity. Therefore, there is ongoing effort to identify affordable and readily available markers that accurately reflect immune activity in these glomerular diseases.
A complete blood count (CBC) test, commonly ordered in clinical practice, measures key blood components like white blood cells (WBCs), neutrophils (NEs), lymphocytes (LYs), and platelets (PLTs). This test provides a general view of the immune system, which is crucial for diagnosing and managing patients. NE and LY are vital for innate and adaptive immunity, respectively. Increased NE indicates inflammation, while decreased LY suggests poor nutrition and prognosis [2]. The neutrophil-to-lymphocyte ratio (NLR) combines these markers to evaluate systemic inflammation. Platelets also play a role in inflammation, leading to the use of the platelet-to-lymphocyte ratio (PLR) as a marker of acute inflammatory and prothrombotic conditions in various diseases, including rheumatic and cardiovascular conditions [2]. Both NLR and PLR have shown prognostic value in coronary artery disease, solid tumors, rheumatoid arthritis, chronic kidney disease (CKD), acute kidney injury (AKI), and rapidly progressive glomerulonephritis [2,3].
Similarly, red cell distribution width (RDW) and platelet distribution width (PDW) are routinely reported in complete blood counts and serve as important prognostic biomarkers [4]. Higher RDW within the normal range is associated with cardiovascular outcomes like myocardial infarction, heart failure, and stroke, which is potentially due to elevated proinflammatory substances affecting erythropoiesis and vascular health [5,6]. The Glasgow prognostic score (GPS), which includes C-reactive protein (CRP) and serum albumin, is a concise tool for assessing systemic inflammation and nutritional status. Extensively validated in oncology, the GPS is also linked to CKD progression and mortality [7,8,9]. However, there are limited data on the value of systemic-inflammation-based prognostic scores and hematological indices of inflammation in IgAN and MN.
In this exploratory study, we aimed to investigate the relationship between various peripheral blood cell scores and indices with kidney and patient survival outcomes in IgAN and MN. We selected six prognostic scores [Glasgow prognostic score (GPS), modified GPS (mGPS), prognostic nutritional index (PNI), NLR, PLR, and lymphocyte-to-C-reactive protein ratio (LCRP)] and two hematological indices [red blood cell distribution width (RDW) and platelet distribution width (PDW)] at diagnosis because they have been previously validated in different nephrological scenarios. Our goal was to enhance the understanding of their predictive value for critical outcomes, specifically end-stage kidney disease (ESKD) and mortality, in these common glomerular diseases.
2. Materials and Methods
2.1. Study Population
We conducted a retrospective, observational study of all adults with biopsy-proven IgAN and MN from January 2008 to December 2017 in the nephrology department of the “Dr. Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania.
From a total of 472 native kidney biopsies at our center with the diagnosis of IgAN and MN, we excluded patients who underwent more than one biopsy during the study period (11 patients—5 with IgAN, 6 with MN), those with solid tumors (6 patients—3 with IgAN, 3 with MN), those with active infections (20 patients—17 with IgAN and 3 with MN), those who underwent biopsy after beginning kidney replacement therapy (1 patient), those who received immunosuppressive treatment prior to the kidney biopsy (37 patients), and those with insufficient material for a complete histopathologic analysis (32 patients) or insufficient clinical data (31 patients). The final cohort for analysis comprised 334 patients—196 with IgAN and 138 with MN.
This study was conducted with the provisions of the Declaration of Helsinki and was approved by the local ethical committee (“Dr. Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania, approval number 40/04.08.2023).
2.2. Clinical Data
Patient demographics and clinical data were retrieved from electronic medical records using a standardized data form. Two analysts conducted the database search to ensure accuracy and reliability, resolving discrepancies through consensus to ensure a rigorous review process.
The collected data included age, Charlson comorbidity index, presence of arterial hypertension (defined as blood pressure >140/90 mmHg or the use of antihypertensive agents), complete blood count, inflammation markers (C-reactive protein, erythrocyte sedimentation rate, fibrinogen), serum albumin, eGFR calculated by the four-variable MDRD formula, proteinuria (quantified by 24-h urine collection and expressed as urinary protein-to-creatinine ratio, in g/g), and hematuria (red blood cells per high power field). Only data at the time of the biopsy were used in the analyses.
