Biochemical Markers in the Prediction of Pregnancy Outcome in Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
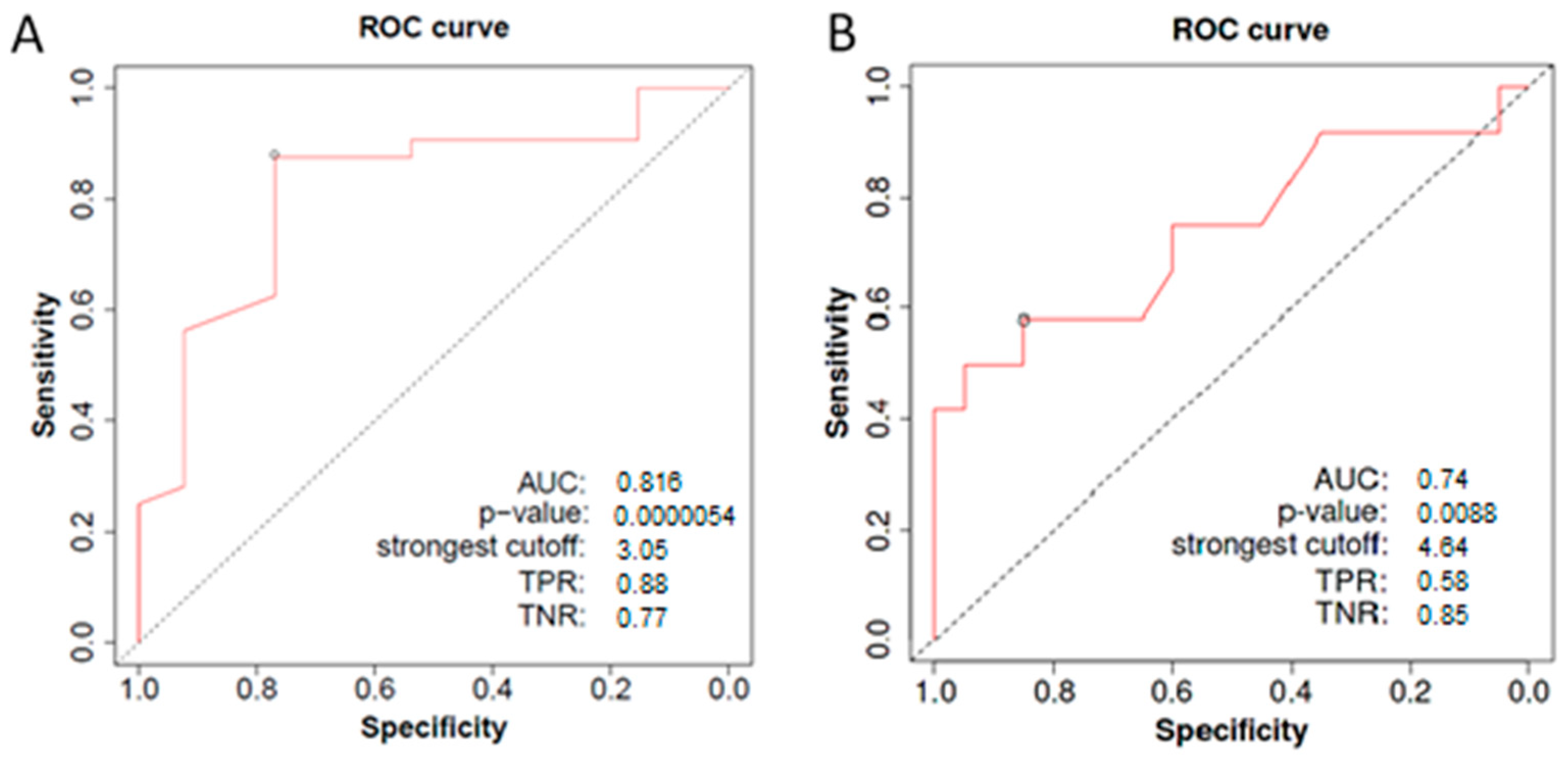
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López Stewart, G. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar]
- Sacks, D.A.; Hadden, D.R.; Maresh, M.; Deerochanawong, C.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Coustan, D.R.; Hod, M.; Oats, J.J.; et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care 2012, 35, 526–528. [Google Scholar] [CrossRef]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2021, 183, 109050. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Schmidt, L.; Damm, P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2464–2470. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Yang, I.V.; Zhang, W.; Davidson, E.J.; Fingerlin, T.E.; Kechris, K.; Dabelea, D. Epigeneticmarks of in utero exposure to gestational diabetes and childhood adiposity outcomes: The EPOCH study. Diabet Med. 2018, 35, 612–620. [Google Scholar] [CrossRef]
- Alesi, S.; Ghelani, D.; Rassie, K.; Mousa, A. Metabolomic Biomarkers in Gestational Diabetes Mellitus: A Review of the Evidence. Int. J. Mol. Sci. 2021, 22, 5512. [Google Scholar] [CrossRef]
- Lu, W.; Hu, C. Molecular biomarkers for gestational diabetes mellitus and postpartum diabetes. Chin. Med. J. 2022, 135, 1940–1951. [Google Scholar] [CrossRef]
- Radojičić, O.; Dobrijević, Z.; Robajac, D.; Gligorijević, N.; Marković, V.M.; Miković, Ž.; Nedić, O. Gestational Diabetes is Associated with an Increased Expression of miR-27a in Peripheral Blood Mononuclear Cells. Mol. Diagn. Ther. 2022, 26, 421–435. [Google Scholar] [CrossRef]
- Zhu, Y.; Barupal, D.K.; Ngo, A.L.; Quesenberry, C.P.; Feng, J.; Fiehn, O.; Ferrara, A. Predictive Metabolomic Markers in Early to Mid-pregnancy for Gestational Diabetes Mellitus: A Prospective Test and Validation Study. Diabetes 2022, 71, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.J.; Venegas, P.; Palmeiro, Y.; Albers, D.; Rice, G.; Roa, J.; Cortez, J.; Monckeberg, M.; Schepeler, M.; Osorio, E.; et al. First trimester prediction of gestational