Predicting Delayed Postoperative Length of Stay Following Robotic Kidney Transplantation: Development and Simulation of Perioperative Risk Factors
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Data Variables
2.3. Anesthesia and Perioperative Management in RAKT
2.4. Primary and Secondary Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Patient Characteristics
3.2. Risk Factors Influencing Delayed Discharge after RAKT
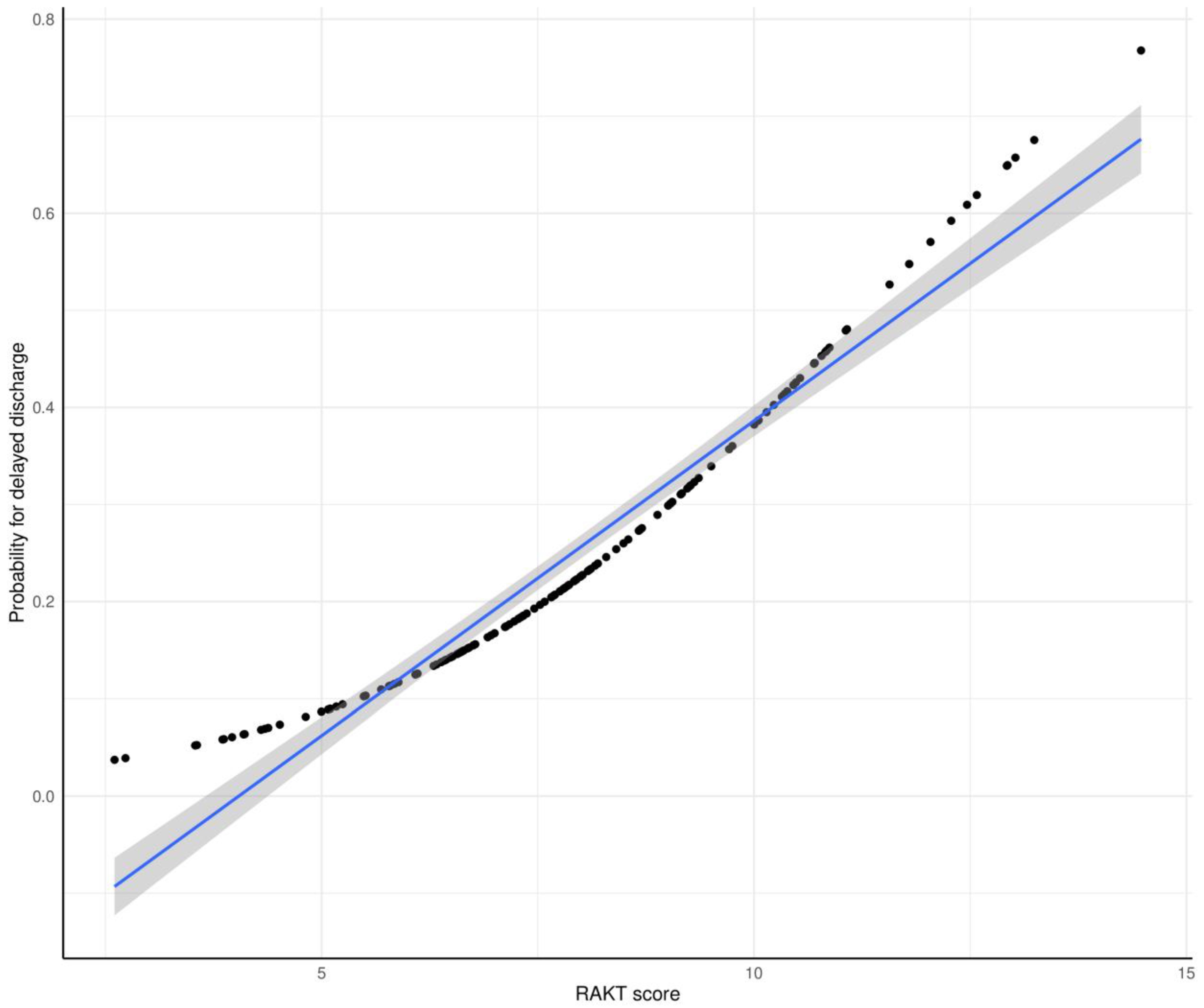
3.3. RAKT Score
3.4. Predictive Performance of RAKT Score for Delayed Postoperative Discharge
3.5. Clinical Outcomes Associated with RAKT Score Distribution
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.P.; Murray, J.E.; Harrison, J.H.; Guild, W.R. Successful homotransplantation of the human kidney between identical twins. J. Am. Med. Assoc. 1956, 160, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.; Gorodner, V.; Sbrana, F.; Tzvetanov, I.; Jeon, H.; Bianco, F.; Kinzer, K.; Oberholzer, J.; Benedetti, E. Robotic transabdominal kidney transplantation in a morbidly obese patient. Am. J. Transplant. 2010, 10, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, J.; Giulianotti, P.; Danielson, K.K.; Spaggiari, M.; Bejarano-Pineda, L.; Bianco, F.; Tzvetanov, I.; Ayloo, S.; Jeon, H.; Garcia-Roca, R.; et al. Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am. J. Transplant. 2013, 13, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Slagter, J.S.; Outmani, L.; Tran, K.; Ijzermans, J.N.M.; Minnee, R.C. Robot-assisted kidney transplantation as a minimally invasive approach for kidney transplant recipients: A systematic review and meta-analyses. Int. J. Surg. 2022, 99, 106264. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiyar, S.S.; Sakowitz, S.; Verma, A.; Richardson, S.; Curry, J.; Chervu, N.L.; Blumberg, J.; Benharash, P. Postoperative length of stay following kidney transplantation in patients without delayed graft function—An analysis of center-level variation and patient outcomes. Clin. Transplant. 2023, 37, e15000. [Google Scholar] [CrossRef] [PubMed]
- Tugcu, V.; Sener, N.C.; Sahin, S.; Yavuzsan, A.H.; Akbay, F.G.; Apaydin, S. Robot-assisted kidney transplantation: Comparison of the first 40 cases of open vs. robot-assisted transplantations by a single surgeon. BJU Int. 2018, 121, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, R.; Sood, A.; Jeong, W.; Ghosh, P.; Keeley, J.; Abdollah, F.; Kher, V.; Olson, P.; Farah, G.; Wurst, H.; et al. Robotic Kidney Transplantation with Regional Hypothermia versus Open Kidney Transplantation for Patients with End Stage Renal Disease: An Ideal Stage 2B Study. J. Urol. 2021, 205, 595–602. [Google Scholar] [CrossRef]
