The Association between ADIPOQ Gene Polymorphisms and Diabetic Retinopathy Risk: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
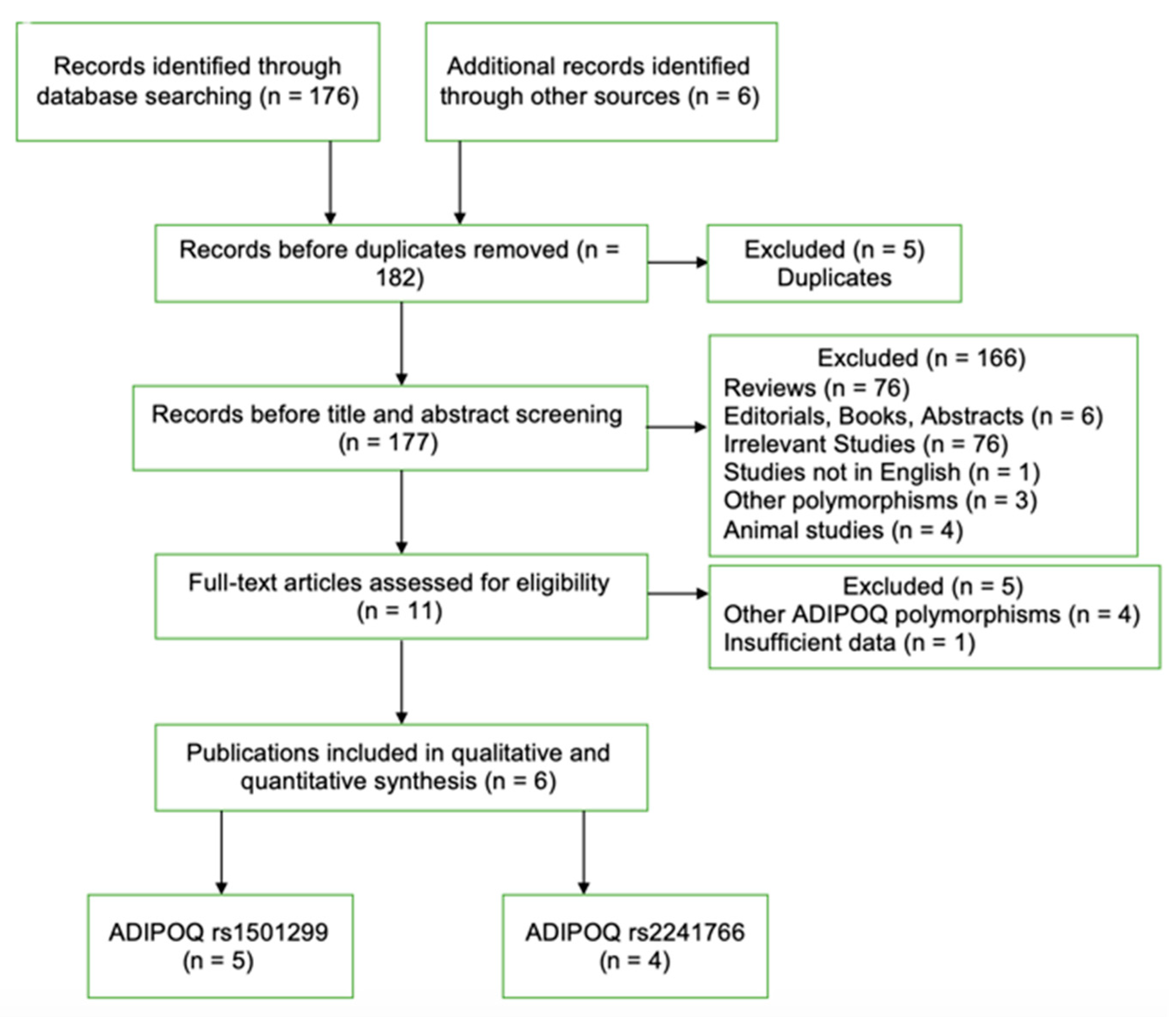
3.1. Literature Search
3.2. Study Characteristics and Summary Statistics
3.3. Quantitative and Subgroup Analyses
3.3.1. Association between rs1501299 Polymorphism and DR Risk
3.3.2. Association between the rs2241766 Polymorphism and DR Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DR | Diabetic retinopathy |
| OR | Odds ratio |
| CI | Confidence interval |
| DM | Diabetes mellitus |
| NPDR | Non-proliferative diabetic retinopathy |
| PDR | Proliferative diabetic retinopathy |
| DME | Diabetic macular edema |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TNF-a | Tumor necrosis factor—a |
| SNP | Single nucleotide polymorphisms |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| HC | Healthy controls |
| NDR | Non-diabetic retinopathy |
| HWE | Hardy–Weinberg equilibrium |
| MAF | Minor allele frequency |
| NOS | Newcastle–Ottawa scale |
| PCR-RFLP | Polymerase chain reaction—restriction fragment length polymorphism |
| N/A | Not available |
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic Retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Banu, R.; Chee, M.L.; Lee, R.; Wang, Y.X.; Tan, G.; Jonas, J.B.; Lamoureux, E.L.; Cheng, C.-Y.; Klein, B.E.K.; et al. Incidence and Progression of Diabetic Retinopathy: A Systematic Review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.Z.; Comyn, O.; Peto, T.; Tadros, C.; Ng, E.; Sivaprasad, S.; Hykin, P.G. Diabetic Retinopathy: Pathogenesis, Clinical Grading, Management and Future Developments. Diabet. Med. 2013, 30, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for Diabetic Retinopathy: New Perspectives and Challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Capitão, M.; Soares, R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell. Biochem. 2016, 117, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Ghamdi, A.H.A. Clinical Predictors of Diabetic Retinopathy Progression; A Systematic Review. Curr. Diabetes Rev. 2019, 16, 242–247. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Hernández, C.; Simó, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019, 62, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Y.; Hsih, W.-H.; Lin, Y.-B.; Wen, C.-Y.; Chang, T.