diabetes mellitus using plasma biomarkers: A case-control study. Jpme 2018, 47, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kotzaeridi, G.; Blätter, J.; Eppel, D.; Rosicky, I.; Mittlböck, M.; Yerlikaya-Schatten, G.; Schatten, C.; Husslein, P.; Eppel, W.; Huhn, E.A.; et al. Performance of early risk assessment tools to predict the later development of gestational diabetes. Eur. J. Clin. Investig. 2021, 51, e13630. [Google Scholar] [CrossRef]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; de Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S251–S260. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Fekete, J.T.; Győrffy, B. ROCplot.org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int. J. Cancer 2019, 145, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.H.; Kim, Y.R.; Kim, N.; Jung, J.E.; Han, S.H.; Cho, H.Y. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef]
- Eidgahi, E.S.; Nasiri, M.; Kariman, N.; Ardebili, N.S.; Salehi, M.; Kazemi, M.; Zayeri, F. Diagnostic accuracy of first and early second trimester multiple biomarkers for prediction of gestational diabetes mellitus: A multivariate longitudinal approach. BMC Pregnancy Childbirth 2022, 22, 13. [Google Scholar] [CrossRef]
- Wang, C.; Lin, L.; Su, R.; Zhu, W.; Wei, Y.; Yan, J.; Feng, H.; Li, B.; Li, S.; Yang, H. Hemoglobin levels during the first trimester of pregnancy are associated with the risk of gestational diabetes mellitus, pre-eclampsia and preterm birth in Chinese women: A retrospective study. BMC Pregnancy Childbirth 2018, 18, 263. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Cheng, Y.; Li, T.; Ma, Z.; Liu, J.; Zhang, Q.; Cheng, H. The utility of HbA1c combined with haematocrit for early screening of gestational diabetes mellitus. Diabetol. Metab. Syndr. 2018, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Fan, K.; Jin, L. Serum uric acid in early pregnancy and risk of gestational diabetes mellitus: A cohort study of 85,609 pregnant women. Diabetes Metab. 2022, 48, 101293. [Google Scholar] [CrossRef] [PubMed]