Clinical syndromes were retrospectively defined as nephrotic syndrome, nephritic syndrome, CKD, AKI, and asymptomatic urinary abnormalities (AUAs).
The selected six prognostic scores—GPS, mGPS, PNI, NLR, PLR, and LCRP—and two hematological indices—RDW and PDW—were defined according to the definitions provided in Table 1.

Table 1.
Inflammation-based scores and hematological markers of inflammation definitions.
2.3. Study Endpoints
The primary endpoint of this study was the incidence of ESKD. This was determined by the need to initiate dialysis or undergo a kidney transplantation in the patient cohort. A patient was considered to have reached the primary endpoint when either of these events was registered in the Romanian Renal Registry.
The secondary endpoint of this study was all-cause mortality. This was measured by tracking the number of deaths, regardless of the cause, in the patient cohort during the follow-up period.
All patients were followed from the start of the study (id est, the moment of kidney biopsy) until 30 April 2024.
2.4. Statistical Analysis
Continuous variables are presented as mean or median with 95% confidence interval (CI) after testing normality with the Shapiro–Wilk test, while categorical variables are presented as percentages. To evaluate differences between IgAN and MN, Student’s t-test or the Mann–Whitney test was applied, depending on the distribution of the variables. For categorical variables, the chi-square test was utilized to determine statistical significance.
The Kaplan—Meier method was employed to analyze the probability of event-free survival, with the log-rank test used for comparisons between IgAN and MN. To identify independent predictors—systemic-inflammation-based prognostic scores and hematological indices—contributing to the progression to ESKD and mortality, both univariate and multivariate analyses (based on the Cox proportional hazard ratio) were performed for each glomerular disease. In the ESKD models, the inflammatory scores were adjusted for eGFR and proteinuria, and, in the mortality models, adjustments were made for eGFR, proteinuria, and Charlson comorbidity score. The results are expressed in terms of hazard ratios (HRs) with a 95% CI. We employed two methods to test for collinearity among our predictor variables: (i) the variance inflation factor (VIF), with a desirable threshold of VIF < 10, and (ii) the absolute value of correlation coefficients, aiming for |r| or |rs| < 0.7. Our analysis indicated no significant collinearity among the variables used in the Cox proportional hazard models.
Statistical analyses were performed using SPSS (IBM SPSS Statistics for Macintosh, Version 27.0. Armonk, NY, USA: IBM Corp) and GraphPad Prism (GraphPad Software (Version 9.5.1 (528)), La Jolla, CA, USA).
3. Results
3.1. Baseline Demographic and Clinical Characteristics
The baseline demographic and clinical characteristics of the cohort are presented in Table 2. Patients with MN were generally older than those with IgAN (55 versus 43 years). As expected, patients with MN more frequently presented with nephrotic syndrome (85% versus 6%), while those with IgAN more often had nephritic syndrome (85 versus 10%). In line with this, arterial hypertension was more common in patients with IgAN, although the Charlson comorbidity score was similar between the two groups.

Table 2.
Patients’ characteristics.
At diagnosis, the patients with IgAN had lower eGFR (39.1 versus 65.0 mL/min), higher hematuria (180 versus 20 RBC/HPF), and lower proteinuria (1.2 versus 5.7 g/g) than patients with MN, reflecting their clinical presentation.
In terms of inflammation parameters, there were no significant differences in hemoglobin levels, neutrophil count, lymphocyte count, or C-reactive protein levels between the two groups. However, patients with MN had higher platelet counts, erythrocyte sedimentation rates, fibrinogen levels, and lower serum albumin levels.
Immunosuppressive treatment was more commonly received by patients with MN, whereas ACEI/ARB usage was similar between the two groups.
The median follow-up time for the entire cohort was 113.0 months (95% CI 108.5–117.4).
3.2. Systemic-Inflammation-Based Prognostic Scores and Hematological Indices
According to the GPS scoring system, including the modified form, the patients with MN had higher GPS scores. Similarly, the PNI was also higher in the MN cohort.
There were no differences in the NLR and LCRP between the glomerular diseases; however, patients with MN had a higher platelet-to-lymphocyte ratio.
Regarding hematological indices, the patients with MN had a higher RDW, while the PDW was similar between the two groups.
3.3. Kidney Survival
The ESKD endpoint occurred more frequently in the IgAN group than in the MN group (37% versus 12%, p = 0.001).
