Conventional and Novel Inflammatory Biomarkers in Chronic Heart Failure Patients with Atrial Fibrillation
Abstract
1. Introduction
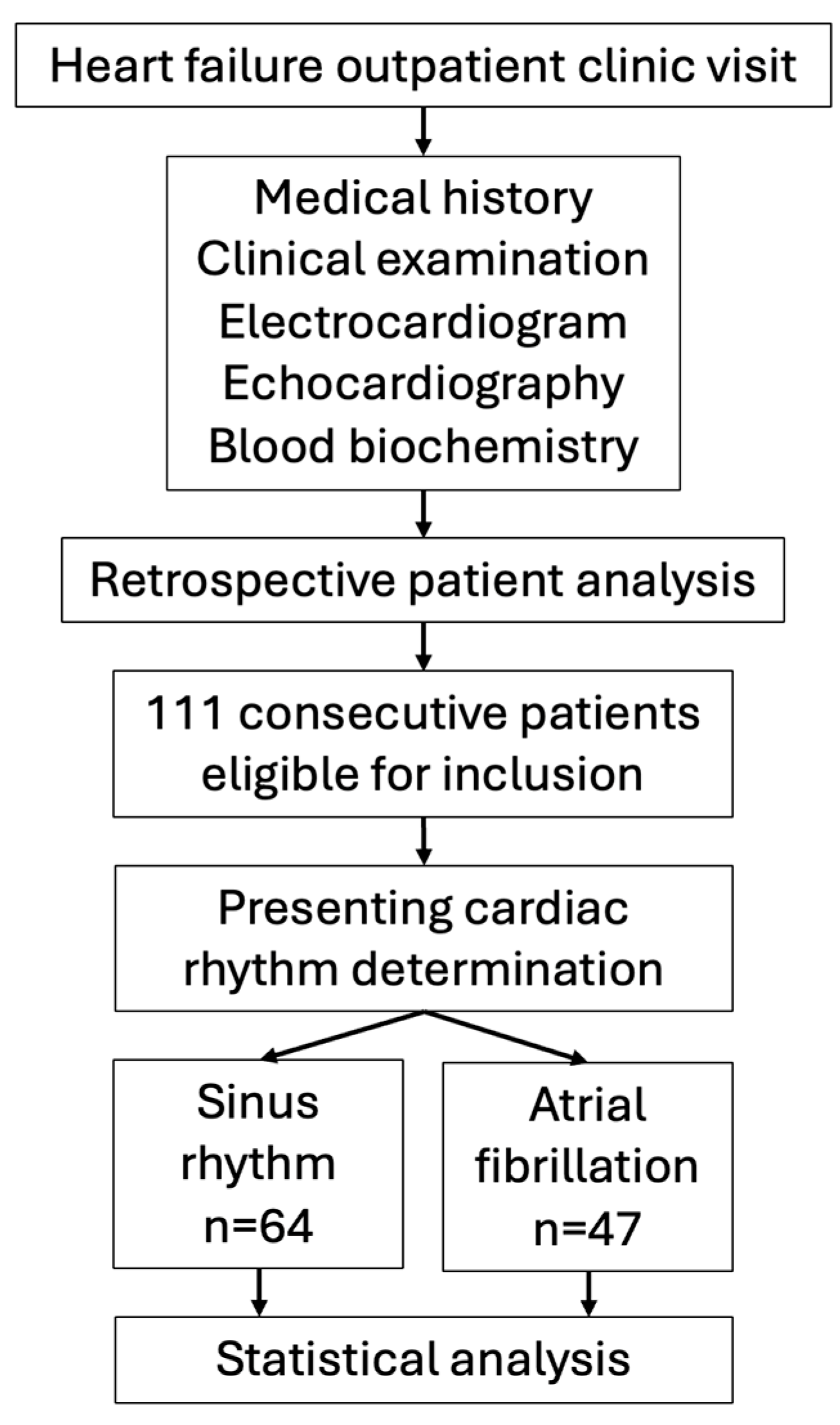
2. Materials and Methods
2.1. Patients, Study Design and Biochemical Analysis
2.2. Statistical Analysis
3. Results
- -
- AF is associated with elevated levels of IL-6 and a higher pro-/anti-inflammatory IL-6/IL-10 ratio in patients with stable chronic HF under the age of 75 years.