- Pleskacova, A.; Bartakova, V.; Chalasova, K.; Pacal, L.; Kankova, K.; Tomandl, J. Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus—The Effect on Adverse Pregnancy Outcomes. Int. J. Mol. Sci. 2018, 19, 3696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, X.; Wang, Z.; Hu, Y. Combination of lipids and uric acid in mid-second trimester can be used to predict adverse pregnancy outcomes. J. Matern. Neonatal Med. 2012, 25, 2633–2638. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, Q.; Wang, Z.; Tao, Y.; Liu, H.; Tang, F.; Liu, S.-M.; Zhang, Y. Establishment and validation of a predictive nomogram for gestational diabetes mellitus during early pregnancy term: A retrospective study. Front. Endocrinol. 2023, 14, 1087994. [Google Scholar] [CrossRef] [PubMed]
- Wolak, T.; Sergienko, R.; Wiznitzer, A.; Paran, E.; Sheiner, E. High uric acid level during the first 20 weeks of pregnancy is associated with higher risk for gestational diabetes mellitus and mild preeclampsia. Hypertens. Pregnancy 2010, 31, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Q.; Yang, Y.; Ma, L. Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J. Diabetes Investig. 2020, 11, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, Q.; Lv, Y.; Song, X.; Qu, H.; Chen, Y. The relationship between iron metabolism, stress hormones, and insulin resistance in gestational diabetes mellitus. Nutr. Diabetes 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Alam, F.; Moin, S.; Noor, N.; Arif, S.H. Role of ferritin and oxidative stress index in gestational diabetes mellitus. J. Diabetes Metab. Disord. 2021, 20, 1615–1619. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, G.; Li, P.; Rao, J.; Wang, L.; Yu, D.; Lin, D.; Fan, D.; Ye, S.; Wu, S.; et al. Prospective association of metal levels with gestational diabetes mellitus and glucose: A retrospective cohort study from South China. Ecotoxicol. Environ. Saf. 2021, 210, 111854. [Google Scholar] [CrossRef]
- Luo, J.; Wu, W.; Zhang, P.; Chen, X.; Feng, Y.; Ma, N.; Yang, H.; Wang, Y.; Li, M.; Xie, B.; et al. Zinc Levels and Birth Weight in Pregnant Women with Gestational Diabetes Mellitus: A Matched Cohort Study in China. J. Clin. Endocrinol. Metab. 2020, 105, e2337–e2345. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, T.; Yu, Y.; Zhang, B. Is serum zinc status related to gestational diabetes mellitus? A meta-analysis. Matern. Child Nutr. 2021, 17, e13239. [Google Scholar] [CrossRef]