-J. Update in the Epidemiology, Risk Factors, Screening, and Treatment of Diabetic Retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The Pathology Associated with Diabetic Retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current Understanding of the Molecular and Cellular Pathology of Diabetic Retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Fung, T.H.; Patel, B.; Wilmot, E.G.; Amoaku, W.M. Diabetic Retinopathy for the Non-Ophthalmologist. Clin. Med. 2022, 22, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Valle, M.L.; Beveridge, C.; Liu, Y.; Sharma, S. Unraveling the Role of Genetics in the Pathogenesis of Diabetic Retinopathy. Eye 2019, 33, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; He, W.; Pang, F.; Hu, Y. ADIPOQ Gene Polymorphisms and Susceptibility to Polycystic Ovary Syndrome: A HuGE Survey and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 161, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Geng, W.; Zhang, D.; Cai, G.; Zhu, H. ADIPOQ Rs2241766 Gene Polymorphism and Predisposition to Diabetic Kidney Disease. J. Diabetes Res. 2020, 2020, 5158497. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef] [PubMed]
- Al-Nbaheen, M.S. Effect of Genetic Variations in the ADIPOQ Gene on Susceptibility to Type 2 Diabetes Mellitus. Diabetes. Metab. Syndr. Obes. 2022, 15, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Alfaqih, M.A.; Al-Hawamdeh, A.; Amarin, Z.O.; Khader, Y.S.; Mhedat, K.; Allouh, M.Z. Single Nucleotide Polymorphism in the ADIPOQ Gene Modifies Adiponectin Levels and Glycemic Control in Type Two Diabetes Mellitus Patients. Biomed Res. Int. 2022, 2022, 6632442. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.F.; Long, K.Z.; Hsu, C.C.; Mamun, A.A.; Chiu, Y.F.; Tu, H.P.; Chen, P.S.; Jhang, H.R.; Hwang, S.J.; Huang, M.C. Adiponectin Gene (ADIPOQ) Polymorphisms Correlate with the Progression of Nephropathy in Taiwanese Male Patients with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Isakova, J.; Talaibekova, E.; Vinnikov, D.; Saadanov, I.; Aldasheva, N. ADIPOQ, KCNJ11 and TCF7L2 Polymorphisms in Type 2 Diabetes in Kyrgyz Population: A Case-Control Study. J. Cell. Mol. Med. 2019, 23, 1628–1631. [Google Scholar] [CrossRef]
- Aioanei, C.S.; Ilies, R.F.; Bala, C.; Petrisor, M.F.; Porojan, M.D.; Popp, R.A.; Catana, A. The Role of Adiponectin and Toll-like Receptor 4 Gene Polymorphisms on Non-Proliferative Retinopathy in Type 2 Diabetes Mellitus Patients. A Casecontrol Study in Romanian Caucasians Patients. Acta Endocrinol. 2019, 15, 32–38. [Google Scholar] [CrossRef]
- Gouliopoulos, N.; Siasos, G.; Bouratzis, N.; Oikonomou, E.; Kollia, C.; Konsola, T.; Oikonomou, D.; Rouvas, A.; Kassi, E.; Tousoulis, D.; et al. Polymorphism Analysis of ADIPOQ Gene in Greek Patients with Diabetic Retinopathy. Ophthalmic Genet. 2022, 43, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sikka, R.; Raina, P.; Matharoo, K.; Bandesh, K.; Bhatia, R.; Chakrabarti, S.; Bhanwer, A.J.S. TNF-α (g.-308 G > A) and ADIPOQ (g. + 45 T > G) Gene Polymorphisms in Type 2 Diabetes and Microvascular Complications in the Region of Punjab (North-West India). Curr. Eye Res. 2014, 39, 1042–1051. [Google Scholar] [CrossRef]
