A Concise Guide to D-Wave Monitoring during Intramedullary Spinal Cord Tumour Surgery
Abstract
1. Introduction and Historical Background
2. Neurophysiological Background
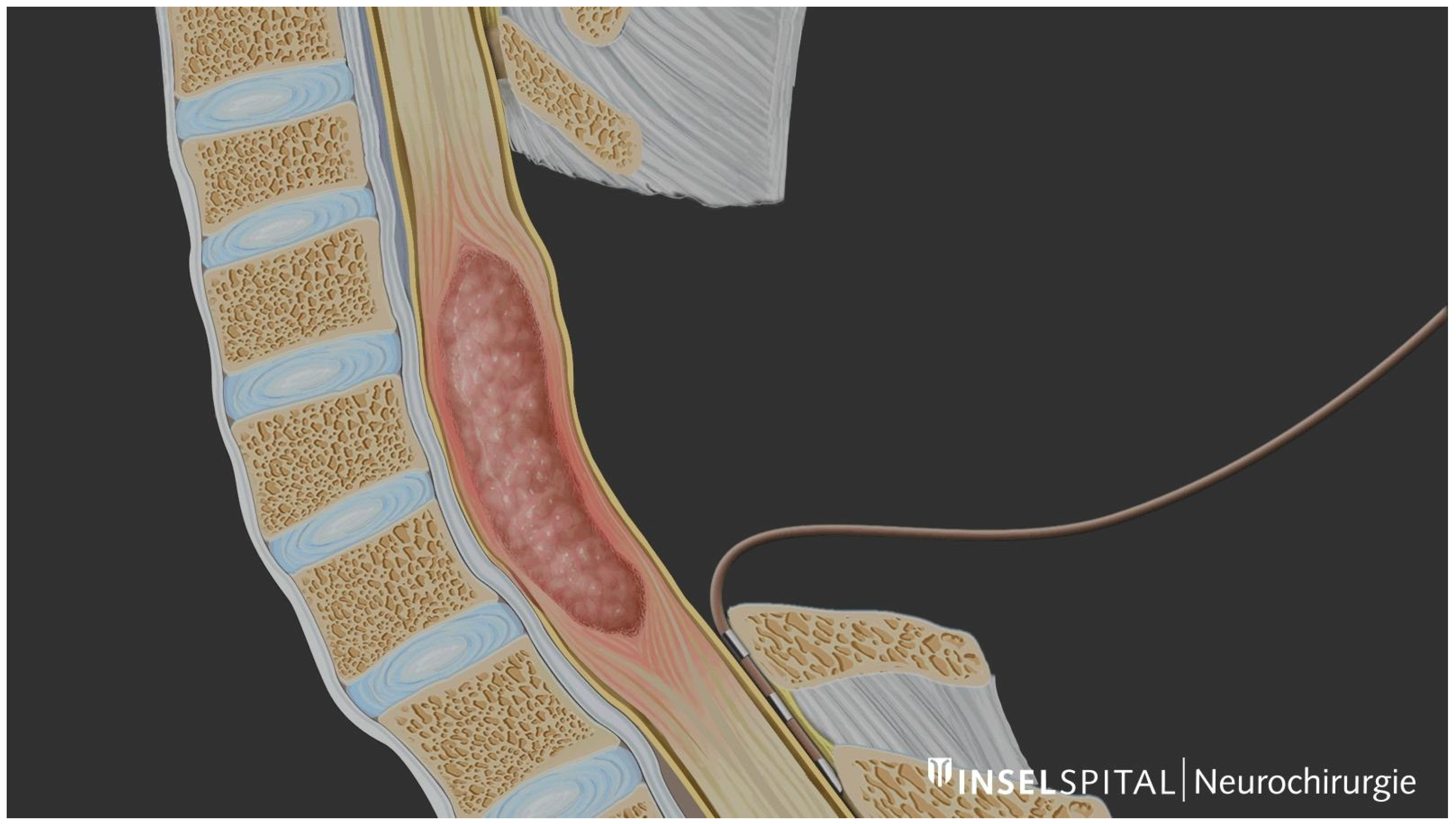
3. Methodology for Intraoperative D-Wave Monitoring
4. D-Waves in Paediatric Spinal Cord Surgery
5. Future Perspectives on CST Monitoring: The Anti D-Wave
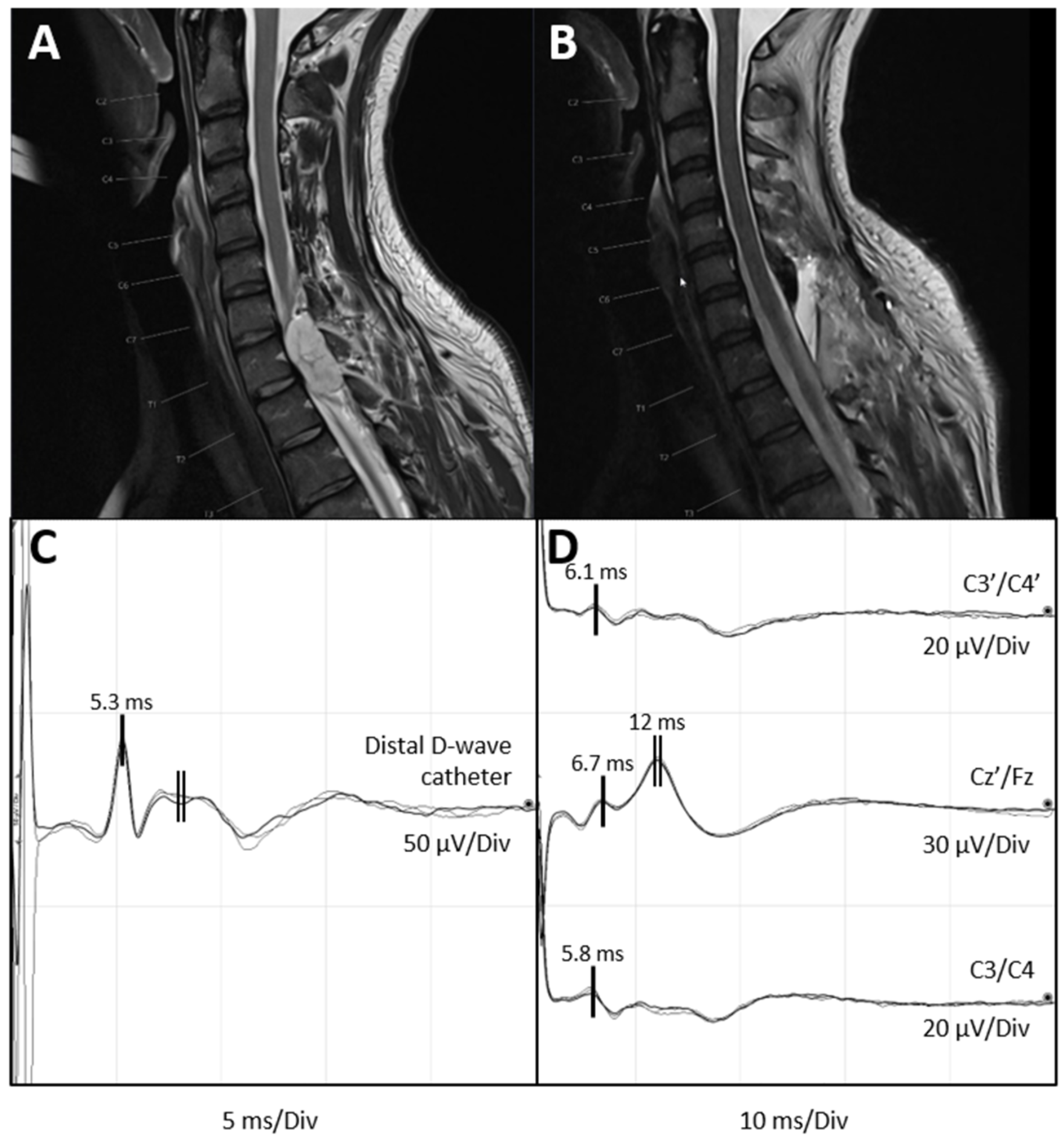
6. Illustrative Case of Feasibility of D-Wave and Anti D-Wave Recordings
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nash, C.L., Jr.; Lorig, R.A.; Schatzinger, L.A.; Brown, R.H. Spinal cord monitoring during operative treatment of the spine. Clin. Orthop. Relat. Res. 1977, 126, 100–105. [Google Scholar] [CrossRef]
- Choi, I.; Hyun, S.J.; Kang, J.K.; Rhim, S.C. Combined muscle motor and somatosensory evoked potentials for intramedullary spinal cord tumour surgery. Yonsei Med. J. 2014, 55, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.B.; Dong, C.; Quatrale, R.; Sala, F.; Skinner, S.; Soto, F.; Szelényi, A. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin. Neurophysiol. 2019, 130, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Macon, J.B.; Poletti, C.E.; Sweet, W.H.; Ojemann, R.G.; Zervas, N.T. Conducted somatosensory evoked potentials during spinal surgery. Part 2: Clinical applications. J. Neurosurg. 1982, 57, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, B.; Haller, G.; Taylor, P. Anterior spinal fusion complicated by paraplegia. A case report of a false-negative somatosensory-evoked potential. Spine 1987, 12, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.H.; Shetter, A.G.; Raudzens, P.A. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials. Case report. J. Neurosurg. 1985, 63, 296–300. [Google Scholar] [CrossRef]
- Lesser, R.P.; Raudzens, P.; Lüders, H.; Nuwer, M.R.; Goldie, W.D.; Morris, H.H., 3rd; Dinner, D.S.; Klem, G.; Hahn, J.F.; Shetter, A.G.; et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann. Neurol. 