Endoscopic Features of Chronic Rhinosinusitis in Patients with Gastroesophageal Reflux Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. CRS Characteristics
- Total Cohort (91 + 41 patients):
- Mucosal Thickening:
- Nasal Polyps:
- Ostiomeatal Complex Obstruction:
2.3. Standardized Questionnaires
2.4. Endoscopic Assisment
- Grade 1: 0–25%
- Grade 2: 26–50%
- Grade 3: 51–75%
- Grade 4: 76–100%
3. Results
3.1. Demographics
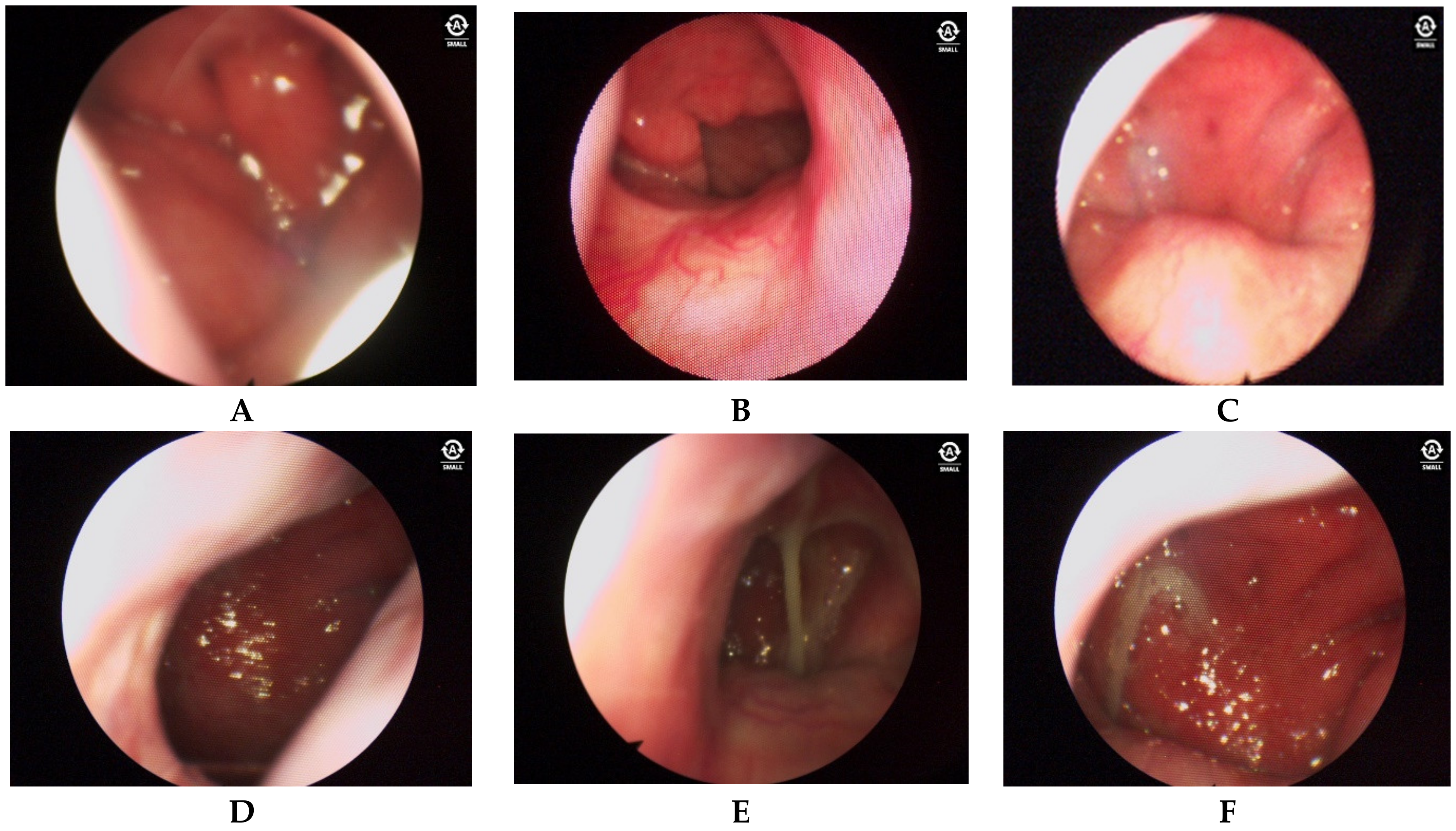
3.2. Endoscopic Findings
- Posterior nasal cavity: Distinct alterations in the mucosa are present, particularly at the posterior end of the inferior nasal concha.
- Anterior nasal cavity: Mucosa may show no changes or grade 1 hypertrophy according to the Camacho classification. Anterior dry rhinitis with crusts may also be observed.
- Posterior nasal cavity: Severe edema, asymmetrical hypertrophy of the posterior ends of the lower nasal bones, and copious mucus production are present.
- Nasal edema detected by two blinded raters is present in a significant percentage of patients (78.9%).
- Increased vascularity: This is noted in a majority of patients (95.7%), indicating possible inflammation or irritation.