- Choe, E.Y.; Wang, H.J.; Kwon, O.; Kim, K.J.; Kim, B.S.; Lee, B.W.; Ahn, C.W.; Cha, B.S.; Lee, H.C.; Kang, E.S.; et al. Variants of the Adiponectin Gene and Diabetic Microvascular Complications in Patients with Type 2 Diabetes. Metabolism 2013, 62, 677–685. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.H.; Jiao, M.L.; Fan, X.H.; Hu, Q.; Hao, Y.H.; Liu, R.H.; Zhang, W.; Cui, Y.; Han, L.Y. Gene-Environment Interaction between Adiponectin Gene Polymorphisms and Environmental Factors on the Risk of Diabetic Retinopathy. J. Diabetes Investig. 2015, 6, 56–66. [Google Scholar] [CrossRef]
- Yoshioka, K.; Yoshida, T.; Takakura, Y.; Umekawa, T.; Kogure, A.; Toda, H.; Yoshikawa, T. Adiponectin Gene Polymorphism (G276T) and Diabetic Retinopathy in Japanese Patients with Type 2 Diabetes. Diabet. Med. 2004, 21, 1158–1159. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hu, Z.; Yuan, S.; Xie, P.; Liu, Q. Association between Transcription Factor 7-like 2 Rs7903146 Polymorphism and Diabetic Retinopathy in Type 2 Diabetes Mellitus: A Meta-Analysis. Diabetes Vasc. Dis. Res. 2015, 12, 436–444. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Y.; Xu, S.; Wu, J.; Yue, S.; Liu, L. Association of FokI, TaqI, BsmI and ApaI Polymorphisms with Diabetic Retinopathy: A Pooled Analysis of Case-Control Studies. Afr. Health Sci. 2018, 18, 891–899. [Google Scholar] [CrossRef]
- Luo, S.; Wang, F.; Shi, C.; Wu, Z. A Meta-Analysis of Association between Methylenetetrahydrofolate Reductase Gene (MTHFR) 677C/T Polymorphism and Diabetic Retinopathy. Int. J. Environ. Res. Public Health 2016, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Horita, N.; Kaneko, T. Genetic Model Selection for a Case-Control Study and a Meta-Analysis. Meta Gene 2015, 5, 1–8. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Jiang, G.B.; Cheng, S.Y.; Song, Y.F.; Deng, C.; Niu, Y.M.; Cai, J.W. Association between PPAR-Γ2 Gene Polymorphisms and Diabetic Retinopathy Risk: A Meta-Analysis. Aging 2021, 13, 5136–5149. [Google Scholar] [CrossRef] [PubMed]
- Montesanto, A.; Bonfigli, A.R.; Crocco, P.; Garagnani, P.; De Luca, M.; Boemi, M.; Marasco, E.; Pirazzini, C.; Giuliani, C.; Franceschi, C.; et al. Genes Associated with Type 2 Diabetes and Vascular Complications. Aging 2018, 10, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chang, Y.W.; Cheng, C.W.; Wu, C.M.; Liao, W.L.; Tsai, F.J. Causal Relationship between Adiponectin and Diabetic Retinopathy: A Mendelian Randomization Study in an Asian Population. Genes 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Rudofsky, G.; Schlimme, M.; Schlotterer, A.; von Eynatten, M.; Reismann, P.; Tafel, J.; Grafe, I.; Morcos, M.; Nawroth, P.; Bierhaus, A.; et al. No Association of the 94T/G Polymorphism in the Adiponectin Gene with Diabetic Complications. Diabetes, Obes. Metab. 