- -
- IL-6 levels were independently associated with AF even after adjusting for other inflammatory biomarkers.
- -
- There was no association between the novel inflammatory markers orosomucoid or endocan and AF in patients with chronic stable HF.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannel, W.B.; Wolf, P.A.; Benjamin, E.J.; Levy, D. Prevalence, Incidence, Prognosis, and Predisposing Conditions for Atrial Fibrillation: Population-Based Estimates. Am. J. Cardiol. 1998, 82, 2N–9N. [Google Scholar] [CrossRef] [PubMed]
- Aviles, R.J.; Martin, D.O.; Apperson-Hansen, C.; Houghtaling, P.L.; Rautaharju, P.; Kronmal, R.A.; Tracy, R.P.; Van Wagoner, D.R.; Psaty, B.M.; Lauer, M.S.; et al. Inflammation as a Risk Factor for Atrial Fibrillation. Circulation 2003, 108, 3006–3010. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, M.D.M.; Svendsen, J.H. Inflammation in the Genesis and Perpetuation of Atrial Fibrillation. Eur. Heart J. 2005, 26, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Boos, C.J.; Anderson, R.A.; Lip, G.Y.H. Is Atrial Fibrillation an Inflammatory Disorder? Eur. Heart J. 2006, 27, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Heijman, J.; Zhou, L.; Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circ. Res. 2020, 127, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Roldan, V.; Marín, F.; Blann, A.; García, A.; Marco, P.; Sogorb, F.; Lip, G.Y.H. Interleukin-6, Endothelial Activation and Thrombogenesis in Chronic Atrial Fibrillation. Eur. Heart J. 2003, 24, 1373–1380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marcus, G.M.; Whooley, M.A.; Glidden, D.V.; Pawlikowska, L.; Zaroff, J.G.; Olgin, J.E. Interleukin-6 and Atrial Fibrillation in Patients with Coronary Artery Disease: Data from the Heart and Soul Study. Am. Heart J. 2008, 155, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ucar, H.; Tok, M.; Atalar, E.; Dogan, O.; Oc, M.; Farsak, B.; Guvener, M.; Yilmaz, M.; Dogan, R.; Demircin, M.; et al. Predictive Significance of Plasma Levels of Interleukin-6 and High-Sensitivity C-Reactive Protein in Atrial Fibrillation after Coronary Artery Bypass Surgery. Heart Surg. Forum 2007, 10, E131–E135. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Mukherjee, M.; Go, A.; Barrows, I.R.; Ramezani, A.; Shoji, J.; Reilly, M.P.; Gnanaraj, J.; Deo, R.; Roas, S.; et al. Interleukin-6 Is a Risk Factor for Atrial Fibrillation in Chronic Kidney Disease: Findings from the CRIC Study. PLoS ONE 2016, 11, e0148189. [Google Scholar] [CrossRef]
