Comparison of Day-Specific Serum LH, Estradiol, and Progesterone with MiraTM Monitor Urinary LH, Estrone-3-glucuronide, and Pregnanediol-3-glucuronide Levels in Ovulatory Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Transvaginal Sonography
2.3. Daily Serum Samples
2.4. Fertility Indicator Equation (FIE) and Area Under the Curve (AUC) Algorithms
3. Results
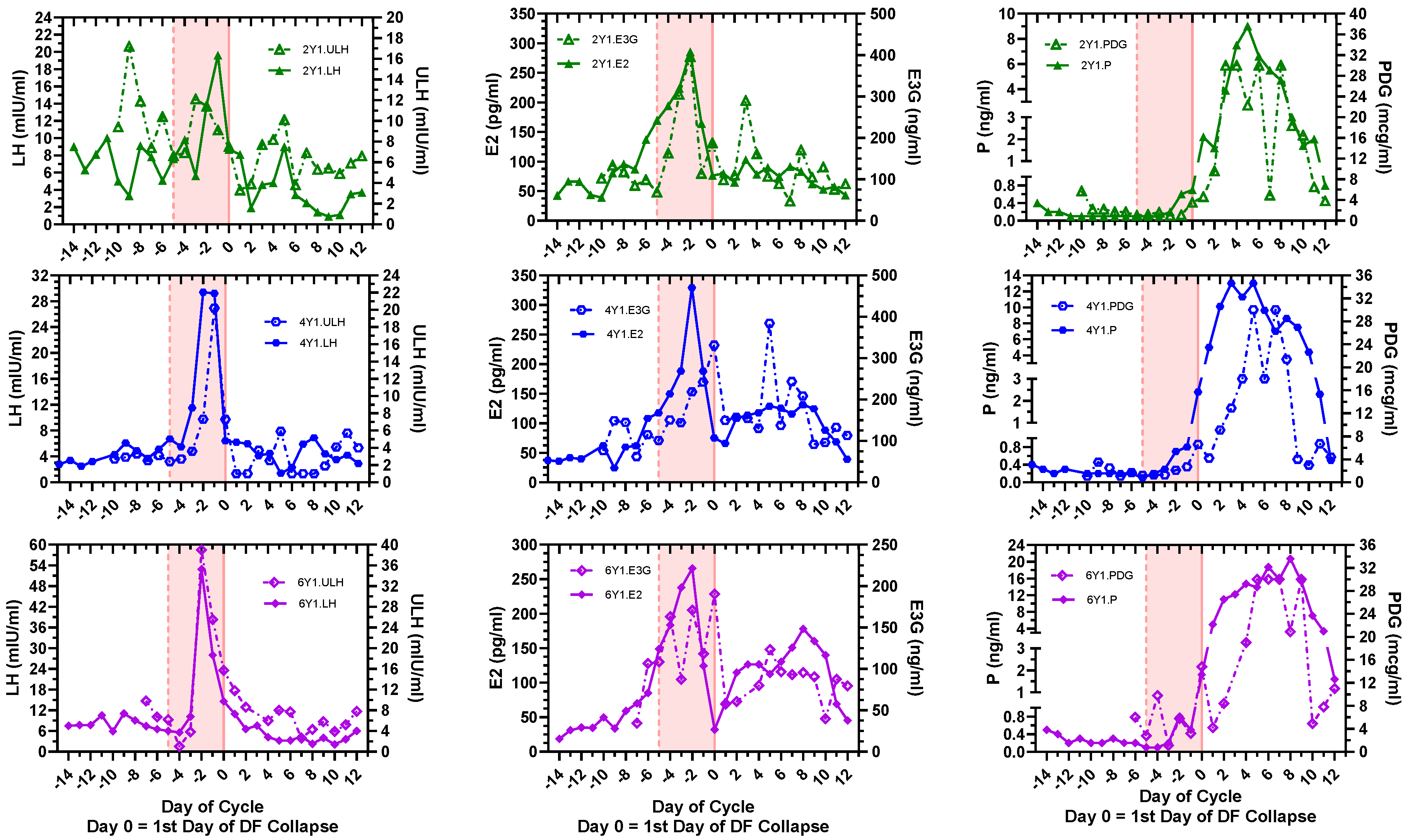
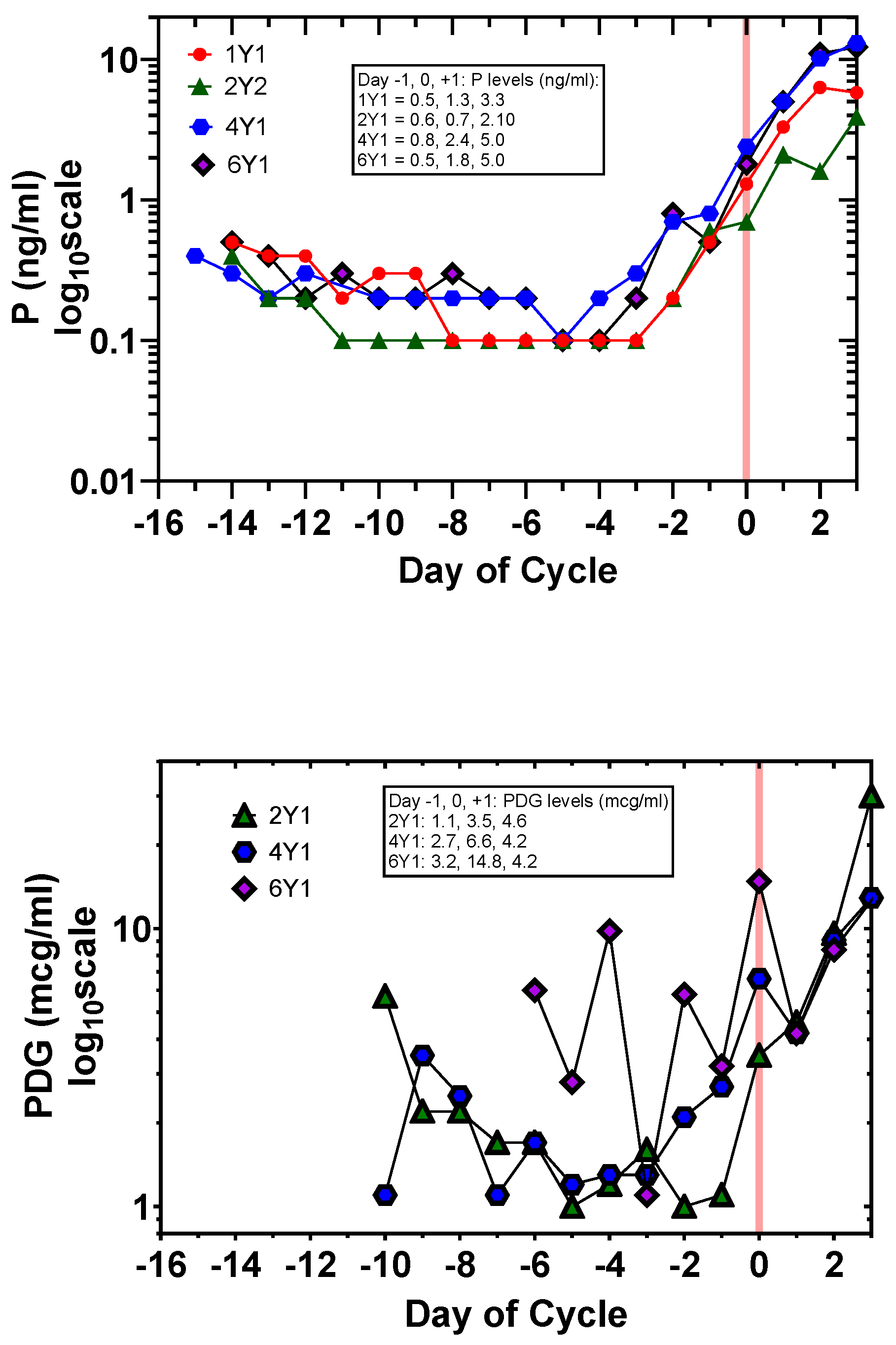
3.1. Day-Specific Serum LH, E2, and P Levels Compared with Day-Specific Urinary LH, E3G, and PDG Levels
| 1Y1 | 2Y1 | 4Y1 | 6Y1 | |
|---|---|---|---|---|
| Dominant Follicle (DF) b Max Size (mm) (Day −1) | 22.3 | 30.3 | 22.4 | 26.9 |
| Cycle length (days) | 25 | 27 | 28 | 27 |
| LH peak day | −1 | −1 | −2, −1 | −2 |
| ULH peak day | - | −9, −3 | −1 | −1 |
| Preovulatory E2 peak day | −2 | −2 | −2 | −2 |
| Preovulatory E3G peak day | - | −2 | 0 | 0 |
| Preovulatory E2 range (pg/mL) (1 pg/mL = 3.67 pmol/L) | 28.1–362.8 | 42.7–285.5 | 24.3–329.2 | 31–265.6 |
| Preovulatory E3G range (ng/mL) (1 ng/mL = 2.23 nmol/L) | - | 84.6–396.1 | 62.4–331.5 | 34.4–190.4 |
3.2. Application of the Fertility Indicator Equation (FIE) to Signal the Fertile Window, Ovulation, and Luteal Phase Using Day-Specific E2 and Day-Specific E3G Levels
3.3. Application of the Area under the Curve (AUC) Algorithm with the Day-Specific E2, P and Day-Specific E3G, PDG Levels to Signal the Time-of-Cycle
3.4. Statistical Validation and Comparison of the FIE(E2) vs. FIE(E3G) Algorithms
3.5. Statistical Validation of the Delta (AUCE2/AUCP) and Delta (AUCE3G/AUCPDG) Algorithm
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duane, M.; Stanford, J.B.; Porucznik, C.A.; Vigil, P. Fertility Awareness-Based Methods for Women’s Health and Family Planning. Front. Med. 2022, 9, 858977. [Google Scholar] [CrossRef]
- Leiva, R.; DiRienzo, L. Combination of Home-Based Hormonal and Mobile Technology for Virtual Monitoring of Menstrual Cycles. Ann. Fam. Med. 2021, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynowicz, A.K.; Beckley, A.; Eyvazzadeh, A.; Levy, G.; Park, J.; Klein, J. Complete Cycle Mapping Using a Quantitative At-Home Hormone Monitoring System in Prediction of Fertile Days, Confirmation of Ovulation, and Screening for Ovulation Issues Preventing Conception. Medicina 2022, 58, 1853. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Das, D.; Venkatesan, V.A.; Rai, A. Predicting Serum Hormone Concentration by Estimation of Urinary Hormones through a Home-Use Device. Hum. Reprod. Open 2023, 2023, hoac058. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Das, D.; Venkatesan, V.A. Validation of Urinary Reproductive Hormone Measurements Using a Novel Smartphone Connected Reader. Sci. Rep. 2023, 13, 9227. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, T.; Yong, P.; Doyle-Baker, P. Establishing a Gold Standard for Quantitative Menstrual Cycle Monitoring. Medicina 2023, 59, 1513. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, T.P. Using Quantitative Hormonal Fertility Monitors to Evaluate the Luteal Phase: Proof of Concept Case Study. Medicina 2023, 59, 140. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Fehring, R.J. A Comparison of Two Hormonal Fertility Monitoring Systems for Ovulation Detection: A Pilot Study. Medicina 2023, 59, 400. [Google Scholar] [CrossRef] [PubMed]
- Earle, S.; Marston, H.R.; Hadley, R.; Banks, D. Use of Menstruation and Fertility App Trackers: A Scoping Review of the Evidence. BMJ Sex. Reprod. Health 2021, 47, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, L.F.; Vigil, P.; Gross, B.; d’Arcangues, C.; Cooke, D.G.; Brown, J.B. Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates of Estrone Glucuronide and Pregnanediol Glucuronide Using the Ovarian Monitor, Part II: Reliability of Home Testing. Hum. Reprod. 2012, 27, 550–557. [Google Scholar] [CrossRef]
- Bouchard, T.P.; Fehring, R.J.; Schneider, M. Pilot Evaluation of a New Urine Progesterone Test to Confirm Ovulation in Women Using a Fertility Monitor. Front. Public Health 2019, 7, 184. [Google Scholar] [CrossRef]
- Leiva, R.; McNamara-Kilian, M.; Niezgoda, H.; Ecochard, R.; Bouchard, T. Pilot Observational Prospective Cohort Study on the Use of a Novel Home-Based Urinary Pregnanediol 3-Glucuronide (PDG) Test to Confirm Ovulation When Used as Adjunct to Fertility Awareness Methods (FAMs) Stage 1. BMJ Open 2019, 9, e028496. [Google Scholar] [CrossRef]