2005, 7, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zietz, B.; Buechler, C.; Kobouch, K.; Neumeier, M.; Schölmerich, J.; Scäffler, A. Serum Levels of Adiponectin Are Associated with Diabetic Retinopathy and with Adiponectin Gene Mutations in Caucasian Patients with Diabetes Mellitus Type 2. Exp. Clin. Endocrinol. Diabetes 2008, 116, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.L.; Chen, Y.H.; Chen, C.C.; Huang, Y.C.; Lin, H.J.; Chen, Y.T.; Ban, B.; Wu, C.M.; Chang, Y.W.; Hsieh, A.R.; et al. Effect of Adiponectin Level and Genetic Variation of Its Receptors on Diabetic Retinopathy A Case-Control Study. Medicine 2019, 98, e14878. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.; Towler, H.M.A. Diabetic Retinopathy. Clin. Cornerstone 2003, 5, 12–21. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Zhang, W.; McDonald, D. Diabetic Retinopathy: Mechanism, Diagnosis, Prevention, and Treatment. Biomed Res. Int. 2015, 2015, 854593. [Google Scholar] [CrossRef]
- Stitt, A.W.; Lois, N.; Medina, R.J.; Adamson, P.; Curtis, T.M. Advances in Our Understanding of Diabetic Retinopathy. Clin. Sci. 2013, 125, 1–17. [Google Scholar] [CrossRef]
- Alimi, M.; Goodarzi, M.T.; Nekoei, M. Association of ADIPOQ Rs266729 and Rs1501299 Gene Polymorphisms and Circulating Adiponectin Level with the Risk of Type 2 Diabetes in a Population of Iran: A Case-Control Study. J. Diabetes Metab. Disord. 2021, 20, 87–93. [Google Scholar] [CrossRef]
- Zhao, N.; Li, N.; Zhang, S.; Ma, Q.; Ma, C.; Yang, X.; Yin, J.; Zhang, R.; Li, J.; Yang, X.; et al. Associations between Two Common Single Nucleotide Polymorphisms (Rs2241766 and Rs1501299) of ADIPOQ Gene and Coronary Artery Disease in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 51994–52005. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Izaola, O.; De La Fuente, B.; Primo, D.; Ovalle, H.F.; Romero, E. Rs1501299 Polymorphism in the Adiponectin Gene and Their Association with Total Adiponectin Levels, Insulin Resistance and Metabolic Syndrome in Obese Subjects. Ann. Nutr. Metab. 2017, 69, 226–231. [Google Scholar] [CrossRef]
- Cai, X.; Gan, Y.; Fan, Y.; Hu, J.; Jin, Y.; Chen, F.; Chen, T.; Sun, Y.; Wang, J.; Qin, W.; et al. The Adiponectin Gene Single-Nucleotide Polymorphism Rs1501299 Is Associated with Hepatocellular Carcinoma Risk. Clin. Transl. Oncol. 2014, 16, 166–172. [Google Scholar] [CrossRef]
- Bieńkiewicz, J.; Smolarz, B.; Malinowski, A. Association between Single Nucleotide Polymorphism +276G > T (Rs1501299) in ADIPOQ and Endometrial Cancer. Pathol. Oncol. Res. 2016, 22, 135–138. [Google Scholar] [CrossRef]