1986, 19, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, D.; Mancarella, C.; Nasi, D.; Tropeano, M.P.; Anania, C.D.; Cataletti, G.; Milani, D.; Fava, E.M.; Ghadirpour, R.; Costa, F.; et al. Intramedullary spinal cord tumors: The value of intraoperative neurophysiological monitoring in a series of 57 cases from two Italian centers. J. Neurosurg. Sci. 2022, 66, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Jecko, V.; Roblot, P.; Mongardi, L.; Ollivier, M.; Piccoli, N.D.; Charleux, T.; Wavasseur, T.; Gimbert, E.; Liguoro, D.; Chotard, G.; et al. Intramedullary Spinal Cord Lesions: A Single-Center Experience. Neurospine 2022, 19, 108–117. [Google Scholar] [CrossRef]
- Macdonald, D.B.; Skinner, S.; Shils, J.; Yingling, C. Intraoperative motor evoked potential monitoring—A position statement by the American Society of Neurophysiological Monitoring. Clin. Neurophysiol. 2013, 124, 2291–2316. [Google Scholar] [CrossRef]
- Rijs, K.; Klimek, M.; Scheltens-de Boer, M.; Biesheuvel, K.; Harhangi, B.S. Intraoperative Neuromonitoring in Patients with Intramedullary Spinal Cord Tumor: A Systematic Review, Meta-Analysis, and Case Series. World Neurosurg. 2019, 125, 498–510.e492. [Google Scholar] [CrossRef]
- Sala, F.; Bricolo, A.; Faccioli, F.; Lanteri, P.; Gerosa, M. Surgery for intramedullary spinal cord tumors: The role of intraoperative (neurophysiological) monitoring. Eur. Spine J. 2007, 16 (Suppl. S2), S130–S139. [Google Scholar] [CrossRef]
- Kothbauer, K.F.; Deletis, V.; Epstein, F.J. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: Correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg. Focus 1998, 4, e1. [Google Scholar] [CrossRef]
- Morota, N.; Deletis, V.; Constantini, S.; Kofler, M.; Cohen, H.; Epstein, F.J. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 1997, 41, 1327–1336. [Google Scholar] [CrossRef]
- Jallo, G.I.; Kothbauer, K.F.; Epstein, F.J. Intrinsic spinal cord tumor resection. Neurosurgery 2001, 49, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Palandri, G.; Basso, E.; Lanteri, P.; Deletis, V.; Faccioli, F.; Bricolo, A. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: A historical control study. Neurosurgery 2006, 58, 1129–1143; discussion 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Skrap, B.; Tramontano, V.; Faccioli, F.; Meglio, M.; Pinna, G.; Sala, F. Surgery for intramedullary spinal cord ependymomas in the neuromonitoring era: Results from a consecutive series of 100 patients. J. Neurosurg. Spine 2022, 36, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Deletis, V.; Rodi, Z.; Amassian, V.E. Neurophysiological mechanisms underlying motor evoked potentials in anesthetized humans. Part 2. Relationship between epidurally and muscle recorded MEPs in man. Clin. Neurophysiol. 