- Pein, U.; Girndt, M.; Markau, S.; Fritz, A.; Breda, A.; Stockle, M.; Mohammed, N.; Kawan, F.; Schumann, A.; Fornara, P.; et al. Minimally invasive robotic versus conventional open living donor kidney transplantation. World J. Urol. 2020, 38, 795–802. [Google Scholar] [CrossRef]
- Lee, S.D.; Rawashdeh, B.; McCracken, E.K.E.; Cantrell, L.A.; Kharwat, B.; Demirag, A.; Agarwal, A.; Brayman, K.L.; Pelletier, S.J.; Goldaracena, N.; et al. Robot-assisted kidney transplantation is a safe alternative approach for morbidly obese patients with end-stage renal disease. Int. J. Med. Robot 2021, 17, e2293. [Google Scholar] [CrossRef]
- Eksi, M.; Sahin, S.; Evren, I.; Arikan, Y.; Akbay, F.G.; Karadag, S.; Guler, A.F.; Celik, Z.; Apaydin, S.; Ihsan Tasci, A.; et al. Can robot-assisted kidney transplantation provide higher quality of life than open kidney transplantation during the early postoperative period? Int. J. Clin. Pract. 2021, 75, e14288. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, N.; Omkarappa, S.; Srinivasan, S.P.; Nair, S.G.; Rajgopal, R.; Eldo, N. Anesthetic Challenges and Perioperative Factors Affecting Delayed Graft Function in Robotic-Assisted Kidney Transplant: A Review of a Single-Center Experience of 100 Cases. Cureus 2022, 14, e28957. [Google Scholar] [CrossRef] [PubMed]
- Wagener, G.; Bezinover, D.; Wang, C.; Kroepfl, E.; Diaz, G.; Giordano, C.; West, J.; Kindscher, J.D.; Moguilevitch, M.; Nicolau-Raducu, R.; et al. Fluid Management during Kidney Transplantation: A Consensus Statement of the Committee on Transplant Anesthesia of the American Society of Anesthesiologists. Transplantation 2021, 105, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, N.K.; Garg, K.; Bose, A.; Aulakh, B.S.; Chahal, H.S.; Aulakh, G.S. Influence of hemodynamics and intra-operative hydration on biochemical outcome of renal transplant recipients. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Yamanaga, S.; Kaba, A.; Tanaka, K.; Ogata, M.; Fujii, M.; Hidaka, Y.; Kawabata, C.; Toyoda, M.; Uekihara, S.; et al. Optimizing Intraoperative Blood Pressure to Improve Outcomes in Living Donor Renal Transplantation. Transplant. Proc. 2020, 52, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Lazar, H.L.; Fitzgerald, C.A.; Ahmad, T.; Bao, Y.; Colton, T.; Shapira, O.M.; Shemin, R.J. Early discharge after coronary artery bypass graft surgery: Are patients really going home earlier? J. Thorac. Cardiovasc. Surg. 2001, 121, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Liska, D.; Novello, M.; Cengiz, B.T.; Holubar, S.D.; Aiello, A.; Gorgun, E.; Steele, S.R.; Delaney, C.P. Enhanced Recovery Pathway Benefits Patients Undergoing Nonelective Colorectal Surgery. Ann. Surg. 2021, 273, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Kowalsky, S.J.; Zenati, M.S.; Steve, J.; Esper, S.A.; Lee, K.K.; Hogg, M.E.; Zeh, H.J., 3rd; Zureikat, A.H. A Combination of Robotic Approach and ERAS Pathway Optimizes Outcomes and Cost for Pancreatoduodenectomy. Ann. Surg. 2019, 269, 1138–1145. [Google Scholar] [CrossRef]
- Lee, L.; Feldman, L.S. Implementation of Enhanced Recovery Pathways in the Real World: Change is Hard. Ann. Surg. 2021, 274, 206–208. [Google Scholar] [CrossRef]
- Calderon, E.; Chang, Y.H.; Chang, J.M.; Velazco, C.S.; Giorgakis, E.; Srinivasan, A.; Moss, A.A.; Khamash, H.; Heilman, R.; Reddy, K.S.; et al. Outcomes and Health Care Utilization after Early Hospital Dismissal in Kidney Transplantation: An Analysis of 1001 Consecutive Cases. Ann. Surg. 2022, 275, e511–e519. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Nataraj, S.A.; Zafar, F.A.; Ghosh, P.; Ahlawat, R. Feasibility and Functional Outcome of Robotic Assisted Kidney Transplantation Using Grafts with Multiple Vessels: Comparison to Propensity Matched Contemporary Open Kidney Transplants Cohort. Front. Surg. 2020, 7, 51. [Google Scholar] [CrossRef]
- Kishore, T.A.; Kuriakose, M.J.; Pathrose, G.; Raveendran, V.; Kumar, K.V.; Unni, V.N. Robotic assisted kidney transplantation in grafts with multiple vessels: Single center experience. Int. Urol. Nephrol. 2020, 52, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Scurt, F.G.; Ewert, L.; Mertens, P.R.; Haller, H.; Schmidt, B.M.W.; Chatzikyrkou, C. Clinical outcomes after ABO-incompatible renal transplantation: A systematic review and meta-analysis. Lancet 2019, 393, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Clymer, J.W.; Po-Han Chen, B.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Tennankore, K.K.; Kim, S.J.; Alwayn, I.P.; Kiberd, B.A. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016, 89, 648–658. [Google Scholar] [CrossRef]
- Prionas, A.; Craddock, C.; Papalois, V. Enhanced Recovery after Renal Transplantation Decreases Recipients’ Urological Complications and Hospital Stay: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2286. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, A.M.; Ghoneim, I.; Moiz, A.; Welch, K.; Brown, J.S. Enhanced Recovery after Surgery Pathway in Kidney Transplantation: The Road Less Traveled. Transplant. Direct. 2022, 8, e1333. [Google Scholar] [CrossRef]