- Hypertrophy: This is observed in a high percentage of patients (87.4%), suggesting chronic inflammation.
- Mucus production: This is present in a majority of patients (81.1%), with varying characteristics of color and consistency.
- Asymmetric hypertrophy of the mucosa of the oropharynx is noted in a significant percentage of patients (65.3%), potentially influenced by sleeping position preference.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Yadlapati, R.; Fass, R.; Katzka, D.; Pandolfino, J.; Savarino, E.; Sifrim, D.; Spechler, S.; Zerbib, F.; Fox, M.R.; et al. Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut 2024, 73, 361–371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. The validity and reliability of the reflux finding score (RFS). Laryngoscope 2001, 111, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Bobin, F.; Muls, V.; Mouawad, F.; Dapri, G.; Dequanter, D.; Horoi, M.; Thill, M.P.; Rodriguez Ruiz, A.; Saussez, S. Changes of Laryngeal and Extralaryngeal Symptoms and Findings in Laryngopharyngeal Reflux Patients. Laryngoscope 2021, 131, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Zeleník, K.; Javorská, Z.; Taimrová, R.; Vrtková, A.; Hránková, V.; Tedla, M.; Lukácová, K.; Lubojacký, J.; Formánek, M.; Komínek, P. Association Between Inferior Turbinate Hypertrophy and Extraesophageal Reflux. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 773–778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chong, L.Y.; Head, K.; Hopkins, C.; Philpott, C.; Schilder, A.G.; Burton, M.J. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2016, 4, CD011996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739, Erratum in: Int. Forum Allergy Rhinol. 2022, 12, 974. https://doi.org/10.1002/alr.22987. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650, Erratum in: Lancet 2019, 394, 1618. https://doi.org/10.1016/S0140-6736(19)32218-4. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Han, M.; Kim, T.H. Therapeutic Strategies of Biologics in Chronic Rhinosinusitis: Current Options and Future Targets. Int. J. Mol. Sci. 2022, 23, 5523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. Validity and reliability of the reflux symptom index (RSI). J. Voice 2002, 16, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Bobin, F.; Rodriguez, A.; Dequanter, D.; Muls, V.; Huet, K.; Harmegnies, B.; Crevier-Buchman, L.; Hans, S.; Saussez, S.; et al. Development and Validation of the Short Version of the Reflux Symptom Score: Reflux Symptom Score-12. Otolaryngol. Head Neck Surg. 2021, 164, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Zaghi, S.; Certal, V.; Abdullatif, J.; Means, C.; Acevedo, J.; Liu, S.; Brietzke, S.E.; Kushida, C.A.; Capasso, R. Inferior turbinate classification system, grades 1 to 4: Development and validation study. Laryngoscope 2015, 125, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Zaghi, S.; Certal, V.; Abdullatif, J.; Modi, R.; Sridhara, S.; Tolisano, A.M.; Chang, E.T.; Cable, B.B.; Capasso, R. Predictors of Nasal Obstruction: Quantification and Assessment Using Multiple Grading Scales. Plast. Surg. Int. 2016, 2016, 6945297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anzić, S.A.; Turkalj, M.; Župan, A.; Labor, M.; Plavec, D.; Baudoin, T. Eight weeks of omeprazole 20 mg significantly reduces both laryngopharyngeal reflux and comorbid chronic rhinosinusitis signs and symptoms: Randomised, double-blind, placebo-controlled trial. Clin. Otolaryngol. 2018, 43, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.N.; Yang, Q.M.; Sheng, Y.; Wang, Z.H.; Zhang, Q.; Yan, J.; Hou, J.; Zhu, K.; Cheng, Y.; Wang, B.T.; et al. Role of pepsin and pepsinogen: Linking laryngopharyngeal reflux with otitis media with effusion in children. Laryngoscope 2014, 124, E294–E300. [Google Scholar] [CrossRef] [PubMed]