- Deng, G.; Chen, H.; Liu, Y.; Zhou, Y.; Lin, X.; Wei, Y.; Sun, R.; Zhang, Z.; Huang, Z. Combined exposure to multiple essential elements and cadmium at early pregnancy on gestational diabetes mellitus: A prospective cohort study. Front. Nutr. 2023, 10, 1278617. [Google Scholar] [CrossRef]
- Cui, S.; Zhu, X.; Li, S.; Zhang, C. Study on the predictive value of serum hypersensitive C-reactive protein, homocysteine, fibrinogen, and omentin-1 levels with gestational diabetes mellitus. Gynecol. Endocrinol. 2023, 39, 2183046. [Google Scholar] [CrossRef]
- Baboolall, U.; Zha, Y.; Gong, X.; Deng, D.R.; Qiao, F.; Liu, H. Variations of plasma D-dimer level at various points of normal pregnancy and its trends in complicated pregnancies: A retrospective observational cohort study. Medicine 2019, 98, e15903. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, X.; Tao, J.; Song, B.; Wu, W.; Li, Y.; Sun, X.; Lu, D.; Zhu, D.; Liu, C.; et al. Gestational diabetes mellitus is associated with antenatal hypercoagulability and hyperfibrinolysis: A case control study of Chinese women. J. Matern. Neonatal Med. 2020, 35, 2995–2998. [Google Scholar] [CrossRef]
- Dong, C.; Gu, X.; Chen, F.; Long, Y.; Zhu, D.; Yang, X.; Qiu, X.; Gao, G.; Qi, W. The variation degree of coagulation function is not responsible for extra risk of hemorrhage in gestational diabetes mellitus. J. Clin. Lab. Anal. 2020, 34, e23129. [Google Scholar] [CrossRef]
- Chen, J.; Yang, X.; Huang, L.; Zhang, Z.; Yao, J.; Liang, H.; Zhou, W. Insulin resistance biomarkers in small-for-gestational-age infants born to mothers with gestational diabetes mellitus. J. Matern. Neonatal Med. 2021, 35, 9061–9065. [Google Scholar] [CrossRef]
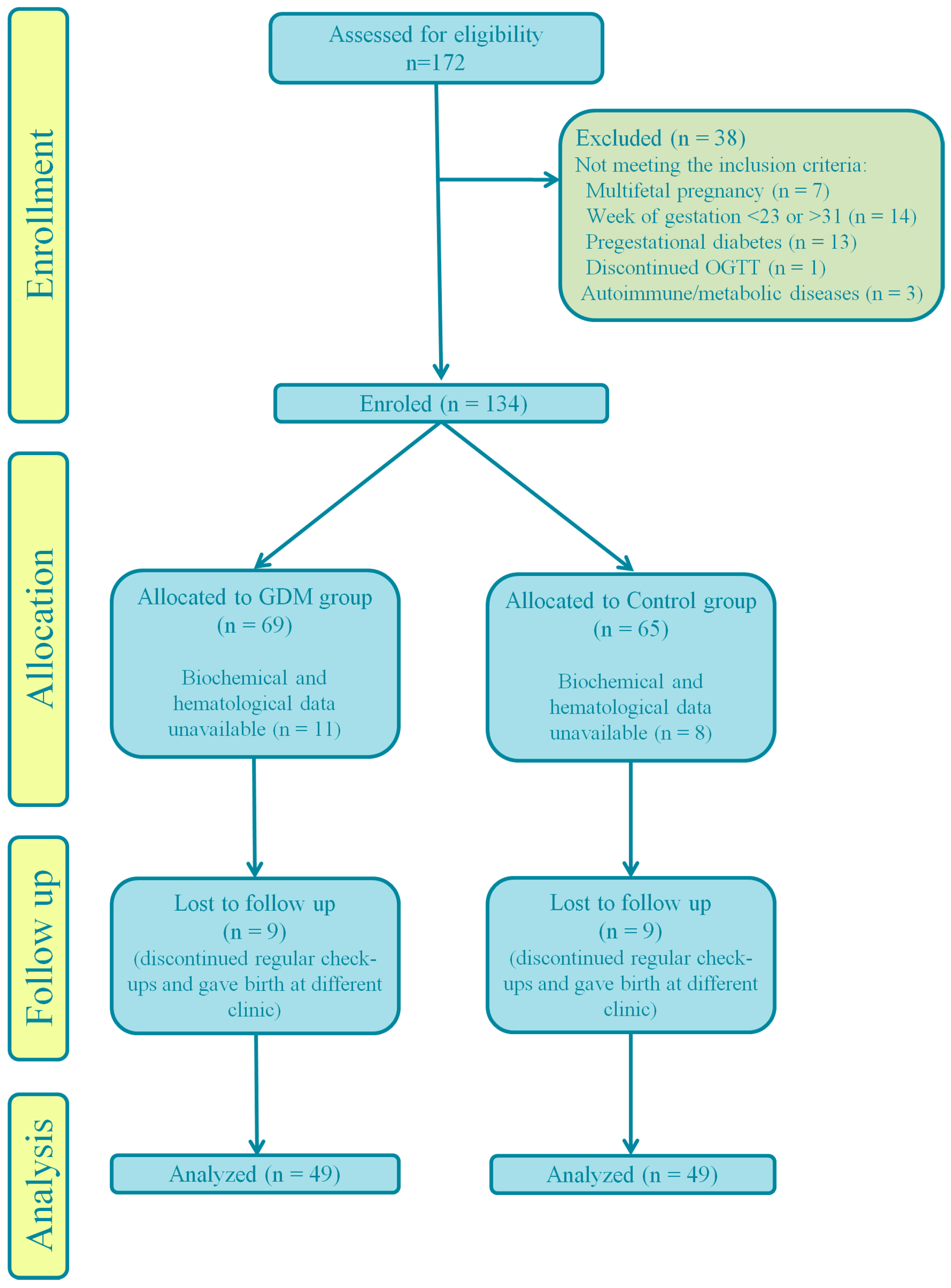
| Inclusion Criteria | Exclusion Criteria | Adverse Outcome |
|---|---|---|
| Indication for OGTT | Multiple pregnancies | Preterm delivery |