- Fujiki, A.; Sakamoto, T.; Nishida, K.; Mizumaki, K.; Inoue, H. Relation of Interleukin-6 and C-Reactive Protein Levels to Sinus Maintenance After Pharmacological Cardioversion in Persistent Atrial Fibrillation. J. Cardiovasc. Pharmacol. 2007, 50, 264–266. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, W.; Wang, C.; Xie, X.; Hou, Y. Association of Pre-Ablation Level of Potential Blood Markers with Atrial Fibrillation Recurrence after Catheter Ablation: A Meta-Analysis. Europace 2017, 19, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Jaroonpipatkul, S.; Trongtorsak, A.; Kewcharoen, J.; Thangjui, S.; Pokawattana, A.; Navaravong, L. High Sensitivity C Reactive Protein Levels and Atrial Fibrillation Recurrence after Catheter Ablation for Atrial Fibrillation: A Systematic Review and Meta-analysis. J. Arrhythmia 2023, 39, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Tu, D.; Liu, X.; Niu, S.; Suo, Y.; Liu, T.; Li, G.; Liu, C. Role of NLRP3-Inflammasome/Caspase-1/Galectin-3 Pathway on Atrial Remodeling in Diabetic Rabbits. J. Cardiovasc. Trans. Res. 2020, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Jalloul, Y.; Refaat, M.M. IL-6 Rapidly Induces Reversible Atrial Electrical Remodeling by Downregulation of Cardiac Connexins. J. Am. Heart Assoc. 2019, 8, e013638. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Acampa, M.; Srivastava, U.; Bertolozzi, I.; Giabbani, B.; Finizola, F.; Vanni, F.; Dokollari, A.; Natale, M.; et al. Systemic Inflammation Rapidly Induces Reversible Atrial Electrical Remodeling: The Role of Interleukin-6–Mediated Changes in Connexin Expression. J. Am. Heart Assoc. 2019, 8, e011006. [Google Scholar] [CrossRef] [PubMed]
- Palà, E.; Bustamante, A.; Pagola, J.; Juega, J.; Francisco-Pascual, J.; Penalba, A.; Rodriguez, M.; De Lera Alfonso, M.; Arenillas, J.F.; Cabezas, J.A.; et al. Blood-Based Biomarkers to Search for Atrial Fibrillation in High-Risk Asymptomatic Individuals and Cryptogenic Stroke Patients. Front. Cardiovasc. Med. 2022, 9, 908053. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, L.; Yu, X.-H.; Hu, M.; Zhang, Y.-K.; Liu, X.; He, P.; Ouyang, X. Endocan: A Key Player of Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 8, 798699. [Google Scholar] [CrossRef]
- Weis, F.; Beiras-Fernandez, A.; Schelling, G.; Briegel, J.; Lang, P.; Hauer, D.; Kreth, S.; Kaufmann, I.; Lamm, P.; Kilger, E. Stress Doses of Hydrocortisone in High-Risk Patients Undergoing Cardiac Surgery: Effects on Interleukin-6 to Interleukin-10 Ratio and Early Outcome. Crit. Care Med. 2009, 37, 1685–1690. [Google Scholar] [CrossRef]
- Fandiño-Vaquero, R.; Fernández-Trasancos, A.; Álvarez, E.; Ahmad, S.; Batista-Oliveira, A.L.; Adrio, B.; Fernández, Á.L.; González-Juanatey, J.R.; Eiras, S. Orosomucoid Secretion Levels by Epicardial Adipose Tissue as Possible Indicator of Endothelial Dysfunction in Diabetes Mellitus or Inflammation in Coronary Artery Disease. Atherosclerosis 2014, 235, 281–288. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.; Kim, S.; Bae, J. Endocan Elicits Severe Vascular Inflammatory Responses In Vitro and In Vivo. J. Cell. Physiol. 2014, 229, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Mundisugih, J.; Franke, K.B.; Tully, P.J.; Munawar, D.A.; Kumar, S.; Mahajan, R. Prevalence and Prognostic Implication of Atrial Fibrillation in Heart Failure Subtypes: Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, 666–677. [Google Scholar] [CrossRef]