2001, 112, 445–452. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Atiba, A.; Attenello, F.; Woodworth, G.F.; Jallo, G.I. Neurological outcome after resection of intramedullary spinal cord tumors in children. Childs Nerv. Syst. 2008, 24, 93–97. [Google Scholar] [CrossRef]
- Constantini, S.; Miller, D.C.; Allen, J.C.; Rorke, L.B.; Freed, D.; Epstein, F.J. Radical excision of intramedullary spinal cord tumors: Surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J. Neurosurg. 2000, 93, 183–193. [Google Scholar] [CrossRef]
- Woodworth, G.F.; Chaichana, K.L.; McGirt, M.J.; Sciubba, D.M.; Jallo, G.I.; Gokaslan, Z.; Wolinsky, J.P.; Witham, T.F. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery 2007, 61, 99–105; discussion 105–106. [Google Scholar] [CrossRef] [PubMed]
- Amassian, V.E. Animal and human motor system neurophysiology related to intraoperative monitoring. In Neurophysiology in Neurosurgery; Elsevier: Amsterdam, The Netherlands, 2002; pp. 3–23. [Google Scholar]
- Di Lazzaro, V.; Profice, P.; Pilato, F.; Dileone, M.; Oliviero, A.; Ziemann, U. The effects of motor cortex rTMS on corticospinal descending activity. Clin. Neurophysiol. 2010, 121, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.B. Intraoperative motor evoked potential monitoring: Overview and update. J. Clin. Monit. Comput. 2006, 20, 347–377. [Google Scholar] [CrossRef] [PubMed]
- Cobb, W.; London, G.B.; Gastaut, H.; Hess Jr, R.; Jung, R.; Magnus, O.; Terzian, H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Deletis, V.; Sala, F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: A review focus on the corticospinal tracts. Clin. Neurophysiol. 2008, 119, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Deletis, V.; Sala, F. The role of intraoperative neurophysiology in the protection or documentation of surgically induced injury to the spinal cord. Ann. N. Y. Acad. Sci. 2001, 939, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ulkatan, S.; Neuwirth, M.; Bitan, F.; Minardi, C.; Kokoszka, A.; Deletis, V. Monitoring of scoliosis surgery with epidurally recorded motor evoked potentials (D wave) revealed false results. Clin. Neurophysiol. 