| Gestational age—24–30 weeks | Gestational age < 23 or >31 weeks | Fetal growth disturbances (macrosomia or FGR) |
| Singleton pregnancy | Metabolic disease | Polyhydramnios |
| Hypertension | Oligohydramnios | |
| Fetal anomaly | Shoulder dystocia |
| With Adverse Outcome N = 18 | Without Adverse Outcome N = 31 | p | |
|---|---|---|---|
| Age (years) a | 33.4 ± 3.6 | 35.3 ± 4.3 | 0.11 |
| Smoking status (%) | 11.7 | 29 | 0.27 |
| Gestational age at sampling (weeks) | 27.2 ± 2.1 | 27.16 ± 2.0 | 0.99 |
| Gravidity, n (%) | |||
| 1 | 9 (50) | 7 (22.6) | 0.09 |
| 2 | 6 (33.3) | 11 (35.5) | |
| ≥3 | 3 (16.7) | 13 (41.9) | |
| Parity, n (%) | |||
| 1 | 12 (66.7) | 11 (35.5) | 0.11 |
| 2 | 4 (22.2) | 14 (45.2) | |
| ≥3 | 2 (11.1) | 6 (19.3) | |
| Family history of diabetes (%) | 47 | 31 | 0.28 |
| Pre-pregnancy weight (kg) a | 70.6 ± 15.6 | 71.1 ± 12.4 | 0.91 |
| Height (cm) a | 167.6 ± 6.5 | 169.1 ± 4.8 | 0.36 |
| Weight gain (kg) a | 6.8 ± 7.5 | 7.5 ± 4.0 | 0.70 |
| Pre-pregnancy BMI | 25.1 ± 5.6 | 24.9 ± 4.6 | 0.88 |
| Delivery, newborn characteristics and obstetric complications a | |||
| Cesarean section, n (%) | 13 (72.2) | 8 (25.8) | 0.005 |
| Weight (g) b | 3900.0 ± 596.2 | 3263.5 ± 313.3 | <0.001 * |
| Length (cm) b | 53.0 ± 2.4 | 50.9 ± 1.5 | 0.005 |
| BMI b | 1.11 ± 0.25 | 0.85 ± 0.13 | <0.001 |
| Apgar score at 1 min | 8.67 ± 0.74 | 9 ± 0.25 | 0.03 |
| Apgar score at 5 min | 9.55 ± 0.76 | 9.93 ± 0.24 | 0.016 |
| Preterm labor, n (%) | 10 (55.6) | - | - |
| Macrosomia, n (%) | 5 (27.8) | - | - |
| FGR, n (%) | 2 (11.1) | - | - |
| Polyhydramnios, n (%) | 2 (11.1) | - | - |
| With Adverse Outcome N = 12 | Without Adverse Outcome N = 32 | p | |
|---|---|---|---|
| Age (years) a | 32.8 ± 4.9 | 31.8 ± 3.9 | 0.50 |
| Smoking status (%) | 16.7 | 21.9 | 0.70 |
| Gestational age at sampling (weeks) | 25.5 ± 1.7 | 27.1 ± 1.6 | 0.009 |
| Gravidity, n (%) | |||
| 1 | 2 (16.7) | 11 (34.4) | 0.32 |
| 2 | 7 (58.3) | 11 (34.4) | |
| ≥3 | 3 (25) | 10 (31.2) | |
| Parity, n (%) | |||
| 1 | 5 (41.7) | 16 (50) | 0.60 |
| 2 | 6 (50.0) | 11 (34.4) | |
| ≥3 | 1 (8.3) | 5 (15.6) | |
| Family history of diabetes (%) | 16.7 | 12.5 | 0.87 |
| Pre-pregnancy weight (kg) a | 65.7 ± 9.9 | 67.1 ± 7.4 | 0.62 |