- Tu, Y.; Yu, Q.; Fan, G.; Yang, P.; Lai, Q.; Yang, F.; Zhang, S.; Wang, W.; Wang, D.; Yu, X.; et al. Assessment of Type 2 Diabetes Risk Conferred by SNPs Rs2241766 and Rs1501299 in the ADIPOQ Gene, a Case/Control Study Combined with Meta-Analyses. Mol. Cell. Endocrinol. 2014, 396, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leon-Cachon, R.B.R.; Salinas-Santander, M.A.; Aguilar-Tamez, D.A.; Marianavaldez-Ortiz, P.; Rios-Ibarra, C.P.; Cepeda-Nieto, A.C.; Suarez-Valencia, V.d.J.; Morlett-Chavez, J.A. ADIPOQ-Rs2241766 Polymorphism Is Associated with Changes in Cholesterol Levels of Mexican Adolescents. J. Appl. Biomed. 2022, 20, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kanu, J.S.; Qiu, S.; Cheng, Y.; Li, R.; Kou, C.; Gu, Y.; Bai, Y.; Shi, J.; Li, Y.; et al. Associations between Three Common Single Nucleotide Polymorphisms (Rs266729, Rs2241766, and Rs1501299) of ADIPOQ and Cardiovascular Disease: A Meta-Analysis. Lipids Health Dis. 2018, 17, 126. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, M.; Wang, S.; Wang, B.Y.; Han, C.Y.; Ren, Y.C.; Zhang, L.; Zhang, H.Y.; Yang, X.Y.; Zhao, Y.; et al. Association of the ADIPOQ Rs2241766 and Rs266729 Polymorphisms with Metabolic Syndrome in the Chinese Population: A Meta-Analysis. Biomed. Environ. Sci. 2016, 29, 505–515. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Meng, K.; Zhang, L. Association of Adiponectin Gene (ADIPOQ) Rs2241766 Polymorphism with Obesity in Adults: A Meta-Analysis. PLoS ONE 2014, 9, e95270. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year of Publication | Country/Ethnicity | Study Design | Genotyping Method | Control Type | Type of DM | DR Grade | Case | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Sex (M/F) | Age (years) | ||||||||
| Aioanei | 2021 | Romania/Caucasian | Case–Control | PCR-RFLP | HC | T2DM | NPDR | 198 | 105/93 | 68.72 ± 11.58 |
| Choe | 2013 | Korea/Asian | Cohort Study | PCR-RFLP | NDR | T2DM | Any DR | 231 | N/A | N/A |
| Gouliopoulos | 2022 | Greece/Caucasian | Case–Control | PCR-RFLP | NDR | T2DM | Any DR | 109 | 74/35 | 67.00 ± 8.00 |
| Li | 2015 | China/Asian | Case–Control | PCR-RFLP | NDR | T2DM | Any DR | 372 | 146/226 | 63.39 ± 10.60 |
| Yoshioka | 2004 | Japan/Asian | Case–Control | PCR-RFLP | HC + NDR | T2DM | Any DR | 104 | 55/49 | 62.05 ± 9.20 |
| Choe | 2013 | Korea/Asian | Cohort Study | PCR-RFLP | NDR | T2DM | Any DR | 225 | N/A | N/A |
| Gouliopoulos | 2022 | Greece/Caucasian | Case–Control | PCR-RFLP | NDR | T2DM | Any DR | 109 | 74/35 | 67.00 ± 8.00 |
| Li | 2015 | China/Asian | Case–Control | PCR-RFLP | NDR | T2DM | Any DR | 372 | 146/226 | 63.39 ± 10.60 |
| Sikka | 2014 | India/Asian | Case–Control | PCR-RFLP | HC+NDR | T2DM | Any DR | 169 | N/A | 58.35 ± 9.01 |