- Iglesias-Álvarez, D.; Fu, X.; Martínez-Cereijo, J.M.; Agra-Bermejo, R.M.; Veiras-Del Río, S.; Selas-Cobos, S.; Rial-Munin, M.V.; Eiras-Mariño, M.; Martínez-Salgado, A.; Taboada-Muñiz, M.; et al. Clinical Profiling and Biomarkers for Post-Operative Atrial Fibrillation Prediction in Patients Undergoing Cardiac Surgery. J. Clin. Med. 2023, 12, 3565. [Google Scholar] [CrossRef] [PubMed]
- Adamsson Eryd, S.; Smith, J.G.; Melander, O.; Hedblad, B.; Engström, G. Inflammation-Sensitive Proteins and Risk of Atrial Fibrillation: A Population-Based Cohort Study. Eur. J. Epidemiol. 2011, 26, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Jug, B.; Salobir, B.G.; Vene, N.; Šebeštjen, M.; Šabovič, M.; Keber, I. Interleukin-6 Is a Stronger Prognostic Predictor than High-Sensitive C-Reactive Protein in Patients with Chronic Stable Heart Failure. Heart Vessel. 2009, 24, 271–276. [Google Scholar] [CrossRef]
- Kosir, G.; Jug, B.; Novakovic, M.; Mijovski, M.B.; Ksela, J. Endocan Is an Independent Predictor of Heart Failure-Related Mortality and Hospitalizations in Patients with Chronic Stable Heart Failure. Dis. Markers 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Li, H.; Song, X.; Liang, Y.; Bai, X.; Liu-Huo, W.-S.; Tang, C.; Chen, W.; Zhao, L. Global, Regional, and National Burden of Disease Study of Atrial Fibrillation/Flutter, 1990–2019: Results from a Global Burden of Disease Study, 2019. BMC Public. Health 2022, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tsigkas, G.; Apostolos, A.; Despotopoulos, S.; Vasilagkos, G.; Kallergis, E.; Leventopoulos, G.; Mplani, V.; Davlouros, P. Heart Failure and Atrial Fibrillation: New Concepts in Pathophysiology, Management, and Future Directions. Heart Fail. Rev. 2022, 27, 1201–1210. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Chen, Y.-J.; Lin, Y.-J.; Chen, S.-A. Inflammation and the Pathogenesis of Atrial Fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Gomez, S.E.; Parizo, J.; Ermakov, S.; Larson, J.; Wallace, R.; Assimes, T.; Hlatky, M.; Stefanick, M.; Perez, M.V. Evaluation of the Association between Circulating IL-1β and Other Inflammatory Cytokines and Incident Atrial Fibrillation in a Cohort of Postmenopausal Women. Am. Heart J. 2023, 258, 157–167. [Google Scholar] [CrossRef]
- Aulin, J.; Siegbahn, A.; Hijazi, Z.; Ezekowitz, M.D.; Andersson, U.; Connolly, S.J.; Huber, K.; Reilly, P.A.; Wallentin, L.; Oldgren, J. Interleukin-6 and C-Reactive Protein and Risk for Death and Cardiovascular Events in Patients with Atrial Fibrillation. Am. Heart J. 2015, 170, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Aulin, J.; Andersson, U.; Alexander, J.H.; Gersh, B.; Granger, C.B.; Hanna, M.; Horowitz, J.; Hylek, E.M.; Lopes, R.D.; et al. Biomarkers of Inflammation and Risk of Cardiovascular