| Height (cm) a | 171.3 ± 7.2 | 172.2 ± 5.9 | 0.67 |
| Weight gain (kg) a | 7.6 ± 2.4 | 7.9 ± 3.6 | 0.80 |
| Pre-pregnancy BMI | 22.3 ± 3.0 | 22.6 ± 2.7 | 0.74 |
| Delivery, newborn characteristics and obstetric complications a | |||
| Cesarean section, n (%) | 5 (41.7) | 8 (25) | 0.005 |
| Weight (g) b | 3859.0 ± 794.1 | 3438.4 ± 323.7 | 0.023 |
| Length (cm) b | 53.3 ± 2.9 | 51.9 ± 1.5 | 0.057 |
| BMI b | 1.12 ± 0.32 | 0.93 ± 0.14 | 0.012 |
| Apgar score at 1 min | 8.75 ± 0.43 | 9.00 ± 0.25 | 0.025 |
| Apgar score at 5 min | 9.75 ± 0.43 | 9.94 ± 0.24 | 0.08 |
| Preterm labor, n (%) | 2 (16.7) | - | - |
| Macrosomia, n (%) | 7 (58.3) | - | - |
| FGR, n (%) | 2 (16.7) | - | - |
| Polyhydramnios, n (%) | 1 (8.3) | - | - |
| With Adverse Outcome N = 18 | Without Adverse Outcome N = 31 | p | |
|---|---|---|---|
| Glycemic status a | |||
| OGTT (mmol/L) | |||
| 0′ | 4.83 ± 0.46 | 4.86 ± 0.78 | 0.85 |
| 60′ | 10.74 ± 1.83 | 10.90 ± 1.50 | 0.74 |
| 120′ | 8.72 ± 1.55 | 9.00 ± 2.34 | 0.66 |
| Fasting insulin (mU/L) | 23.32 ± 17.16 | 15.53 ± 6.13 | 0.76 |
| HOMA-IR | 5.04 ± 5.13 | 3.46 ± 1.75 | 0.70 |
| HOMA-β | 3.86 ± 4.86 | 2.66 ± 1.78 | 0.53 |
| HbA1c (%) | 4.79 ± 0.15 | 4.75 ± 0.24 | 0.64 |
| Lipid profile a | |||
| Triglycerides (TG) (mmol/L) | 2.45 ± 0.81 | 2.36 ± 0.82 | 0.76 |
| Cholesterol (mmol/L) | 7.30 ± 1.36 | 6.92 ± 1.82 | 0.53 |
| HDL (mmol/L) | 1.98 ± 0.43 | 1.92 ± 0.45 | 0.68 |
| LDL (mmol/L) | 4.28 ± 1.12 | 3.90 ± 1.86 | 0.51 |
| TG/HDL | 1.34 ± 0.58 | 1.31 ± 0.65 | 0.89 |
| LDL/HDL | 2.25 ± 0.72 | 2.16 ± 1.53 | 0.85 |
| Other biochemical parameters a | |||
| Total proteins (g/L) | 64.12 ± 3.64 | 64.08 ± 2.91 | 0.96 |
| Albumin (g/L) | 35.13 ± 1.77 | 35.25 ± 2.07 | 0.86 |
| Urea (mmol/L) | 3.29 ± 0.92 | 3.13 ± 0.89 | 0.59 |
| Creatinine (µmol/L) | 52.12 ± 8.81 | 50.08 ± 6.26 | 0.40 |
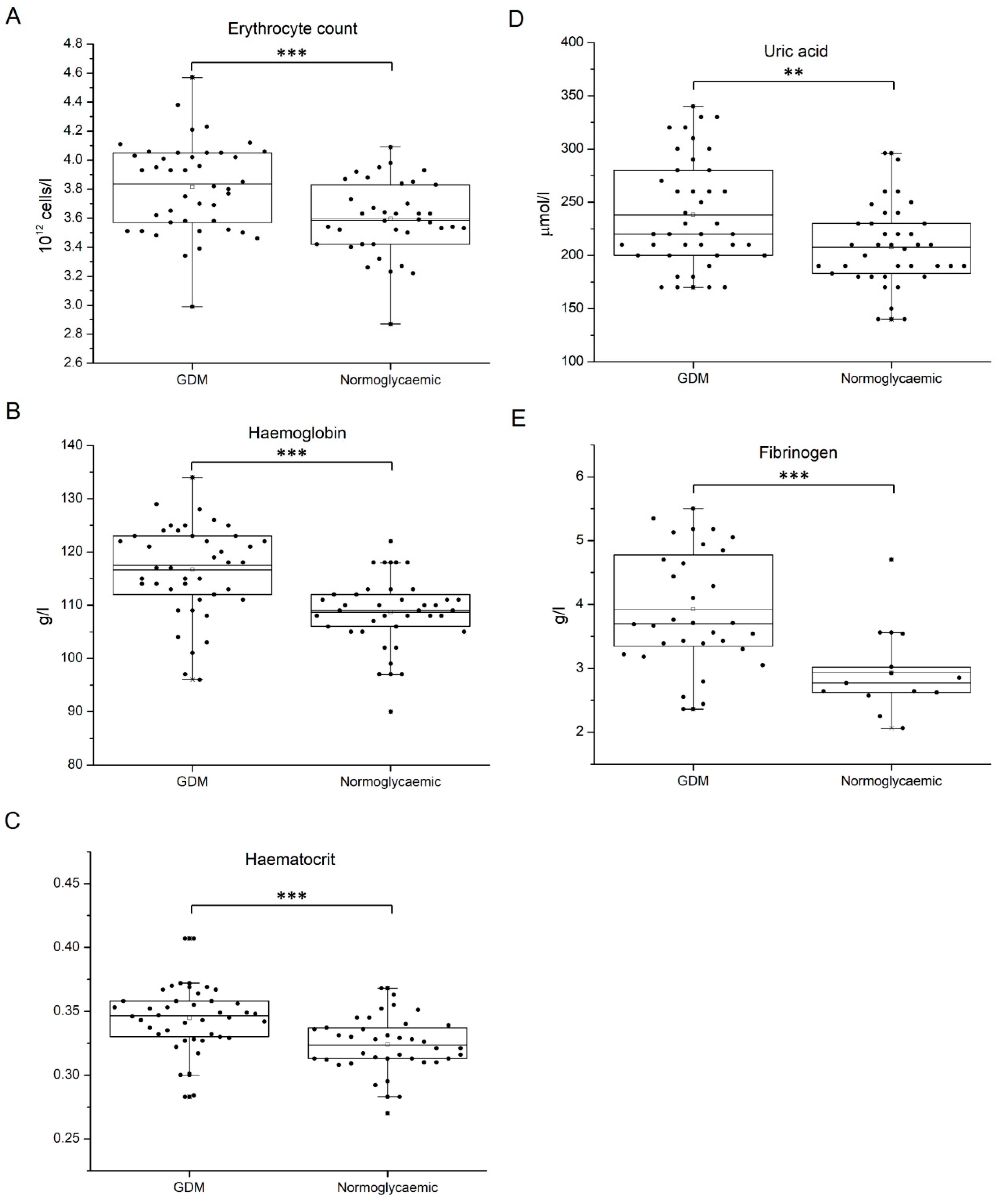
| Uric acid (µmol/L) | 235.00 ± 47.17 | 240.00 ± 52.33 | 0.76 |
| CRP (mg/L) | 9.77 ± 13.09 | 6.26 ± 5.08 | 0.58 |
| AST (U/L) | 18.19 ± 4.90 | 16.69 ± 5.45 | 0.39 |
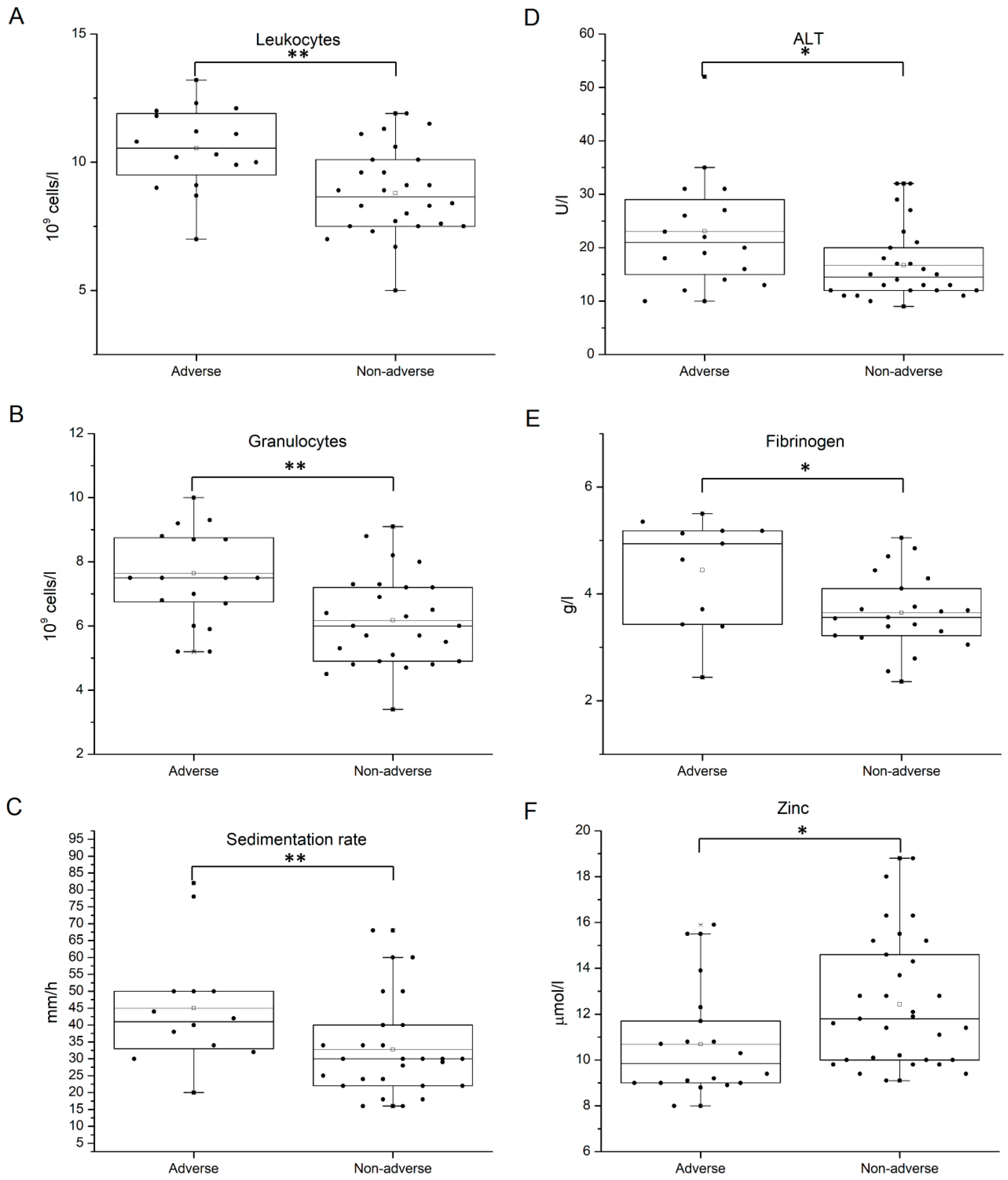
| ALT (U/L) | 23.06 ± 10.33 | 16.73 ± 6.62 | 0.023 |