In the IgAN cohort, the mean kidney survival time was 10.7 years (95% CI: 9.8–11.7). Renal survival rates at 1, 3, 5, and 10 years were 92%, 84%, 73%, and 60%, respectively. In the MN group, the mean kidney survival time was 13.8 years (95% CI: 13.0–14.5). The renal survival rates at 1, 3, 5, and 10 years were 99%, 95%, 91%, and 85%, respectively (Figure 1A).
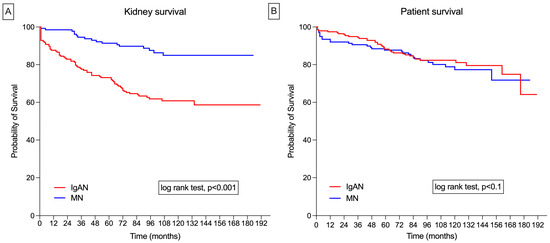
Figure 1.
(A) Nonadjusted Kaplan–Meier survival curves for IgA nephropathy (IgAN) and membranous nephropathy (MN) for kidney survival; (B) nonadjusted Kaplan–Meier survival curves for IgAN and MN for patient survival.
In univariate Cox regression analysis, GPS and PNI were associated with kidney survival in patients with IgAN. However, after adjusting for eGFR and proteinuria, only a lower NLR and a higher LCRP remained significant risk factors for ESKD (Table 3).

Table 3.
Relationship between inflammation-based scores and hematological markers of inflammation with kidney survival.
In the MN cohort, none of the systemic-inflammation-based prognostic scores or hematological indices studied were associated with kidney survival in either univariate or multivariate analysis (Table 3).
3.4. Patient Survival
A total of 38 deaths (19%) occurred in the IgAN group compared to 29 deaths (21%) in the MN group, indicating no significant difference in the mortality rates between the two glomerular diseases. The primary cause of mortality in the IgAN group was cardiovascular diseases (60%), followed by infectious (20%), gastroenterological (13%), and neoplastic (9%) diseases. Similarly, in the MN group, cardiovascular diseases (45%) and infectious diseases (26%) were the main causes of death.
In the IgAN group, the mean survival time was 13.4 years (95% CI: 12.6–14.1), with survival rates at 1, 3, 5, and 10 years of 94%, 85%, 83%, and 82%, respectively. In the MN group, the mean survival time was 12.7 years (95% CI: 11.8–13.8), with survival rates at 1, 3, 5, and 10 years of 91%, 90%, 87%, and 77%, respectively (Figure 1B).
In the univariate Cox regression analysis for the IgAN group, GPS, mGPS, PNI, PLR, and PDW were associated with mortality. However, after adjusting for age, the Charlson comorbidity score, eGFR, and proteinuria, only a lower PLR was associated with a higher risk of mortality (Table 4). In patients with MN, PNI, NLR, PLR, LCRP, and RDW were associated with mortality in the univariate Cox regression analysis. However, in the multivariate analysis, only higher NLR, PLR, and RDW were associated with mortality (Table 4).

Table 4.
Relationship between inflammation-based scores and hematological markers of inflammation with survival.
4. Discussion
In the present study, we aimed to explore the relationship between common systemic-inflammation-based prognostic scores and hematological indices at diagnosis with long-term outcomes—specifically kidney and patient survival—in IgAN and MN, which are the most frequent glomerular diseases diagnosed by kidney biopsy. The main findings of the study are as follows: (i) In IgAN, NLR and LCRP were identified as risk factors for progression to ESKD. Additionally, PLR was associated with increased mortality. (ii) In MN, there were no systemic-inflammation-based prognostic scores or hematological indices that were associated with kidney survival. However, both NLR and PLR were associated with increased mortality in patients with MN.
4.1. Kidney Survival
Previous studies from China identified a higher NLR at diagnosis as an independent risk factor for ESKD in IgAN; however, the median eGFR at baseline in these studies was between 80 and 96 mL/min [16,17,18]. In contrast, in our European population, the median eGFR was 39 mL/min. In our multivariate analysis adjusted for eGFR and proteinuria, a lower NLR emerged as an independent risk factor for ESKD. These findings could be explained by the lower eGFR at diagnosis in our population. In this context, a lower NLR might reflect chronic inflammation, immune exhaustion, and malnutrition. Thus, in earlier stages of IgAN, a higher NLR could reflect the “disease-specific” immune-mediated nephron loss, while, in advanced stages, a lower NLR might reflect the generic response to nephron loss (id est, CKD progression).