2006, 117, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Kothbauer, K.; Deletis, V.; Epstein, F.J. Intraoperative spinal cord monitoring for intramedullary surgery: An essential adjunct. Pediatr. Neurosurg. 1997, 26, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kothbauer, K. Intraoperative Neurophysiological Monitoring for Spinal Cord Surgery. Intraoperative Monit. 2008, 3, 56–58. [Google Scholar] [CrossRef]
- McIntyre, I.W.; Francis, L.; McAuliffe, J.J. Transcranial Motor-Evoked Potentials Are More Readily Acquired Than Somatosensory-Evoked Potentials in Children Younger Than 6 Years. Anesth. Analg. 2016, 122, 212–218. [Google Scholar] [CrossRef]
- Brody, B.A.; Kinney, H.C.; Kloman, A.S.; Gilles, F.H. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J. Neuropathol. Exp. Neurol. 1987, 46, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Hömberg, V.; Lenard, H.G. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalogr. Clin. Neurophysiol. 1991, 81, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Lyon, R.; Feiner, J.; Diab, M.; Gregory, G.A. The effect of age on motor evoked potentials in children under propofol/isoflurane anesthesia. Anesth. Analg. 2006, 103, 316–321, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Rivet, D.J.; O’Brien, D.F.; Park, T.S.; Ojemann, J.G. Distance of the motor cortex from the coronal suture as a function of age. Pediatr. Neurosurg. 2004, 40, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Szelényi, A.; Bueno de Camargo, A.; Deletis, V. Neurophysiological evaluation of the corticospinal tract by D-wave recordings in young children. Childs Nerv. Syst. 2003, 19, 30–34. [Google Scholar] [CrossRef]
- Fekete, G.; Bognár, L.; Novák, L. D-wave recording during the surgery of a 10-month-old child. Childs Nerv. Syst. 2014, 30, 2135–2138. [Google Scholar] [CrossRef] [PubMed]
