Evaluation of the Effects of Repetitive Anaesthesia Administration on the Brain Tissues and Cognitive Functions of Rats with Experimental Alzheimer’s Disease †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Groups and Protocol
2.2. Biochemical Assessment
2.3. Histopathological Evaluation
2.4. Immunohistochemical Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mota, S.I.; Ferreira, I.L.; Rego, A.C. Dysfunctional synapse in Alzheimer’s disease—A focus on NMDA receptors. Neuropharmacology 2014, 76, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, C.; Chaparro, R.E.; Karlnoski, R.; Erasso, D.; Gordon, M.; Morgan, D.; Bosco, G.; Rubini, A.; Parmagnani, A.; Paoli, A.; et al. Effects of repetitive exposure to anesthetics and analgesics in the Tg2576 mouse Alzheimer’s model. Neurotox. Res. 2014, 26, 414–421. [Google Scholar] [CrossRef]
- Culley, D.J.; Xie, Z.; Crosby, G. General anesthetic-induced neurotoxicity: An emerging problem for the young and old? Curr. Opin. Anesthesiol. 2007, 20, 408–413. [Google Scholar] [CrossRef]
- Baranov, D.; Bickler, P.E.; Crosby, G.J.; Culley, D.J.; Eckenhoff, M.F.; Eckenhoff, R.G.; Hogan, K.J.; Jevtovic-Todorovic, V.; Palotás, A.; Perouansky, M.; et al. Consensus statement: First international workshop on anesthetics and Alzheimer’s disease. Anesth. Analg. 2009, 108, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gupta, R.; Martín-Ávila, A.; Cui, M.; Xie, Z.; Yang, G. Anesthesia-induced hippocampal-cortical hyperactivity and tau hyperphosphorylation impair remote memory retrieval in Alzheimer’s disease. Alzheimers Dement. 2023; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Anesthesia-Alzheimer disease link probed. JAMA 2007, 297, 1760. [Google Scholar] [CrossRef]
- Querzfurth, H. Review article. Mechanism of disease Alzheimer’disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar]
- Marques, A.; Lapa, T. Anesthesia and Alzheimer disease—Current perceptions. Braz. J. Anesthesiol. 2018, 68, 174–182. [Google Scholar] [CrossRef]
- Papon, M.A.; Whittington, R.A.; El-Khoury, N.B.; Planel, E. Alzheimer’s disease and anesthesia. Front. Neurosci. 2011, 4, 272. [Google Scholar] [CrossRef]
- Xie, Z.; Culley, D.J.; Dong, Y.; Zhang, G.; Zhang, B.; Moir, R.D.; Frosch, M.P.; Crosby, G.; Tanzi, R.E. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann. Neurol. 2008, 64, 618–627. [Google Scholar] [CrossRef]
- Planel, E.; Richter, K.E.; Nolan, C.E.; Finley, J.E.; Liu, L.; Wen, Y.; Krishnamurthy, P.; Herman, M.; Wang, L.; Schachter, J.B.; et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 2007, 27, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Agren-Wilsson, A.; Lekman, A.; Sjöberg, W.; Rosengren, L.; Blennow, K.; Bergenheim, A.T.; Malm, J. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2007, 116, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Palotás, A.; Reis, H.J.; Bogáts, G.; Babik, B.; Racsmány, M.; Engvau, L.; Kecskeméti, E.; Juhász, A.; Vieira, L.B.; Teixeira, A.L.; et al. Coronary artery bypass surgery provokes Alzheimer’s disease-like changes in the cerebrospinal fluid. J. Alzheimers Dis. 2010, 21, 1153–1164. [Google Scholar] [CrossRef]
- Hussain, M.; Berger, M.; Eckenhoff, R.G.; Seitz, D.P. General anesthetic and the risk of dementia in elderly patients: Current insights. Clin. Interv. Aging 2014, 9, 1619–1628. [Google Scholar] [PubMed]
- Fodale, V.; Santamaria, L.B.; Schifilliti, D.; Mandal, P.K. Anaesthetics and postoperative cognitive dysfunction: A pathological mechanism mimicking Alzheimer’s disease. Anaesthesia 2010, 65, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, X.J.; Huang, Y.; Sun, E.Y.; Sun, M. Ketamine protects gamma oscillations by inhibiting hippocampal LTD. PLoS ONE 2016, 11, e0159192. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Hashimoto, K. (R)-ketamine as prophylactic and therapeutic drug for neurological disorders: Beyond depression. Neurosci. Biobehav. Rev. 2022, 139, 104762. [Google Scholar] [CrossRef]
- Limon, A.; Reyes-Ruiz, J.M.; Miledi, R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. USA 2012, 109, 10071–10076. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, Y.; Dong, Y.; Xu, Z.; Yue, Y.; Golde, T.E.; Tanzi, R.E.; Moir, R.D.; Xie, Z. Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Aβ oligomerization. PLoS ONE 2011, 6, e27019. [Google Scholar] [CrossRef]