- Behre, H.M.; Kuhlage, J.; Gassner, C.; Sonntag, B.; Schem, C.; Schneider, H.P.; Nieschlag, E. Prediction of Ovulation by Urinary Hormone Measurements with the Home Use ClearPlan Fertility Monitor: Comparison with Transvaginal Ultrasound Scans and Serum Hormone Measurements. Hum. Reprod. 2000, 15, 2478–2482. [Google Scholar] [CrossRef]
- Tanabe, K.; Susumu, N.; Hand, K.; Nishii, K.; Ishikawa, I.; Nozawa, S. Prediction of the Potentially Fertile Period by Urinary Hormone Measurements Using a New Home-Use Monitor: Comparison with Laboratory Hormone Analyses. Hum. Reprod. 2001, 16, 1619–1624. [Google Scholar] [CrossRef]
- Bouchard, T.P.; Fehring, R.J.; Mu, Q. Quantitative versus Qualitative Estrogen and Luteinizing Hormone Testing for Personal Fertility Monitoring. Expert. Rev. Mol. Diagn. 2021, 21, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J.; Weinberg, C.R.; Baird, D.D. Timing of Sexual Intercourse in Relation to Ovulation. Effects on the Probability of Conception, Survival of the Pregnancy, and Sex of the Baby. N. Engl. J. Med. 1995, 333, 1517–1521. [Google Scholar] [CrossRef]
- Dunson, D.B.; Colombo, B.; Baird, D.D. Changes with Age in the Level and Duration of Fertility in the Menstrual Cycle. Hum. Reprod. 2002, 17, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Stanford, J.B.; Dunson, D.B. Effects of Sexual Intercourse Patterns in Time to Pregnancy Studies. Am. J. Epidemiol. 2007, 165, 1088–1095. [Google Scholar] [CrossRef]
- Colombo, B.; Mion, A.; Passarin, K.; Scarpa, B. Cervical Mucus Symptom and Daily Fecundability: First Results from a New Database. Stat. Methods Med. Res. 2006, 15, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, L.F.; Vigil, P.; Cooke, D.G.; d’Arcangues, C.; Brown, J.B. Monitoring of Ovarian Activity by Daily Measurement of Urinary Excretion Rates of Oestrone Glucuronide and Pregnanediol Glucuronide Using the Ovarian Monitor, Part III: Variability of Normal Menstrual Cycle Profiles. Hum. Reprod. 2013, 28, 3306–3315. [Google Scholar] [CrossRef]
- Alliende, M.E. Mean versus Individual Hormonal Profiles in the Menstrual Cycle. Fertil. Steril. 2002, 78, 90–95. [Google Scholar] [CrossRef]
- Usala, S.J.; Alliende, M.E.; Trindade, A.A. Algorithms with Area under the Curve for Daily Urinary Estrone-3-Glucuronide and Pregnanediol-3-Glucuronide to Signal the Transition to the Luteal Phase. Medicina 2022, 58, 119. [Google Scholar] [CrossRef] [PubMed]