While a higher NLR typically reflects acute inflammation (high neutrophil counts), a lower NLR might reflect a shift toward chronic inflammation and immune dysregulation, which are detrimental in long-term IgAN progression [19]. This scenario is consistent with the finding that a higher LCRP (higher lymphocyte counts relative to CRP) was also associated with ESKD.
In our study, none of the systemic-inflammation-based prognostic scores or hematological indices were associated with ESKD in patients with membranous nephropathy (MN). This finding suggests that the progression to ESKD in MN may be influenced more by other factors beyond systemic inflammation and hematological parameters [20]. MN is primarily characterized by immune complex deposition in the glomeruli, leading to podocyte damage and proteinuria, which may not be directly reflected in the systemic inflammatory markers measured [20]. Moreover, the progression of MN can be heavily influenced by other factors such as the degree of proteinuria, response to immunosuppressive therapy, and underlying comorbidities like hypertension and diabetes [21]. These factors may have a more significant impact on renal outcomes in MN than the systemic inflammatory response, which is why the prognostic scores and hematological indices based on inflammation did not show a significant association with ESKD in this patient group. This underscores the need for more specific biomarkers or indicators that directly reflect the pathophysiological mechanisms of MN.
4.2. Patient Survival
In relation to the PLR, there are not enough data to define a definitive cut-off point to predict mortality. Patients with IgAN had a significantly lower PLR than patients with MN. Moreover, in the multivariate survival analysis, a lower PLR value was associated with mortality in the IgAN group, while a higher PLR value was associated with mortality in the MN group. These findings suggest a J-curve pattern for death in relation to PLR, which might also reflect the different pathophysiological mechanisms underlying the two diseases.
Specifically, IgAN is characterized by chronic immune-mediated inflammation, where lower PLR values indicate severe disease and immune dysregulation. This is because IgAN involves the deposition of IgA immune complexes in the glomeruli, leading to local inflammation and increased lymphocyte activity, which can reduce the PLR. On the other hand, MN is driven by autoimmune processes targeting glomerular podocytes, resulting in a prothrombotic state. The higher PLR values in MN reflect increased platelet activation and aggregation, which are associated with a higher risk of thrombosis and systemic inflammation, thereby increasing the mortality risk [22].
Supporting our findings, a recent study of over 100,000 incident patients on hemodialysis established a J-curve pattern for mortality in relation to PLR: patients with PLR values below 100 and above 300 had higher mortality rates compared to those with PLR values in the range of 100–150 s [23].
In addition to PLR, a higher NLR was also associated with mortality in patients with MN. Studies suggest that both a high baseline NLR and increases in NLR over time are linked to higher mortality rates. Some authors proposed a cut-off point of NLR ≥ 3.5, beyond which the risk of mortality significantly increases [24]. The strong association between high NLR and low serum albumin levels further supports using NLR as a reliable mortality marker. Therefore, in patients with MN presenting with nephrotic syndrome, NLR may have greater predictive utility than albumin because NLR increases in the blood much faster (6–8 h) compared to the slower decrease in albumin levels (19–21 days) [23,25].
The RDW level may be a useful predictive biomarker for estimating the response to immunosuppressive therapy and reflecting an increased inflammatory response in nephrotic syndrome (NS). Turgutalep et al. found that the RDW level is a powerful and independent predictor of treatment response in patients with NS caused by primary glomerular diseases, including MN [26]. Patients with low or normal RDW levels had a high response rate. Similarly, our study showed that a higher RDW level was associated with increased mortality, further supporting the use of RDW as a prognostic marker in MN.
4.3. Limitations
Our study has several limitations that should be taken into account when interpreting the findings. Firstly, being a single-center observational study, the scope and generalizability of our results are inherently limited. The specific patient population and treatment practices at our center may not represent the broader global patient population or the practices at other healthcare centers. Therefore, our results may not be universally applicable to all patients with IgAN or MN.