- Antkowiak, L.; Putz, M.; Sordyl, R.; Pokora, S.; Mandera, M. Relevance of intraoperative motor evoked potentials and D-wave monitoring for the resection of intramedullary spinal cord tumors in children. Neurosurg. Rev. 2022, 45, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Deletis, V.; Raabe, A.; Lutz, K.; Schucht, P. Intraoperative Neurophysiologic Monitoring and Mapping During Surgery on Intramedullary Spinal Cord Tumors in Children and Adolescents. J. Clin. Neurophysiol. 2024, 41, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Berić, A.; Dimitrijević, M.R.; Sharkey, P.C.; Sherwood, A.M. Cortical potentials evoked by epidural stimulation of the cervical and thoracic spinal cord in man. Electroencephalogr. Clin. Neurophysiol. 1986, 65, 102–110. [Google Scholar] [CrossRef]
- Phillips, L.H., 2nd; Blanco, J.S.; Sussman, M.D. Direct spinal stimulation for intraoperative monitoring during scoliosis surgery. Muscle Nerve 1995, 18, 319–325. [Google Scholar] [CrossRef]
- Ertekin, C.; Sarica, Y.; Uçkardeşler, L. Somatosensory cerebral potentials evoked by stimulation of the lumbo-sacral spinal cord in normal subjects and in patients with conus medullaris and cauda equina lesions. Electroencephalogr. Clin. Neurophysiol. 1984, 59, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Deletis, V. Cortical activity after stimulation of the corticospinal tract in the spinal cord. Clin. Neurophysiol. 2016, 127, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurita Perea, S.N.; Alvarez Abut, P.A.; Seidel, K. A Concise Guide to D-Wave Monitoring during Intramedullary Spinal Cord Tumour Surgery. Medicina 2024, 60, 1242. https://doi.org/10.3390/medicina60081242
Zurita Perea SN, Alvarez Abut PA, Seidel K. A Concise Guide to D-Wave Monitoring during Intramedullary Spinal Cord Tumour Surgery. Medicina. 2024; 60(8):1242. https://doi.org/10.3390/medicina60081242
Chicago/Turabian StyleZurita Perea, Santos Nicolás, Pablo Abel Alvarez Abut, and Kathleen Seidel. 2024. "A Concise Guide to D-Wave Monitoring during Intramedullary Spinal Cord Tumour Surgery" Medicina 60, no. 8: 1242. https://doi.org/10.3390/medicina60081242
APA StyleZurita Perea, S. N., Alvarez Abut, P. A., & Seidel, K. (2024). A Concise Guide to D-Wave Monitoring during Intramedullary Spinal Cord Tumour Surgery. Medicina, 60(8), 1242. https://doi.org/10.3390/medicina60081242