- Ecochard, R.; Bouchard, T.; Leiva, R.; Abdulla, S.; Dupuis, O.; Duterque, O.; Garmier Billard, M.; Boehringer, H.; Genolini, C. Characterization of Hormonal Profiles during the Luteal Phase in Regularly Menstruating Women. Fertil. Steril. 2017, 108, 175–182. [Google Scholar] [CrossRef]
- Blackwell, L.F.; Vigil, P.; Alliende, M.E.; Brown, S.; Festin, M.; Cooke, D.G. Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates Using the Ovarian Monitor, Part IV: The Relationship of the Pregnanediol Glucuronide Threshold to Basal Body Temperature and Cervical Mucus as Markers for the Beginning of the Post-Ovulatory Infertile Period. Hum. Reprod. 2016, 31, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, L.F.; Cooke, D.G.; Brown, S. The Use of Estrone-3-Glucuronide and Pregnanediol-3-Glucuronide Excretion Rates to Navigate the Continuum of Ovarian Activity. Front. Public Health 2018, 6, 153. [Google Scholar] [CrossRef]
- Alliende, M.E.; Arraztoa, J.A.; Guajardo, U.; Mellado, F. Towards the Clinical Evaluation of the Luteal Phase in Fertile Women: A Preliminary Study of Normative Urinary Hormone Profiles. Front. Public Health 2018, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Keay, N. Quantitative Hormone Analysis Reveals Sources of Variability in the Menstrual Cycle. Women Sport Phys. Act. J. 2024, 32 (Suppl. S1), wspaj.2023-0062. [Google Scholar] [CrossRef]
- Usala, S.J.; Trindade, A.A. A Novel Fertility Indicator Equation Using Estradiol Levels for Assessment of Phase of the Menstrual Cycle. Medicina 2020, 56, 555. [Google Scholar] [CrossRef] [PubMed]
- Usala, S.J.; Alliende, M.E.; Trindade, A.A. The Fertility Indicator Equation Using Serum Progesterone and Urinary Pregnanediol-3-Glucuronide for Assessment of Ovulatory to Luteal Phase Transition. Medicina 2021, 57, 134. [Google Scholar] [CrossRef]
- AIUM Practice Parameter for the Performance of a Focused Ultrasound Examination in Reproductive Endocrinology and FemaleInfertility. J. Ultrasound Med. 2019, 38, E1–E3. [CrossRef]
- Fehring, R.J.; Schneider, M. Comparison of Abstinence and Coital Frequency Between 2 Natural Methods of Family Planning. J. Midwifery Womens Health 2014, 59, 528–532. [Google Scholar] [CrossRef]