Secondly, we only analyzed patients with IgAN and MN at the time of presentation, i.e., at the time of kidney biopsy. This means that our findings do not consider potential changes in disease status or treatment responses over time, which could affect long-term outcomes. Moreover, while the chosen confounders are pertinent, other potential confounders (e.g., medication adherence and lifestyle factors, such as diet and physical activity) were not accounted for, which could influence the study outcomes.
Additionally, our study’s sample size restricted our ability to conduct in-depth subgroup analyses for each systemic-inflammation-based prognostic score and hematological index. This highlights the need for larger future studies to comprehensively address these subgroup variations.
5. Conclusions
This study highlights the importance of systemic-inflammation-based scores in predicting the outcomes of IgAN and MN. In IgAN, NLR and LCRP are significant predictors for ESKD progression, while PLR is associated with increased mortality. In MN, NLR, PLR, and RDW predict mortality but not kidney survival. These findings underscore the necessity of disease-specific biomarkers and indicate that systemic inflammatory responses influence the progression and outcomes of these glomerular diseases differently. Future studies with larger cohorts are essential for validating these markers and investigating the mechanisms that affect them.
Author Contributions
Conceptualization, N.P., G.Ș. and C.C.; methodology, N.P., G.Ș., T.P. and O.C.; validation, S.H.S. and C.C.; formal analysis, G.Ș. and N.P.; resources, T.P., O.C. and C.C.; writing—original draft preparation, N.P. and G.Ș.; writing—review and editing, C.C.; visualization, S.H.S., C.C., T.P. and O.C.; supervision, S.H.S. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted with the provisions of the Declaration of Helsinki, and the study was approved by the local ethics committee (study reference 40/4 August 2023).
Informed Consent Statement
Since all data were anonymized, informed consent was not obtained from individual patients.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Acknowledgments
The publication of this paper was supported by the University of Medicine and Pharmacy “Carol Davila” through the institutional program “Publish not Perish”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef] [PubMed]
- Valga, F.; Monzon, T.; Henriquez, F.; Anton-Perez, G. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as biological markers of interest in kidney disease. Nefrologia (Engl. Ed.) 2019, 39, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, J.; Peng, Z.; Zhou, Q.; Xiao, X.; Xie, Y.; Wang, W.; Huang, L.; Tang, W.; Sun, D.; et al. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J. Transl. Med. 2019, 17, 86. [Google Scholar] [CrossRef]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Bahreyni, A.; Ghandehari, M.; Shafiee, M.; Rahmani, F.; Parizadeh, M.R.; Seifi, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors 2019, 45, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; James, M.T.; Naugler, C.; Manns, B.J.; Klarenbach, S.W.; Hemmelgarn, B.R. Red cell distribution width associations with clinical outcomes: A population-based cohort study. PLoS ONE 2019, 14, e0212374. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.C.; Lin, Y.T.; Ting, I.W.; Huang, H.C.; Chiang, H.Y.; Chung, C.W.; Chang, S.N.; Kuo, C.C. Variability of red blood cell size predicts all-cause mortality, but not progression to dialysis, in patients with chronic kidney disease: A 13-year pre-ESRD registry-based cohort. Clin. Chim. Acta 2019, 497, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Stefan, G.; Zugravu, A.; Stancu, S. Glasgow prognostic score as an outcome predictor for patients initiating hemodialysis. Ther. Apher. Dial. 2024, 28, 34–41. [Google Scholar] [CrossRef]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- Stefan, G.; Stancu, S.; Zugravu, A.; Capusa, C. Inflammation-based modified Glasgow prognostic score and renal outcome in chronic kidney disease patients: Is there a relationship? Intern. Med. J. 2022, 52, 968–974. [Google Scholar] [CrossRef]