Events in Anticoagulated Patients with Atrial Fibrillation. Heart 2016, 102, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Silvain, J.; Kerneis, M.; Zeitouni, M.; Lattuca, B.; Galier, S.; Brugier, D.; Mertens, E.; Procopi, N.; Suc, G.; Salloum, T.; et al. Interleukin-1β and Risk of Premature Death in Patients with Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Abe, I.; Gotoh, K.; Fukui, A.; Takanari, H.; Ishii, Y.; Ikebe, Y.; Kira, S.; Oniki, T.; Saito, S.; et al. Interleukin 10 Treatment Ameliorates High-Fat Diet–Induced Inflammatory Atrial Remodeling and Fibrillation. Circ. Arrhythmia Electrophysiol. 2018, 11, e006040. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.L.; Duarte, R.C.F.; Vieira, É.L.M.; Rocha, N.P.; Figueiredo, E.L.; Silveira, F.R.; Caiaffa, J.R.S.; Lanna, R.P.; Carvalho, M.D.G.; Palotás, A.; et al. Evaluation of New Potential Inflammatory Markers in Patients with Nonvalvular Atrial Fibrillation. Int. J. Mol. Sci. 2023, 24, 3326. [Google Scholar] [CrossRef] [PubMed]
- Povar-Echeverría, M.; Auquilla-Clavijo, P.E.; Andrès, E.; Martin-Sánchez, F.J.; Laguna-Calle, M.V.; Calvo-Elías, A.E.; Lorenzo-Villalba, N.; Méndez-Bailón, M. Interleukin-6 Could Be a Potential Prognostic Factor in Ambulatory Elderly Patients with Stable Heart Failure: Results from a Pilot Study. J. Clin. Med. 2021, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Schrage, B.; Geelhoed, B.; Niiranen, T.J.; Gianfagna, F.; Vishram-Nielsen, J.K.K.; Costanzo, S.; Söderberg, S.; Ojeda, F.M.; Vartiainen, E.; Donati, M.B.; et al. Comparison of Cardiovascular Risk Factors in European Population Cohorts for Predicting Atrial Fibrillation and Heart Failure, Their Subsequent Onset, and Death. J. Am. Heart Assoc. 2020, 9, e015218. [Google Scholar] [CrossRef]
- Zhang, L.; He, G.; Huo, X.; Tian, A.; Ji, R.; Pu, B.; Peng, Y. Long-Term Cumulative High-Sensitivity C-Reactive Protein and Mortality Among Patients with Acute Heart Failure. J. Am. Heart Assoc. 2023, 12, e029386. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, P.; Pereira, J.; Ribeiro, A.; Ferreira-Coimbra, J.; Barroso, I.; Guimarães, J.-T.; Leite-Moreira, A.; Bettencourt, P. C-Reactive Protein Decrease Associates with Mortality Reduction Only in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Med. 2019, 20, 23–29. [Google Scholar] [CrossRef]
- Hatem, S.N.; Sanders, P. Epicardial Adipose Tissue and Atrial Fibrillation. Cardiovasc. Res. 2014, 102, 205–213. [Google Scholar] [CrossRef]
- Wang, P.; Feng, J.; Zhang, Z.; Chen, Y.; Qin, Z.; Dai, X.; Wei, J.; Hu, B.; Zhang, W.; Sun, Y.; et al. The Adipokine Orosomucoid Alleviates Adipose Tissue Fibrosis via the AMPK Pathway. Acta Pharmacol. Sin. 2022, 43, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Faridi, L.; Nikniaz, L.; Daneshvar, S.; Naseri, A.; Taban-Sadeghi, M.; Manaflouyan, H.; Shahabi, J.; Sarrafzadegan, N. Effect of Endothelial Adhesion Molecules on Atrial Fibrillation: A Systematic Review and Meta-Analysis. Heart Int. 2022, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Willeit, K.; Pechlaner, R.; Willeit, P.; Skroblin, P.; Paulweber, B.; Schernthaner, C.; Toell, T.; Egger, G.; Weger, S.; Oberhollenzer, M.; et al. Association Between Vascular Cell Adhesion Molecule 1 and Atrial Fibrillation. JAMA Cardiol. 