- Peragallo Urrutia, R.; Polis, C.B.; Jensen, E.T.; Greene, M.E.; Kennedy, E.; Stanford, J.B. Effectiveness of Fertility Awareness-Based Methods for Pregnancy Prevention: A Systematic Review. Obstet. Gynecol. 2018, 132, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Fehring, R.J.; Schneider, M.; Barron, M.L. Efficacy of the Marquette Method of Natural Family Planning. MCN Am. J. Matern. Child. Nurs. 2008, 33, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Burke, E.E.; Beqaj, S.; Douglas, N.C.; Luo, R. Concordance of Fingerstick and Venipuncture Sampling for Fertility Hormones. Obstet. Gynecol. 2019, 133, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Wang, D.; Arain, H.; Mohan, C. Design of Gold Nanoparticle Vertical Flow Assays for Point-of-Care Testing. Diagnostics 2022, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Y.; Xu, Y. Determining Procalcitonin at Point-of-Care; A Method Comparison Study of Four Commercial PCT Assays. Pract. Lab. Med. 2021, 25, e00214. [Google Scholar] [CrossRef]
- Corstjens, P.L.A.M.; van Hooij, A.; Tjon Kon Fat, E.M.; Alam, K.; Vrolijk, L.B.; Dlamini, S.; da Silva, M.B.; Spencer, J.S.; Salgado, C.G.; Richardus, J.H.; et al. Fingerstick Test Quantifying Humoral and Cellular Biomarkers Indicative for M. Leprae Infection. Clin. Biochem. 2019, 66, 76–82. [Google Scholar] [CrossRef]
- Li, H.; Han, D.; Hegener, M.A.; Pauletti, G.M.; Steckl, A.J. Flow Reproducibility of Whole Blood and Other Bodily Fluids in Simplified No Reaction Lateral Flow Assay Devices. Biomicrofluidics 2017, 11, 024116. [Google Scholar] [CrossRef]
- Prajapati, A.; Verma, N.; Pandya, A. Highly Sensitive Vertical Flow Based Point-of-Care Immunokit for Rapid and Early Detection of Human CRP as a Cardiovascular Risk Factor. Biomed. Microdevices 2020, 22, 28. [Google Scholar] [CrossRef]
- Li, H.; Steckl, A.J. Paper Microfluidics for Point-of-Care Blood-Based Analysis and Diagnostics. Anal. Chem. 2019, 91, 352–371. [Google Scholar] [CrossRef]
- Lykins, J.; Li, X.; Levigne, P.; Zhou, Y.; El Bissati, K.; Clouser, F.; Wallon, M.; Morel, F.; Leahy, K.; El Mansouri, B.; et al. Rapid, Inexpensive, Fingerstick, Whole-Blood, Sensitive, Specific, Point-of-Care Test for Anti-Toxoplasma Antibodies. PLoS Negl. Trop. Dis. 2018, 12, e0006536. [Google Scholar] [CrossRef] [PubMed]