| Fibrinogen (g/L) | 4.44 ± 0.98 | 3.65 ± 0.70 | 0.016 |
| Iron (µmol/L) | 17.13 ± 5.66 | 14.25 ± 4.56 | 0.10 |
| Ferritin (µg/L) | 22.38 ± 8.53 | 18.31 ± 13.08 | 0.39 |
| TIBC (µmol/L) | 62.32 ± 10.77 | 65.85 ± 10.96 | 0.29 |
| Zinc (µmol/L) | 10.68 ± 2.28 | 12.42 ± 2.66 | 0.027 |
| Complete blood count a | |||
| Erythrocytes (1012 cells/L) | 3.80 ± 0.34 | 3.82 ± 0.28 | 0.83 |
| Hemoglobin (g/L) | 115.50 ± 9.95 | 117.38 ± 7.26 | 0.49 |
| Hematocrit | 0.344 ± 0.029 | 0.345 ± 0.023 | 0.96 |
| Sedimentation rate (mm/h) | 45.00 ± 17.64 | 32.74 ± 13.69 | 0.028 |
| MCV (fl) | 90.64 ± 2.93 | 90.30 ± 4.81 | 0.80 |
| MCH (pg/cell) | 30.41 ± 1.30 | 30.77 ± 1.82 | 0.51 |
| MCHC (g/L) | 335.25 ± 7.75 | 340.73 ± 9.27 | 0.06 |
| Leukocytes (109 cells/L) | 10.54 ± 1.55 | 8.79 ± 1.64 | 0.002 |
| Thrombocytes (109 cells/L) | 253.00 ± 52.01 | 220.46 ± 51.52 | 0.06 |
| Granulocytes (109 cells/L) | 7.64 ± 1.32 | 6.17 ± 1.40 | 0.002 |
| Lymphocytes (109 cells/L) | 2.20 ± 0.36 | 2.04 ± 0.31 | 0.15 |
| With Adverse Outcome N = 12 | Without Adverse Outcome N = 32 | p | |
|---|---|---|---|
| Glycemic status a | |||
| OGTT (mmol/L) | |||
| 0′ | 4.39 ± 0.31 | 4.38 ± 0.38 | 0.93 |
| 60′ | 8.04 ± 1.27 | 7.26 ± 1.14 | 0.06 |
| 120′ | 6.86 ± 0.76 | 6.55 ± 1.08 | 0.38 |
| Fasting insulin (mU/L) | 15.78 ± 6.66 | 16.46 ± 10.59 | 0.68 |
| HOMA-IR | 3.08 ± 1.38 | 3.26 ± 2.20 | 0.56 |
| HOMA-β | 4.51 ± 3.52 | 4.56 ± 4.21 | 0.89 |
| HbA1c (%) | 4.69 ± 0.21 | 4.70 ± 0.29 | 0.86 |
| Lipid profile a | |||
| Triglycerides (TG) (mmol/L) | 2.21 ± 0.67 | 2.20 ± 0.62 | 0.95 |
| Cholesterol (mmol/L) | 7.22 ± 1.05 | 6.74 ± 0.80 | 0.15 |
| HDL (mmol/L) | 2.31 ± 0.44 | 2.05 ± 0.46 | 0.10 |
| LDL (mmol/L) | 3.87 ± 1.13 | 3.73 ± 0.80 | 0.67 |
| TG/HDL | 1.06 ± 0.72 | 1.18 ± 0.68 | 0.66 |
| LDL/HDL | 1.72 ± 0.56 | 1.90 ± 0.78 | 0.51 |
| Other biochemical parameters a | |||
| Total proteins (g/L) | 65.78 ± 3.32 | 63.93 ± 3.68 | 0.20 |
| Albumin (g/L) | 35.81 ± 2.80 | 35.82 ± 2.21 | 0.99 |
| Urea (mmol/L) | 2.73 ± 0.52 | 2.84 ± 0.93 | 0.76 |
| Creatinine (µmol/L) | 50.11 ± 5.86 | 53.14 ± 6.58 | 0.24 |
| Uric acid (µmol/L) | 221.11 ± 38.71 | 203.55 ± 33.82 | 0.21 |