- Kato, A.; Tsuji, T.; Sakao, Y.; Ohashi, N.; Yasuda, H.; Fujimoto, T.; Takita, T.; Furuhashi, M.; Kumagai, H. A comparison of systemic inflammation-based prognostic scores in patients on regular hemodialysis. Nephron. Extra 2013, 3, 91–100. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, S.; Chen, Z.; Li, D.; Shan, Y.; Tao, Y.; Gao, M.; Shen, X.; Zhang, W.; Xia, S.; et al. Glasgow prognostic score and its derived scores predicts contrast-associated acute kidney injury in patients undergoing coronary angiography. Heliyon 2023, 9, e22284. [Google Scholar] [CrossRef] [PubMed]
- Miyasato, Y.; Hanna, R.M.; Morinaga, J.; Mukoyama, M.; Kalantar-Zadeh, K. Prognostic Nutritional Index as a Predictor of Mortality in 101,616 Patients Undergoing Hemodialysis. Nutrients 2023, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Nakayama, M.; Sakoh, T.; Fukui, A.; Katafuchi, E.; Seki, M.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren. Fail. 2019, 41, 238–243. [Google Scholar] [CrossRef]
- Gan, W.; Guan, Q.; Hu, X.; Zeng, X.; Shao, D.; Xu, L.; Xiao, W.; Mao, H.; Chen, W. The association between platelet-lymphocyte ratio and the risk of all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2022, 54, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, W.; Diao, Z.; Huang, H.; Liu, W. Lymphocyte-to-C reactive protein ratio as novel inflammatory marker for predicting outcomes in hemodialysis patients: A multicenter observational study. Front. Immunol. 2023, 14, 1101222. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, L.; Pei, G.; Jiang, Z.; Qin, A.; Tan, J.; Tang, Y.; Qin, W. High Neutrophil-To-Lymphocyte Ratio Is an Independent Risk Factor for End Stage Renal Diseases in IgA Nephropathy. Front. Immunol. 2021, 12, 700224. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Cai, K.; Wang, K.; Luo, Q. Relationship between blood neutrophil-lymphocyte ratio and renal tubular atrophy/interstitial fibrosis in IgA nephropathy patients. J. Clin. Lab. Anal. 2021, 35, e23774. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, P.; Shi, S.; Liu, L.; Lv, J.; Zhu, L.; Zhang, H. Neutrophil-to-lymphocyte ratio as an independent inflammatory indicator of poor prognosis in IgA nephropathy. Int. Immunopharmacol. 2020, 87, 106811. [Google Scholar] [CrossRef] [PubMed]
- Esteve Cols, C.; Graterol Torres, F.A.; Quirant Sanchez, B.; Marco Rusinol, H.; Navarro Diaz, M.I.; Ara Del Rey, J.; Martinez Caceres, E.M. Immunological Pattern in IgA Nephropathy. Int. J. Mol. Sci. 2020, 21, 1389. [Google Scholar] [CrossRef]
- Hoxha, E.; Reinhard, L.; Stahl, R.A.K. Membranous nephropathy: New pathogenic mechanisms and their clinical implications. Nat. Rev. Nephrol. 2022, 18, 466–478. [Google Scholar] [CrossRef]
- Stefan, G.; Stancu, S.; Zugravu, A.; Popa, O.; Zubidat, D.; Petre, N.; Mircescu, G. Negative anti-phospholipase A2 receptor antibody status at three months predicts remission in primary membranous nephropathy. Ren. Fail. 2022, 44, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Dai, G.X.; Zhang, Q.D.; Huang, H.D.; Liu, W.H. The Value of Peripheral Blood Cell Ratios in Primary Membranous Nephropathy: A Single Center Retrospective Study. J. Inflamm. Res. 2023, 16, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Catabay, C.; Obi, Y.; Streja, E.; Soohoo, M.; Park, C.; Rhee, C.M.; Kovesdy, C.P.; Hamano, T.; Kalantar-Zadeh, K. Lymphocyte Cell Ratios and Mortality among Incident Hemodialysis Patients. Am. J. Nephrol. 2017, 46, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Tatar, E.; Mirili, C.; Isikyakar, T.; Yaprak, M.; Guvercin, G.; Ozay, E.; Asci, G. The association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with clinical outcomes in geriatric patients with stage 3-5 chronic kidney disease. Acta Clin. Belg. 2016, 71, 221–226. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; Liu, W.T.; Huang, Y.; Huang, Y.L.; Jin, F.Y.; Wu, Q.Y.; Wang, G.H.; et al. The ratio of neutrophils to lymphocytes effectively predicts clinical outcomes in idiopathic membranous nephropathy. Clin. Nephrol. 2024, 101, 101–108. [Google Scholar] [CrossRef]
- Turgutalp, K.; Kiykim, A.; Bardak, S.; Demir, S.; Karabulut, U.; Ozcan, T.; Helvaci, I.; Gozukara, Y. Is the red cell distribution width strong predictor for treatment response in primary glomerulonephritides? Ren. Fail. 2014, 36, 1083–1089. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).