2017, 2, 516. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Bukowska, A.; Lendeckel, U.; Erxleben, M.; Hammwöhner, M.; Strugala, D.; Pfeiffenberger, J.; Röhl, F.-W.; Huth, C.; Ebert, M.P.A.; et al. Angiotensin II Receptor Blockade Reduces Tachycardia-Induced Atrial Adhesion Molecule Expression. Circulation 2008, 117, 732–742. [Google Scholar] [CrossRef]
- Shirazi, L.F.; Bissett, J.; Romeo, F.; Mehta, J.L. Role of Inflammation in Heart Failure. Curr. Atheroscler. Rep. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Mann, D.L.; Deswal, A. Biomarkers of Inflammation in Heart Failure. Heart Fail. Rev. 2010, 15, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the Role of Inflammation in Heart Failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Sanders-van Wijk, S.; Van Empel, V.; Davarzani, N.; Maeder, M.T.; Handschin, R.; Pfisterer, M.E.; Brunner-La Rocca, H.P.; for the TIME-CHF Investigators. Circulating Biomarkers of Distinct Pathophysiological Pathways in Heart Failure with Preserved vs. Reduced Left Ventricular Ejection Fraction. Eur. J. Heart Fail. 2015, 17, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.S.S.; Da Paixão, A.B.F.; Da Rocha Junior, L.F.; Branco Pinto Duarte, A.L.; Pereira, M.C.; Barreto De Melo Rêgo, M.J.; Da Rocha Pitta, I.; Da Rocha Pitta, M.G. Atorvastatin Inhibits IL-17A, TNF, IL-6, and IL-10 in PBMC Cultures from Patients with Severe Rheumatoid Arthritis. Immunobiology 2020, 225, 151908. [Google Scholar] [CrossRef]
- Saeed, H.; Mateen, S.; Moin, S.; Khan, A.Q.; Owais, M. Cardiac Glycoside Digoxin Ameliorates Pro-Inflammatory Cytokines in PBMCs of Rheumatoid Arthritis Patients in Vitro. Int. Immunopharmacol. 2020, 82, 106331. [Google Scholar] [CrossRef]

| All | Sinus Rhythm | AF | p-Value | |
|---|---|---|---|---|
| Patients (n) | 111 | 64 (57.7%) | 47 (42.3%) | / |
| Age (years) | 71.2 ± 10.7 | 69.6 ± 11.4 | 73.5 ± 9.4 | 0.116 |
| Sex (male, %) | 64.9 | 68.8 | 59.6 | 0.317 |
| AF (%) | 42.3 | 0 | 100 | / |
| EF (%) | 35 (27.5–40) | 34 (27–40) | 35 (30–40) | 0.206 |
| CAD (%) | 49.6 | 54.7 | 42.6 | 0.206 |
| CVD (%) | 22.5 | 20.3 | 25.5 | 0.515 |
| PAD (%) | 19.8 | 18.8 | 21.3 | 0.741 |
| DM (%) | 33.3 | 34.4 | 31.9 | 0.786 |
| AH (%) | 71.2 | 73.4 | 68.1 | 0.538 |
| HLP (%) | 47.7 | 53.1 | 40.4 | 0.186 |
| NYHA I/II/III (%) | 3.6/65.8/30.6 | 6.3/68.8/25.0 | 0/61.7/38.3 | 0.095 |
| ACEI/ARB (%) | 97.3 | 98.4 | 95.7 | 0.387 |
| Beta blocker (%) | 90.1 | 87.5 | 93.6 | 0.287 |
| MRA (%) | 50.5 | 45.3 | 57.4 | 0.206 |
| Loop diuretic (%) | 64.0 | 56.3 | 74.5 | 0.048 |