| CRP (mg/L) | 5.34 ± 3.45 | 5.21 ± 3.10 | 0.96 |
| AST (U/L) | 17.14 ± 6.92 | 17.54 ± 5.50 | 0.88 |
| ALT (U/L) | 18.14 ± 12.84 | 14.86 ± 7.29 | 0.42 |
| Fibrinogen (g/L) | 3.68 ± 0.64 | 2.60 ± 0.28 | 0.002 |
| Iron (µmol/L) | 14.66 ± 6.77 | 14.82 ± 6.66 | 0.95 |
| Ferritin (µg/L) | 17.24 ± 13.06 | 13.74 ± 9.49 | 0.46 |
| TIBC (µmol/L) | 61.10 ± 11.10 | 61.32 ± 11.00 | 0.84 |
| Zinc (µmol/L) | 13.18 ± 3.25 | 11.29 ± 2.78 | 0.07 |
| Complete blood count a | |||
| Erythrocytes (1012 cells/L) | 3.72 ± 0.21 | 3.56 ± 0.25 | 0.09 |
| Hemoglobin (g/L) | 112.00 ± 5.60 | 107.62 ± 6.11 | 0.07 |
| Hematocrit | 0.332 ± 0.022 | 0.322 ± 0.020 | 0.20 |
| Sedimentation rate (mm/h) | 36.60 ± 15.57 | 32.43 ± 10.98 | 0.38 |
| MCV (fl) | 89.19 ± 1.97 | 90.98 ± 6.22 | 0.41 |
| MCH (pg/cell) | 30.14 ± 0.66 | 30.38 ± 2.07 | 0.75 |
| MCHC (g/L) | 338.00 ± 8.54 | 335.00 ± 7.47 | 0.33 |
| Leukocytes (109 cells/L) | 9.06 ± 1.77 | 9.24 ± 1.66 | 0.78 |
| Thrombocytes (109 cells/L) | 257.33 ± 52.80 | 236.34 ± 43.77 | 0.25 |
| Granulocytes (109 cells/L) | 6.59 ± 1.60 | 6.61 ± 1.39 | 0.96 |
| Lymphocytes (109 cells/L) | 1.80 ± 0.27 | 1.99 ± 0.43 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić-Marković, V.; Dobrijević, Z.; Robajac, D.; Miljuš, G.; Šunderić, M.; Penezić, A.; Nedić, O.; Ardalić, D.; Miković, Ž.; Radojičić, O.; et al. Biochemical Markers in the Prediction of Pregnancy Outcome in Gestational Diabetes Mellitus. Medicina 2024, 60, 1250. https://doi.org/10.3390/medicina60081250
Mandić-Marković V, Dobrijević Z, Robajac D, Miljuš G, Šunderić M, Penezić A, Nedić O, Ardalić D, Miković Ž, Radojičić O, et al. Biochemical Markers in the Prediction of Pregnancy Outcome in Gestational Diabetes Mellitus. Medicina. 2024; 60(8):1250. https://doi.org/10.3390/medicina60081250
Chicago/Turabian StyleMandić-Marković, Vesna, Zorana Dobrijević, Dragana Robajac, Goran Miljuš, Miloš Šunderić, Ana Penezić, Olgica Nedić, Danijela Ardalić, Željko Miković, Ognjen Radojičić, and et al. 2024. "Biochemical Markers in the Prediction of Pregnancy Outcome in Gestational Diabetes Mellitus" Medicina 60, no. 8: 1250. https://doi.org/10.3390/medicina60081250