- Pryor, K.; Blackstock-Bernstein, A.S.; Feiler, D.; Vortsman, E.; Root, J.C. Administration of propofol after learning improves memory performance in human subjects via loss of competitive consolidation: Evidence that propofol amnesia occurs at the induction of consolidation. Anesthesiology 2012, 117, BOC09. [Google Scholar]
- Shao, H.; Zhang, Y.; Dong, Y.; Yu, B.; Xia, W.; Xie, Z. Chronic treatment with anesthetic propofol improves cognitive function and attenuates caspase activation in both aged and Alzheimer’s disease transgenic mice. J. Alzheimer’s Dis. 2014, 41, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ng, O.T.; Ho, Y.S.; Irwin, M.G.; Chang, R.C.; Wong, G.T. Effect of continuous propofol infusion in rat on tau phosphorylation with or without temperature control. J. Alzheimer’s Dis. 2016, 51, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.B.; Fu, Y.T.; Wang, G.; Sun, L.H.; Fan, Y.Y.; Yang, T.W. Hyperphosphorylation of protein Tau in hippocampus may cause cognitive dysfunction of propofol-anesthetized rats. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3577–3585. [Google Scholar]
- Pang, R.; Quartermain, D.; Rosman, E.; Turndorf, H. Effect of propofol on memory in mice. Pharmacol. Biochem. Behav. 1993, 44, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, F.J.; Xue, Q.S.; Zhao, X.; Yu, B.W. Bilateral inhibition of γ-aminobutyric acid type A receptor function within the basolateral amygdala blocked propofol-induced amnesia and activity-regulated cytoskeletal protein expression inhibition in the hippocampus. J. Am. Soc. Anesthesiol. 2008, 109, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, M.; Sia, E.; Giannouli, V. Anesthesia and dementia: An up-to-date review of the existing literature. Appl. Neuropsychol. Adult 2022, 31, 181–190. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Xu, Z.P.; Li, L.; Bao, J.; Wang, Z.H.; Zeng, J.; Liu, E.J.; Li, X.G.; Huang, R.X.; Gao, D.; Li, M.Z.; et al. Magnesium Protects Cognitive Functions and Synaptic Plasticity in Streptozotocin-Induced Sporadic Alzheimer’s Model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef]
- Ozer, A.B.; Ceribasi, S.; Ceribasi, A.O.; Demirel, I.; Bayar, M.K.; Ustundag, B.; Ileri, A.; Erhan, O.L. Effects of sevoflurane on apoptosis, BDNF and cognitive functions in neonatal rats. Bratisl. Lek. Listy 2017, 118, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.S.; Samuelson, R.J. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Emik, U.; Unal, Y.; Arslan, M.; Demirel, C.B. The effects of memantine on recovery, cognitive functions, and pain after propofol anesthesia. Braz. J. Anesthesiol. 2016, 66, 485–491. [Google Scholar] [CrossRef]
- Yu, D.J.; Gao, H.Y. Effect of propofol on mitochondrial ATP content and ATPase activity in hippocampus of rats with cerebral ischemia-reperfusion injury. Saudi J. Biol. Sci. 2017, 24, 246–250. [Google Scholar] [CrossRef]
- Arras, M.; Autenried, P.; Rettich, A.; Spaeni, D.; Rülicke, T. Optimization of intraperitoneal injection anesthesia in mice: Drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 2001, 51, 443–456. [Google Scholar] [PubMed]
- Reves, J.G.; Glass PS, A.; Lubarsky, D.A.; McEvoy, M.D. Intravenous Anesthetics in Miller’s Anesthesia; Elsevier: Amsterdam, The Netherlands, 2010; pp. 317–377. [Google Scholar]
- Sanou, J.; Goodall, G.; Capuron, L.; Bourdalle-Badie, C.; Maurette, P. Cognitive sequelae of propofol anaesthesia. Neuroreport 1996, 7, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Zhang, C.; Dong, Y.; Zhang, Y.; Nakazawa, H.; Kaneki, M.; Zheng, H.; Shen, Y.; Marcantonio, E.R.; Xie, Z. Battery of behavioral tests in mice to study postoperative delirium. Sci. Rep. 2016, 6, 29874. [Google Scholar] [CrossRef]
- Ozdemirkan, A.; Kurtipek, A.C.; Kucuk, A.; Ozdemir, C.; Yesil, S.; Sezen, S.C.; Kavutcu, M.; Arslan, M. Effect of Cerium Oxide on Kidney and Lung Tissue in Rats with Testicular Torsion/Detorsion. Biomed. Res. Int. 2022, 2022, 3176455. [Google Scholar] [CrossRef]
- Kartal, S.; Kip, G.; Küçük, A.; Atan, A.; Erdem, Ö.; Arslan, M.; Kavutçu, M. The efficacy of dexmedetomidine on lung injury induced by renal ischemia/reperfusion in diabetic rats. Anaesth. Pain Intensive Care 2020, 24, 272–278. [Google Scholar] [CrossRef]