| First Author | Control | Genotype Distribution | HWE p-Value | MAF | NOS (Stars) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Sex (M/F) | Age (years) | Case | Control | Control | Case | Control | ||||||
| rs1501299 G/T | |||||||||||||
| GG | GT | TT | GG | GT | TT | ||||||||
| Aioanei | 200 | 143/57 | 58.10 ± 9.00 | 93 | 79 | 26 | 92 | 88 | 20 | 0.876 | 0.33 | 0.32 | 7 |
| Choe | 440 | N/A | N/A | 109 | 102 | 20 | 222 | 178 | 40 | 0.616 | 0.31 | 0.29 | 7 |
| Gouliopoulos | 109 | 75/34 | 66.00 ± 9.00 | 40 | 58 | 11 | 59 | 37 | 13 | 0.069 | 0.37 | 0.29 | 7 |
| Li | 145 | 49/96 | 62.34 ± 10.75 | 164 | 169 | 39 | 82 | 55 | 8 | 0.756 | 0.33 | 0.24 | 8 |
| Yoshioka | 340 | 219/121 | 59.70 ± 10.10 | 50 | 42 | 12 | 163 | 147 | 30 | 0.699 | 0.32 | 0.30 | 7 |
| rs2241766 T/G | |||||||||||||
| TT | TG | GG | TT | TG | GG | ||||||||
| Choe | 442 | N/A | N/A | 111 | 96 | 18 | 213 | 194 | 35 | 0.315 | 0.29 | 0.30 | 7 |
| Gouliopoulos | 109 | 75/34 | 66.00 ± 9.00 | 84 | 23 | 2 | 74 | 32 | 3 | 0.836 | 0.12 | 0.17 | 7 |
| Li | 145 | 49/96 | 62.34 ± 10.75 | 206 | 140 | 25 | 82 | 53 | 10 | 0.720 | 0.26 | 0.25 | 8 |
| Sikka | 355 | N/A | 53.16 ±12.15 | 158 | 9 | 2 | 292 | 58 | 5 | 0.285 | 0.04 | 0.09 | 6 |
| rs1501299 | Study | Sample Size | Studies (n) | Test of Association | Test of Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) | Z | p-Value | χ2 | p-Value | I2 (%) | T2 | |||
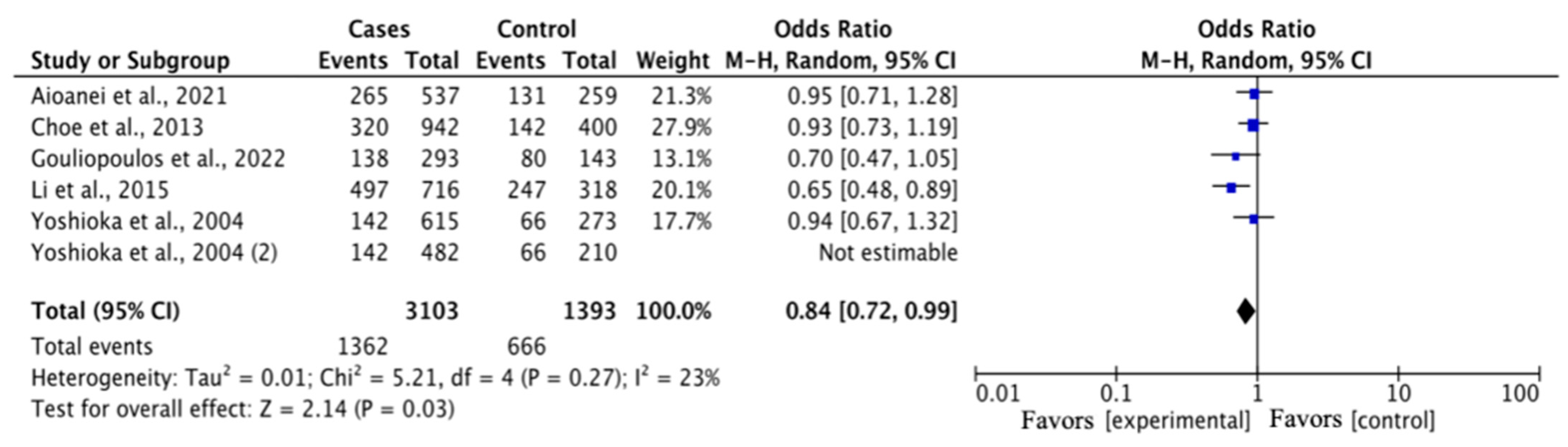
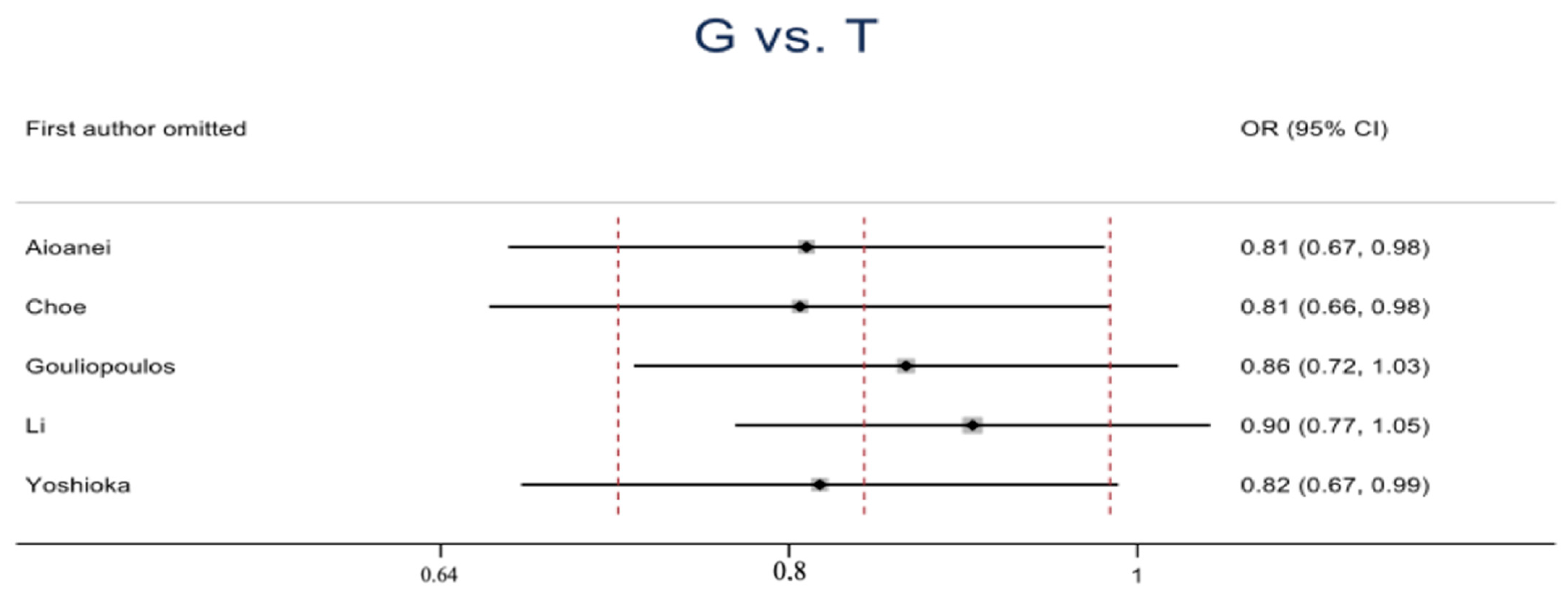
| G vs. T | Overall | 3103 | 1393 | 5 | 0.84 (0.72–0.99) | 2.14 | 0.03 | 5.21 | 0.27 | 23 | 0.01 |
| Asian | 2273 | 991 | 3 | 0.84 (0.66–1.05) | 1.51 | 0.13 | 3.76 | 0.15 | 47 | 0.02 | |
| Caucasian | 830 | 402 | 2 | 0.84 (0.63–1.13) | 1.14 | 0.25 | 1.44 | 0.23 | 31 | 0.01 | |
| DR vs. DM | 2433 | 1071 | 4 | 0.81 (0.67–0.97) | 2.28 | 0.02 | 4.13 | 0.25 | 27 | 0.01 | |
| DR vs. HC | 812 | 388 | 2 | 0.97 (0.76–1.24) | 0.21 | 0.83 | 0.07 | 0.79 | 0 | 0.00 | |
| GG vs. TT | Overall | 1074 | 219 | 5 | 0.76 (0.55–1.04) | 1.73 | 0.08 | 3.02 | 0.55 | 0 | 0.00 |
| Asian | 790 | 149 | 3 | 0.72 (0.44–1.18) | 1.32 | 0.19 | 2.99 | 0.22 | 33 | 0.06 | |
| Caucasian | 284 | 70 | 2 | 0.79 (0.46–1.33) | 0.90 | 0.37 | 0.00 | 0.96 | 0 | 0.00 | |
| DR vs. DM | 846 | 165 | 4 | 0.75 (0.52–1.08) | 1.55 | 0.12 | 3.01 | 0.39 | 0 | 0.00 | |
| DR vs. HC | 278 | 66 | 2 | 0.78 (0.45–1.34) | 0.91 | 0.36 | 0.00 | 1.00 | 0 | 0.00 | |
| GT vs. TT | Overall | 955 | 219 | 5 | 0.90 (0.63–1.29) | 0.56 | 0.57 | 4.82 | 0.31 | 17 | 0.03 |
| Asian | 693 | 149 | 3 | 0.87 (0.58–1.30) | 0.70 | 0.48 | 1.70 | 0.43 | 0 | 0.00 | |
| Caucasian | 262 | 70 | 2 | 1.08 (0.41–2.81) | 0.15 | 0.88 | 3.00 | 0.08 | 67 | 0.32 | |