- Tan, J.H.S.; Bhatia, K.; Sharma, V.; Swamy, M.; van Dellen, D.; Dhanda, R.; Khambalia, H. Enhanced recovery after surgery recommendations for renal transplantation: Guidelines. Br. J. Surg. 2022, 110, 57–59. [Google Scholar] [CrossRef]


| Total | Early (<8 Days) | Delayed (≥8 Days) | p-Value | |
|---|---|---|---|---|
| Number of patients, n (%) | 146 | 110 (75.3) | 36 (24.7) | |
| Demographic data | ||||
| Age, years | 44.3 ± 13.3 | 43.9 ± 13.2 | 45.4 ± 13.5 | 0.489 |
| Female sex, n (%) | 63 (43.2) | 49 (44.5) | 14 (38.9) | 0.688 |
| BMI, kg/m2 | 23.1 ± 4.7 | 22.7 ± 4.6 | 24.4 ± 5.0 | 0.050 |
| Comorbidities | ||||
| HTN, n (%) | 122 (84.1) | 93 (85.3) | 29 (80.6) | 0.678 |
| DM, n (%) | 41 (28.1) | 30 (27.3) | 11 (30.6) | 0.868 |
| Coronary artery disease, n (%) | 2 (1.4) | 1 (0.9) | 1 (2.8) | 0.991 |
| Congestive heart failure, n (%) | 28 (19.2) | 17 (15.5) | 11 (30.6) | 0.079 |
| Cerebrovascular accidents, n (%) | 1 (0.7) | 0 (0.0) | 1 (2.8) | 0.562 |
| mFI-5 | 1.1 ± 0.7 | 1.1 ± 0.7 | 1.3 ± 0.7 | 0.662 |
| ABO incompatibility, n (%) | 38 (26.0) | 24 (21.8) | 14 (38.9) | 0.071 |
| Preoperative hemodialysis, n (%) | 111 (77.1) | 78 (72.2) | 33 (91.7) | 0.030 |
| Cause of CRF | 0.571 | |||
| HTN, n (%) | 19 (13.0) | 15 (13.6) | 4 (11.1) | |
| DM, n (%) | 31 (21.2) | 21 (19.1) | 10 (27.8) | |
| IgA, n (%) | 34 (23.3) | 23 (20.9) | 11 (30.6) | |
| FSGS, n (%) | 10 (6.8) | 8 (7.3) | 2 (5.6) | |
| PCKD, n (%) | 27 (18.5) | 22 (20.0) | 5 (13.9) | |
| Unknown, n (%) | 25 (17.1) | 21 (19.1) | 4 (11.1) | |
| Medication history | ||||
| ACE inhibitor or ARB, n (%) | 57 (39.0) | 45 (40.9) | 12 (33.3) | 0.541 |
| Beta blocker, n (%) | 60 (41.1) | 41 (37.3) | 19 (52.8) | 0.148 |
| CCB, n (%) | 97 (66.4) | 74 (67.3) | 23 (63.9) | 0.865 |
| Preoperative laboratory data | ||||
| Hemoglobin, g/dL | 10.6 ± 1.8 | 10.6 ± 1.7 | 10.3 ± 1.9 | 0.378 |
| Creatinine, mg/dL | 7.6 ± 2.8 | 7.8 ± 2.8 | 7.2 ± 2.8 | 0.406 |
| BUN, mg/dL | 60.7 ± 22.4 | 64.0 ± 23.4 | 50.5 ± 15.5 | 0.004 |
| Albumin, g/dL | 3.8 ± 0.7 | 3.8 ± 0.7 | 3.8 ± 0.5 | 0.213 |
| Sodium, mmol/L | 136.3 ± 3.4 | 136.0 ± 3.4 | 137.0 ± 3.4 | 0.229 |
| Potassium, mmol/L | 4.8 ± 0.9 | 4.9 ± 0.9 | 4.5 ± 0.8 | 0.005 |
| eGFR, mL/min/1.73 m2 | 8.1 ± 4.1 | 7.7 ± 3.4 | 9.2 ± 5.5 | 0.175 |
| Intraoperative data | ||||
| Operation time, min | 371.0 ± 63.7 | 362.2 ± 52.6 | 397.7 ± 85.1 | 0.009 |
| Cold ischemia time, min | 106.1 ± 38.0 | 102.3 ± 33.5 | 118.4 ± 48.3 | 0.062 |
| Rewarming time, min | 58.8 ± 15.3 | 58.1 ± 12.3 | 61.2 ± 22.6 | 0.779 |
| Inotropes, n (%) | 9 (6.2) | 5 (4.5) | 4 (11.1) | 0.307 |
| Vasodilator, n (%) | 31 (21.2) | 18 (16.4) | 13 (36.1) | 0.023 |