| Digoxin (%) | 21.6 | 9.4 | 38.3 | <0.001 |
| Statin (%) | 37.8 | 46.9 | 25.5 | 0.022 |
| NT-proBNP (pg/mL) | 1697 (705–4353) | 1352 (494–4670) | 2095 (1423–3992) | 0.046 |
| hsCRP (mg/L) | 2.7 (1.4–6.6) | 2.9 (1.4–6.2) | 2.4 (1.4–7.5) | 0.988 |
| IL-6 (ng/L) | 5.1 (2.5–8.2) | 3.9 (2.1–7.6) | 6.1 (3.5–8.9) | 0.083 |
| IL-10 (ng/L) | 11.6 (9.4–13.7) | 11.2 (9.0–13.5) | 12.1 (9.9–14.2) | 0.233 |
| IL6-/IL-10 | 0.428 (0.187–0.674) | 0.382 (0.158–0.645) | 0.488 (0.320–0.713) | 0.160 |
| Orosomucoid (mcg/L) | 597 (392–1062) | 602 (434–952) | 566 (349–1223) | 0.818 |
| Endocan (mcg/L) | 3.3 (2.4–4.9) | 3.2 (2.3–4.6) | 3.6 (2.9–5.0) | 0.300 |
| All | Sinus Rhythm | AF | p-Value | |
|---|---|---|---|---|
| Patients (n) | 70 | 43 | 27 | |
| NT-proBNP (pg/mL) | 1563 (570–4583) | 729 (332–4447) | 2095 (1491–4764) | 0.022 |
| hsCRP (mg/L) | 2.8 (1.3–6.7) | 2.8 (1.5–6.5) | 2.4 (1.2–7.9) | 0.914 |
| IL-6 (ng/L) | 5.3 (2.6–7.8) | 4.1 (2.0–6.9) | 7.0 (5.3–9.5) | 0.012 |
| IL-10 (ng/L) | 12.2 (9.5–14.3) | 12.0 (9.3–13.7) | 12.4 (10.8–16.6) | 0.178 |
| IL-6/IL-10 | 0.427 (0.192–0. 663) | 0.348 (0.154–0.558) | 0.488 (0.352–0.728) | 0.044 |
| Orosomucoid (mcg/L) | 604 (371–1033) | 550 (383–959) | 649 (322–1431) | 0.638 |
| Endocan (mcg/L) | 3.2 (2.2–4.4) | 2.9 (2.2–4.0) | 3.4 (2.3–4.8) | 0.242 |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| IL-6 | 1.175 (1.013–1.363) | 0.034 | 1.327 (1.068–1.650) | 0.011 |
| IL-10 | 1.002 (0.959–1.047) | 0.931 | 0.998 (0.953–1.044) | 0.914 |
| IL-6/IL-10 | 3.435 (0.745–15.836) | 0.113 | NA | NA |
| Orosomucoid | 1.000 (1.000–1.001) | 0.319 | 1.000 (0.999–1.001) | 0.645 |
| Endocan | 1.151 (0.875–1.515) | 0.315 | 1.039 (0.767–1.407) | 0.805 |
| NT-proBNP | 1.000 (1.000–1.000) | 0.782 | 1.000 (1.000–1.000) | 0.228 |
| hsCRP | 0.993 (0.931–1.059) | 0.820 | 0.939 (0.864–1.020) | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vercek, G.; Jug, B.; Novakovic, M.; Antonic, M.; Djordjevic, A.; Ksela, J. Conventional and Novel Inflammatory Biomarkers in Chronic Heart Failure Patients with Atrial Fibrillation. Medicina 2024, 60, 1238. https://doi.org/10.3390/medicina60081238
Vercek G, Jug B, Novakovic M, Antonic M, Djordjevic A, Ksela J. Conventional and Novel Inflammatory Biomarkers in Chronic Heart Failure Patients with Atrial Fibrillation. Medicina. 2024; 60(8):1238. https://doi.org/10.3390/medicina60081238
Chicago/Turabian StyleVercek, Gregor, Borut Jug, Marko Novakovic, Miha Antonic, Anze Djordjevic, and Jus Ksela. 2024. "Conventional and Novel Inflammatory Biomarkers in Chronic Heart Failure Patients with Atrial Fibrillation" Medicina 60, no. 8: 1238. https://doi.org/10.3390/medicina60081238
APA StyleVercek, G., Jug, B., Novakovic, M., Antonic, M., Djordjevic, A., & Ksela, J. (2024). Conventional and Novel Inflammatory Biomarkers in Chronic Heart Failure Patients with Atrial Fibrillation. Medicina, 60(8), 1238. https://doi.org/10.3390/medicina60081238