| DR vs. DM | 741 | 165 | 4 | 1.00 (0.66–1.52) | 0.01 | 0.99 | 3.64 | 0.30 | 18 | 0.03 | |
| DR vs. HC | 256 | 66 | 2 | 0.66 (0.38–1.14) | 1.49 | 0.14 | 0.06 | 0.81 | 0 | 0.00 | |
| GG vs. GT | Overall | 1074 | 955 | 5 | 0.80 (0.60–1.08) | 1.44 | 0.15 | 9.55 | 0.05 | 58 | 0.07 |
| Asian | 790 | 693 | 3 | 0.83 (0.64–1.08) | 1.40 | 0.16 | 2.60 | 0.27 | 23 | 0.01 | |
| Caucasian | 284 | 262 | 2 | 0.71 (0.28–1.82) | 0.71 | 0.48 | 6.94 | 0.008 | 86 | 0.39 | |
| DR vs. DM | 846 | 741 | 4 | 0.72 (0.53–0.98) | 2.06 | 0.04 | 5.85 | 0.12 | 49 | 0.05 | |
| DR vs. HC | 278 | 256 | 2 | 1.18 (0.84–1.66) | 0.97 | 0.33 | 0.16 | 0.69 | 0 | 0.00 | |
| GG vs. GT + TT | Overall | 1074 | 1174 | 5 | 0.79 (0.61–1.03) | 1.76 | 0.08 | 8.15 | 0.09 | 51 | 0.04 |
| Asian | 790 | 842 | 3 | 0.81 (0.61–1.07) | 1.48 | 0.14 | 3.34 | 0.19 | 40 | 0.02 | |
| Caucasian | 284 | 332 | 2 | 0.73 (0.35–1.52) | 0.83 | 0.40 | 4.81 | 0.03 | 79 | 0.22 | |
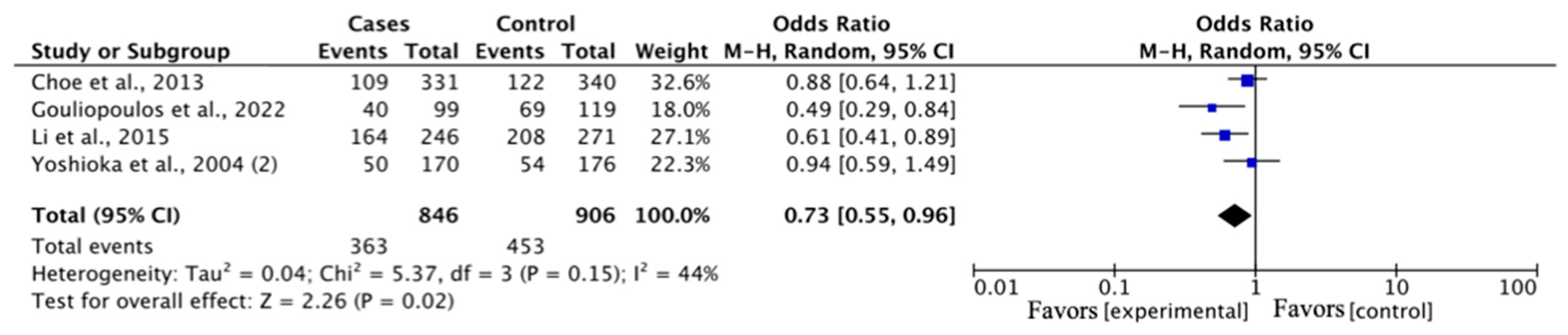
| DR vs. DM | 846 | 906 | 4 | 0.73 (0.55–0.96) | 2.26 | 0.02 | 5.37 | 0.15 | 44 | 0.04 | |
| DR vs. HC | 278 | 322 | 2 | 1.09 (0.79–1.50) | 0.50 | 0.61 | 0.14 | 0.71 | 0 | 0.00 | |
| GG + GT vs. TT | Overall | 2031 | 219 | 5 | 0.82 (0.61–1.12) | 1.26 | 0.21 | 3.31 | 0.51 | 0 | 0.00 |
| Asian | 1485 | 149 | 3 | 0.78 (0.51–1.19) | 1.14 | 0.26 | 2.38 | 0.30 | 16 | 0.02 | |
| Caucasian | 546 | 70 | 2 | 0.87 (0.53–1.44) | 0.53 | 0.59 | 0.85 | 0.36 | 0 | 0.00 | |
| DR vs. DM | 1587 | 165 | 4 | 0.86 (0.60–1.23) | 0.81 | 0.42 | 3.06 | 0.38 | 2 | 0.00 | |
| DR vs. HC | 534 | 66 | 2 | 0.72 (0.43–1.21) | 1.25 | 0.21 | 0.02 | 0.90 | 0 | 0.00 | |
| GG + TT vs. GT | Overall | 1293 | 955 | 5 | 0.85 (0.63–1.13) | 1.13 | 0.26 | 9.80 | 0.04 | 59 | 0.06 |
| Asian | 939 | 693 | 3 | 0.87 (0.70–1.08) | 1.23 | 0.22 | 1.99 | 0.37 | 0 | 0.00 | |
| Caucasian | 354 | 262 | 2 | 0.75 (0.29–1.91) | 0.61 | 0.54 | 7.78 | 0.005 | 87 | 0.40 | |
| DR vs. DM | 1011 | 741 | 4 | 0.76 (0.57–1.02) | 1.82 | 0.13 | 5.72 | 0.13 | 48 | 0.04 | |
| DR vs. HC | 344 | 256 | 2 | 1.24 (0.90–1.72) | 1.30 | 0.19 | 0.16 | 0.69 | 0 | 0.00 | |
| rs2241766 | Study | Sample Size | Studies (n) | Test of Association | Test of Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) | Z | p-Value | χ2 | p-Value | I2 (%) | T2 | |||
| T vs. G | Overall | 3045 | 805 | 4 | 1.30 (1.00–2.09) | 1.42 | 0.15 | 10.03 | 0.02 | 70 | 0.09 |
| Asian | 2674 | 740 | 3 | 1.27 (0.82–1.96) | 1.06 | 0.29 | 8.93 | 0.01 | 78 | 0.11 | |
| DR vs. DM | 2681 | 763 | 4 | 1.21 (0.90–1.64) | 1.27 | 0.20 | 6.76 | 0.08 | 56 | 0.05 | |
| TT vs. GG | Overall | 1220 | 100 | 4 | 1.34 (0.87–2.07) | 1.31 | 0.19 | 1.74 | 0.63 | 0 | 0.00 |
| Asian | 1062 | 95 | 3 | 1.32 (0.84–2.07) | 1.21 | 0.23 | 1.66 | 0.44 | 0 | 0.00 | |