| Loop diuretic dose (Furosemide), mg | 41.0 ± 9.2 | 39.9 ± 6.3 | 44.4 ± 14.4 | 0.014 |
| Volume of Crystalloid, mL | 1514.5 ± 675.2 | 1405.5 ± 560.7 | 1847.8 ± 870.9 | 0.009 |
| Volume of Colloid, mL | 466.6 ± 209.2 | 481.8 ± 206.8 | 420.2 ± 212.6 | 0.190 |
| Transfusion (RBC), unit | 0.2 ± 0.6 | 0.2 ± 0.5 | 0.3 ± 0.7 | 0.343 |
| Urine output, mL | 669.5 ± 537.2 | 701.8 ± 563.9 | 570.7 ± 437.9 | 0.220 |
| Blood loss, mL | 88.5 ± 56.3 | 84.9 ± 48.8 | 99.4 ± 74.6 | 0.227 |
| HR (mean), bpm | 68.6 ± 9.2 | 68.2 ± 8.9 | 69.9 ± 10.0 | 0.351 |
| HR (SD), bpm | 6.9 ± 2.7 | 6.9 ± 2.6 | 6.8 ± 3.0 | 0.426 |
| ΔHR (mean), bpm | 2.1 ± 1.0 | 2.1 ± 0.9 | 2.1 ± 1.1 | 0.531 |
| ΔHR (SD), bpm | 3.3 ± 1.9 | 3.4 ± 1.9 | 3.2 ± 1.9 | 0.310 |
| SBP (mean), mmHg | 137.0 ± 14.8 | 135.8 ± 13.5 | 140.6 ± 18.0 | 0.149 |
| SBP (SD), mmHg | 13.9 ± 4.1 | 13.8 ± 4.2 | 14.3 ± 3.7 | 0.533 |
| ΔSBP (mean), mmHg | 6.7 ± 2.3 | 6.4 ± 2.2 | 7.3 ± 2.6 | 0.056 |
| ΔSBP (SD), mmHg | 8.0 ± 3.5 | 7.6 ± 2.9 | 9.1 ± 4.7 | 0.113 |
| DBP (mean), mmHg | 72.1 ± 8.9 | 72.6 ± 8.6 | 70.5 ± 9.6 | 0.215 |
| DBP (SD), mmHg | 8.4 ± 3.2 | 8.2 ± 2.7 | 9.0 ± 4.4 | 0.305 |
| ΔDBP (mean), mmHg | 3.9 ± 1.5 | 3.7 ± 1.4 | 4.3 ± 1.8 | 0.055 |
| ΔDBP (SD), mmHg | 5.3 ± 4.2 | 5.0 ± 3.1 | 6.3 ± 6.6 | 0.241 |
| MBP (mean), mmHg | 93.7 ± 10.3 | 94.0 ± 10.1 | 92.9 ± 10.9 | 0.586 |
| MBP (SD), mmHg | 10.7 ± 3.4 | 10.4 ± 3.1 | 11.5 ± 4.2 | 0.116 |
| ΔMBP (mean), mmHg | 5.0 ± 1.9 | 4.8 ± 1.7 | 5.5 ± 2.2 | 0.057 |
| ΔMBP (SD), mmHg | 6.3 ± 4.2 | 5.9 ± 3.1 | 7.6 ± 6.4 | 0.080 |
| CVP (mean), mmHg | 10.6 ± 3.8 | 10.9 ± 3.7 | 9.8 ± 3.9 | 0.110 |
| CVP (SD), mmHg | 3.9 ± 1.8 | 4.0 ± 1.8 | 3.5 ± 1.8 | 0.088 |
| Postoperative complications | ||||
| DGF, n (%) | 3 (2.1) | 0 (0) | 3 (8.3) | 0.017 |
| Rejection, n (%) | 3 (2.1) | 2 (1.8) | 1 (2.8) | 1.000 |
| MACE, n (%) | 1 (0.7) | 0 (0) | 1 (2.8) | 0.544 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, years | 1.01 (0.98–1.04) | 0.549 | ||
| Female sex | 0.79 (0.37–1.71) | 0.552 | ||
| BMI | 1.07 (0.99–1.16) | 0.074 | ||
| Preoperative hemodialysis | 4.23 (1.21–14.8) | 0.024 | ||
| HTN | 0.71 (0.27–1.90) | 0.499 | ||
| DM | 1.17 (0.52–2.68) | 0.704 | ||
| Coronary artery disease | 3.12 (0.19–51.2) | 0.426 | ||
| Congestive heart failure | 2.41 (1.00–5.79) | 0.050 | ||
| ABO incompatibility | 2.28 (1.02–5.12) | 0.046 | 2.20 (0.97–4.96) | 0.059 |
| Hemoglobin | 0.91 (0.74–1.12) | 0.376 | ||
| Albumin | 1.16 (0.66–2.04) | 0.600 | ||
| BUN | 0.97 (0.95–0.99) | 0.002 | 0.97 (0.95–0.99) | 0.005 |
| Creatinine | 0.92 (0.80–1.07) | 0.274 | ||