| Day of Cycle Day 0 = 1st Day of DF Collapse | 1Y1 FIE-E2 | 2Y1 FIE-E2 | 4Y1 FIE-E2 | 6Y1 FIE-E2 | 2Y1 FIE-E3G | 4Y1 FIE-E3G | 6Y1 FIE-E3G |
|---|---|---|---|---|---|---|---|
| −12 | 0 | 0 | 0 | 8.91 | |||
| −11 | 0 | −0.25 | −0.13 | 0 | |||
| −10 | 0 | −3.65 | 0 | 0 | |||
| −9 | −4.45 | 0 | 0 | 0 | |||
| −8 | 0 | 20.34 | 0 | 0 | 0 | 0 | |
| −7 | 0 | 0 | 5.11 | 14.22 | −3.39 | −1.67 | |
| −6 | 0 | 0 | 2.66 | 3.99 | 0 | 0 | |
| −5 | 27.66 | 13.85 | 6.36 | 15.97 | 0 | 0 | 3.74 |
| −4 | 1.66 | 3.51 | 2.3 | 17.51 | 0 | 0 | 0.89 |
| −3 | 0.35 | 2.2 | 7.05 | 6.87 | 120.06 | 0 | 0 |
| −2 | 33.3 | 4.15 | 19.31 | 3.45 | 26 | 0 | 0 |
| −1 | 0 | 0 | 0 | 0 | 0 | 5.52 | 0 |
| 0 | −5.01 | −22.73 | −25.7 | −39.41 | 0 | 3.95 | 0 |
| 1 | −19.4 | 0 | −7.41 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 73.39 | 0 | 0 | 0 |
| 3 | 0 | 0 | 3.19 | 6.17 | 19.37 | 0 | 0 |
| 4 | 0 | 0 | 0.18 | 0 | 0 | −0.188 | 0 |
| 5 | 0 | 0 | 0.35 | 0 | −15.04 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | −5.62 | 0 | 0 |
| 7 | 0 | 0 | −0.21 | 2.46 | −7.75 | 0 | −0.744 |
| 8 | −14.37 | 0 | 0 | 2.86 | 0 | 0 | 0 |
| 9 | −24.4 | −2.22 | 0 | 0 | 0 | −8.03 | 0 |
| 10 | −3.95 | −1.52 | −1.28 | 0 | 0 | −2.92 | |
| 11 | 0 | −6.58 | −6.48 | 0 | 1.82 | 0 | |
| 12 | 0 | −9.71 | −17.44 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usala, S.J.; Vineyard, D.D.; Kastis, M.; Trindade, A.A.; Gill, H.S. Comparison of Day-Specific Serum LH, Estradiol, and Progesterone with MiraTM Monitor Urinary LH, Estrone-3-glucuronide, and Pregnanediol-3-glucuronide Levels in Ovulatory Cycles. Medicina 2024, 60, 1207. https://doi.org/10.3390/medicina60081207
Usala SJ, Vineyard DD, Kastis M, Trindade AA, Gill HS. Comparison of Day-Specific Serum LH, Estradiol, and Progesterone with MiraTM Monitor Urinary LH, Estrone-3-glucuronide, and Pregnanediol-3-glucuronide Levels in Ovulatory Cycles. Medicina. 2024; 60(8):1207. https://doi.org/10.3390/medicina60081207
Chicago/Turabian StyleUsala, Stephen J., David D. Vineyard, Maria Kastis, A. Alexandre Trindade, and Harvinder Singh Gill. 2024. "Comparison of Day-Specific Serum LH, Estradiol, and Progesterone with MiraTM Monitor Urinary LH, Estrone-3-glucuronide, and Pregnanediol-3-glucuronide Levels in Ovulatory Cycles" Medicina 60, no. 8: 1207. https://doi.org/10.3390/medicina60081207