| DR vs. DM | 1056 | 97 | 4 | 1.33 (0.86–2.07) | 1.28 | 0.20 | 1.74 | 0.63 | 0 | 0.00 | |
| TG vs. GG | Overall | 605 | 100 | 4 | 0.94 (0.60–1.49) | 0.26 | 0.79 | 1.06 | 0.79 | 0 | 0.00 |
| Asian | 550 | 95 | 3 | 0.93 (0.58–1.50) | 0.29 | 0.77 | 1.03 | 0.60 | 0 | 0.00 | |
| DR vs. DM | 569 | 97 | 4 | 0.96 (0.60–1.52) | 0.18 | 0.86 | 0.70 | 0.87 | 0 | 0.00 | |
| TT vs. TG + GG | Overall | 1220 | 705 | 4 | 1.38 (0.89–2.15) | 1.45 | 0.15 | 10.61 | 0.01 | 72 | 0.14 |
| Asian | 1062 | 645 | 3 | 1.35 (0.78–2.35) | 1.07 | 0.28 | 9.69 | 0.008 | 79 | 0.18 | |
| DR vs. DM | 1056 | 666 | 4 | 1.29 (0.89–1.87) | 1.35 | 0.18 | 7.21 | 0.07 | 58 | 0.08 | |
| TT vs. TG | Overall | 1220 | 605 | 4 | 1.41 (0.88–2.25) | 1.43 | 0.15 | 10.92 | 0.01 | 73 | 0.16 |
| Asian | 1062 | 550 | 3 | 1.39 (0.76–2.53) | 1.08 | 0.28 | 10.13 | 0.006 | 80 | 0.22 | |
| DR vs. DM | 1056 | 569 | 4 | 1.31 (0.88–1.95) | 1.33 | 0.18 | 7.54 | 0.06 | 60 | 0.09 | |
| TT + TG vs. GG | Overall | 1845 | 100 | 4 | 0.99 (0.64–1.54) | 0.03 | 0.97 | 0.33 | 0.95 | 0 | 0.00 |
| Asian | 1632 | 95 | 3 | 0.97 (0.62–1.52) | 0.14 | 0.88 | 0.11 | 0.95 | 0 | 0.00 | |
| DR vs. DM | 1645 | 97 | 4 | 0.99 (0.63–1.53) | 0.06 | 0.95 | 0.30 | 0.96 | 0 | 0.00 | |
| TT + GG vs. TG | Overall | 1320 | 605 | 4 | 1.40 (0.88–2.21) | 1.42 | 0.16 | 10.91 | 0.01 | 72 | 0.15 |
| Asian | 1157 | 550 | 3 | 1.38 (0.77–2.49) | 1.08 | 0.28 | 10.14 | 0.006 | 80 | 0.21 | |
| DR vs. DM | 1153 | 569 | 4 | 1.30 (0.88–1.92) | 1.31 | 0.19 | 7.51 | 0.06 | 60 | 0.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flindris, K.; Markozannes, G.; Moschos, M.; Gazouli, M.; Christodoulou, A.; Tsilidis, K.; Kitsos, G. The Association between ADIPOQ Gene Polymorphisms and Diabetic Retinopathy Risk: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 1254. https://doi.org/10.3390/medicina60081254
Flindris K, Markozannes G, Moschos M, Gazouli M, Christodoulou A, Tsilidis K, Kitsos G. The Association between ADIPOQ Gene Polymorphisms and Diabetic Retinopathy Risk: A Systematic Review and Meta-Analysis. Medicina. 2024; 60(8):1254. https://doi.org/10.3390/medicina60081254
Chicago/Turabian StyleFlindris, Konstantinos, Georgios Markozannes, Marilita Moschos, Maria Gazouli, Aikaterini Christodoulou, Konstantinos Tsilidis, and Georgios Kitsos. 2024. "The Association between ADIPOQ Gene Polymorphisms and Diabetic Retinopathy Risk: A Systematic Review and Meta-Analysis" Medicina 60, no. 8: 1254. https://doi.org/10.3390/medicina60081254
APA StyleFlindris, K., Markozannes, G., Moschos, M., Gazouli, M., Christodoulou, A., Tsilidis, K., & Kitsos, G. (2024). The Association between ADIPOQ Gene Polymorphisms and Diabetic Retinopathy Risk: A Systematic Review and Meta-Analysis. Medicina, 60(8), 1254. https://doi.org/10.3390/medicina60081254