- Brunworth, J.D.; Mahboubi, H.; Garg, R.; Johnson, B.; Brandon, B.; Djalilian, H.R. Nasopharyngeal acid reflux and Eustachian tube dysfunction in adults. Ann. Otol. Rhinol. Laryngol. 2014, 123, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.; Watters, C.; Watson, N.; Birchall, M.; Karagama, Y. Ear, nose and throat (ENT) manifestations and complications of reflux. Frontline Gastroenterol. 2022, 13, e57–e64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, G.; Guo, W.; Liu, S.; Wang, Y.; Zhang, X. Causal analysis between gastroesophageal reflux disease and chronic rhinosinusitis. Eur. Arch. Otorhinolaryngol. 2024, 281, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Rodriguez Ruiz, A.; Dequanter, D.; Bobin, F.; Mouawad, F.; Muls, V.; Huet, K.; Harmegnies, B.; Remacle, S.; Finck, C.; et al. Validity and Reliability of the Reflux Sign Assessment. Ann. Otol. Rhinol. Laryngol. 2020, 129, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.J.; Zhao, Y.; Wang, J.; Ren, X.; Xu, Y.; Tang, W.; He, Z. PepsinA as a Marker of Laryngopharyngeal Reflux Detected in Chronic Rhinosinusitis Patients. Otolaryngol. Head Neck Surg. 2017, 156, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Chang, Y.T.; Chen, S.F.; Lin, W.C.; Su, Y.Y.; Luo, S.D. The symptom burden of autonomic dysfunction is positively associated with chronic rhinosinusitis status. Rhinology 2018, 56, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ting, F.; Hopkins, C. Outcome Measures in Chronic Rhinosinusitis. Curr. Otorhinolaryngol. Rep. 2018, 6, 271–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeo, N.K.; Park, S.J.; An, T.H. Laryngopharyngeal reflux in chronic rhinosinusitis patients and the role of endoscopic sinus surgery. Auris Nasus Larynx 2022, 49, 663–669. [Google Scholar] [CrossRef] [PubMed]
- DelGaudio, J.M. Direct nasopharyngeal reflux of gastric acid is a contributing factor in refractory chronic rhinosinusitis. Laryngoscope 2005, 115, 946–957. [Google Scholar] [CrossRef] [PubMed]
| Group | |||
|---|---|---|---|
| Main | Control 1 | Control 2 | |
| Male (n = 75) | 46 | 23 | 6 |
| % in group | 48.4 | 56.1 | 60 |
| Female (n = 71) | 49 | 18 | 4 |
| % in group | 51.6 | 43.9 | 40 |
| Main | Control 1 | Control 2 | ||||
|---|---|---|---|---|---|---|
| Mean | CI | Mean | CI | Mean | CI | |
| Age | 48.9 | 2.8 | 36.8 | 3.2 | 32.5 | 4.0 |
| RSS-12 | 29.0 | 1.2 | 9.8 | 0.8 | 7.6 | 1.4 |
| RSI | 186.8 | 7.4 | 14.5 | 1.4 | 14.0 | 1.8 |
| Age | p-Value 1 | RSS-12 | p-Value 1 | RSI | p-Value 1 | ||
|---|---|---|---|---|---|---|---|
| Middle | Middle | Middle | |||||
| Sex | Male | 43.23 (CI 3.29) | 0.252 | 22.36 (CI 2.60) | 0.641 | 125.39 (CI 21.47) | 0.828 |
| Female | 45.65 (CI 3.11) | 21.87 (CI 2.34) | 127.76 (CI 19.32) | ||||
| Race | M | 44.75 (CI 2.77) | 0.809 | 22.80 (CI 2.21) | 0.075 | 128.07 (CI 18.14) | 0.099 |
| E | 43.49 (CI 3.89) | 20.39 (CI 2.56) | 122.63 (CI 22.15) | ||||
| Group | Main | 48.94 (CI 2.77) | 0.000 2 | 28.96 (CI 1.20) | 0.000 2 | 186.76 (CI 7.40) | 0.000 2 |
| Control 1 | 36.78 (CI 3.19) | 9.83 (CI 0.82) | 14.46 (CI 1.42) | ||||
| Control 2 | 32.50 (CI 3.99) | 7.60 (CI 1.44) | 14.00 (CI 1.85) |
| Signs of Exposure to Nasopharyngeal Reflux | 0 | 1 | Total Score | |
|---|---|---|---|---|
| Nose | Asymmetry between the anterior and posterior regions of the nasal cavity | |||
| Predominantly unilateral hypertrophy of the posterior end of the inferior turbinate Absence of mucus in the middle nasal passage | ||||
| Nasopharynx | Hypertrophy of the posterior wall of the nasopharynx | |||
| Hypertrophy of the Eustachian junction | ||||
| Increased vascular pattern | ||||
| Presence of mucus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagandykova, K.; Papulova, N.; Azhenov, T.; Darbekova, A.; Aigozhina, B.; Lechien, J.R. Endoscopic Features of Chronic Rhinosinusitis in Patients with Gastroesophageal Reflux Disease. Medicina 2024, 60, 1257. https://doi.org/10.3390/medicina60081257
Sagandykova K, Papulova N, Azhenov T, Darbekova A, Aigozhina B, Lechien JR. Endoscopic Features of Chronic Rhinosinusitis in Patients with Gastroesophageal Reflux Disease. Medicina. 2024; 60(8):1257. https://doi.org/10.3390/medicina60081257
Chicago/Turabian StyleSagandykova, Kalamkas, Nataliya Papulova, Talapbek Azhenov, Aliya Darbekova, Bayan Aigozhina, and Jerome R. Lechien. 2024. "Endoscopic Features of Chronic Rhinosinusitis in Patients with Gastroesophageal Reflux Disease" Medicina 60, no. 8: 1257. https://doi.org/10.3390/medicina60081257
APA StyleSagandykova, K., Papulova, N., Azhenov, T., Darbekova, A., Aigozhina, B., & Lechien, J. R. (2024). Endoscopic Features of Chronic Rhinosinusitis in Patients with Gastroesophageal Reflux Disease. Medicina, 60(8), 1257. https://doi.org/10.3390/medicina60081257