| Sodium | 1.10 (0.97–1.23) | 0.132 | ||
| Potassium | 0.55 (0.33–0.93) | 0.025 | ||
| eGFR | 1.08 (0.99–1.18) | 0.077 | ||
| Beta blocker | 1.88 (0.88–4.02) | 0.103 | ||
| BMI of donor | 1.08 (0.97–1.20) | 0.147 | ||
| Operation time | 1.01 (1.00–1.01) | 0.008 | 1.01 (1.00–1.01) | 0.007 |
| Cold ischemia time | 1.01 (1.00–1.02) | 0.036 | ||
| Rewarming time | 1.01 (0.99–1.04) | 0.305 | ||
| Inotropes | 2.63 (0.67–10.4) | 0.168 | ||
| Vasodilator | 2.89 (1.24–6.74) | 0.014 | 2.41 (1.05–5.55) | 0.038 |
| Transfusion (RBC) | 1.53 (0.57–4.11) | 0.400 | ||
| ΔHR, mean | 0.94 (0.63–1.39) | 0.755 | ||
| ΔHR, SD | 0.93 (0.75–1.16) | 0.521 | ||
| ΔSBP, mean | 1.18 (1.01–1.38) | 0.044 | ||
| ΔSBP, SD | 1.11 (1.00–1.24) | 0.043 | ||
| ΔDBP, mean | 1.26 (0.99–1.60) | 0.058 | ||
| ΔDBP, SD | 1.07 (0.98–1.16) | 0.134 | ||
| ΔMBP, mean | 1.23 (1.01–1.50) | 0.037 | ||
| ΔMBP, SD | 1.09 (0.99–1.19) | 0.070 | ||
| Outcomes | Score ≤ 7 | 7 < Score ≤ 9 | 9 < Score | p-Value |
|---|---|---|---|---|
| (N = 52) | (N = 46) | (N = 48) | ||
| Postoperative length of stay, days | 6.2 ± 4.3 | 7.2 ± 3.8 | 11.9 ± 24.0 | <0.001 |
| eGFR at postoperative day 7, mL/min/1.73 m2 | 75.8 ± 21.0 | 67.7 ± 25.0 | 61.4 ± 23.8 | 0.009 |
| eGFR at discharge, mL/min/1.73 m2 | 75.5 ± 19.0 | 67.0 ± 25.3 | 62.7 ± 22.5 | 0.015 |
| Creatinine at discharge, mg/dL | 1.08 ± 0.37 | 1.44 ± 1.19 | 1.54 ± 1.26 | 0.024 |
| Postop. DGF, n (%) | 0 (0.0) | 2 (4.3) | 1 (2.1) | 0.318 |
| MACE, n (%) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0.350 |
| Rejection, n (%) | 1 (1.9) | 2 (4.3) | 0 (0.0) | 0.331 |
| Readmission within 90 days, n (%) | 11 (21.2) | 7 (15.2) | 13(27.1) | 0.372 |
| Postop. ICU admission, n (%) | 0 (0.0) | 2 (4.5) | 8 (17.0) | 0.003 |
| Overall mortality, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Kim, K.-S.; Kim, S.-H.; Sim, J.-Y. Predicting Delayed Postoperative Length of Stay Following Robotic Kidney Transplantation: Development and Simulation of Perioperative Risk Factors. Medicina 2024, 60, 1255. https://doi.org/10.3390/medicina60081255
Lee S-W, Kim K-S, Kim S-H, Sim J-Y. Predicting Delayed Postoperative Length of Stay Following Robotic Kidney Transplantation: Development and Simulation of Perioperative Risk Factors. Medicina. 2024; 60(8):1255. https://doi.org/10.3390/medicina60081255
Chicago/Turabian StyleLee, Sang-Wook, Kyoung-Sun Kim, Sung-Hoon Kim, and Ji-Yeon Sim. 2024. "Predicting Delayed Postoperative Length of Stay Following Robotic Kidney Transplantation: Development and Simulation of Perioperative Risk Factors" Medicina 60, no. 8: 1255. https://doi.org/10.3390/medicina60081255