- Van Ye, T.M.; Roza, A.M.; Pieper, G.M.; Henderson, J., Jr.; Johnson, C.P.; Adams, M.B. Inhibition of Intestinal Lipid Peroxidation Does Not Minimize Morphologic Damage. J. Surg. Res. 1993, 55, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA; London, UK, 1974. [Google Scholar]
- Brites, F.D.; Verona, J.; Schreier, L.E.; Fruchart, J.C.; Castro, G.R.; Wikinski, R.L. Paraoxonase 1 and platelet-activating factor acetylhydrolase activities in patients with low hdl-cholesterol levels with or without primary hypertriglyceridemia. Arch. Med. Res. 2004, 35, 235–240. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Pearn, M.L.; Hu, Y.; Niesman, I.R.; Patel, H.H.; Drummond, J.C.; Roth, D.M.; Akassoglou, K.; Patel, P.M.; Head, B.P. Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology 2012, 116, 352–361. [Google Scholar] [CrossRef]
- Whittington, R.A.; Virág, L.; Marcouiller, F.; Papon, M.A.; El Khoury, N.B.; Julien, C.; Morin, F.; Emala, C.W.; Planel, E. Propofol directly increases tau phosphorylation. PLoS ONE 2011, 6, e16648. [Google Scholar] [CrossRef] [PubMed]
- Krzisch, M.; Sultan, S.; Sandell, J.; Demeter, K.; Vutskits, L.; Toni, N. Propofol anesthesia impairs the maturation and survival of adult-born hippocampal neurons. Anesthesiology 2013, 118, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Bayona, N.A.; Gelb, A.W.; Jiang, Z.; Wilson, J.X.; Urquhart, B.L.; Cechetto, D.F. Propofol neuroprotection in cerebral ischemia and its effects on low-molecular-weight antioxidants and skilled motor tasks. Anesthesiology 2004, 100, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, R.; Theresia, S.; Austin, R.; Tiara, R. Propofol effects in rodent models of traumatic brain injury: A systematic review. Asian Biomed. (Res. Rev. News) 2021, 15, 253–265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wakita, M.; Kotani, N.; Nonaka, K.; Shin, M.C.; Akaike, N. Effects of propofol on GABAergic and glutamatergic transmission in isolated hippocampal single nerve-synapse preparations. Eur. J. Pharmacol. 2013, 718, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Twaroski, D.M.; Yan, Y.; Olson, J.M.; Bosnjak, Z.J.; Bai, X. Down-regulation of microRNA-21 is involved in the propofol-induced neurotoxicity observed in human stem cell-derived neurons. Anesthesiology 2014, 121, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Pontén, E.; Gordh, T.; Eriksson, P.; Fredriksson, A.; Sharma, A. Propofol promotes blood-brain barrier breakdown and heat shock protein (HSP 72 kd) activation in the developing mouse brain. CNS Neurol Disord. Drug Targets 2014, 13, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, T.E.; Wang, Y.T. The intersections of NMDAR-dependent synaptic plasticity and cell survival. Neuropharmacology 2013, 74, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, A.; Pontén, E.; Gordh, T.; Eriksson, P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology 2007, 107, 427–436. [Google Scholar] [CrossRef]
- Morgan, C.J.; Riccelli, M.; Maitland, C.H.; Curran, H.V. Long-term effects of ketamine: Evidence for a persisting impairment of source memory in recreational users. Drug. Alcohol. Depend. 2004, 75, 301–308. [Google Scholar] [CrossRef]
- Huang, S.; Dai, Y.; Zhang, Z.; Hao, W.; Chen, H. Docosahexaenoic acid intake ameliorates ketamine-induced impairment of spatial cognition and learning ability in ICR mice. Neurosci. Lett. 2014, 580, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kapila, A.K.; Watts, H.R.; Wang, T.; Ma, D. The impact of surgery and anesthesia on post-operative cognitive decline and Alzheimer’s disease development: Biomarkers and preventive strategies. J. Alzheimers Dis. 2014, 41, 1–13. [Google Scholar] [CrossRef]
- Nykänen, N.P.; Kysenius, K.; Sakha, P.; Tammela, P.; Huttunen, H.J. γ-Aminobutyric acid type A (GABAA) receptor activation modulates tau phosphorylation. J. Biol. Chem. 2012, 287, 6743–6752. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Arima, H.; Sugiura, T.; Hirate, H.; Taniura, H.; Suzuki, K.; Sobue, K. Propofol and thiopental suppress amyloid fibril formation and GM1 ganglioside expression through the γ-aminobutyric acid A receptor. Anesthesiology 2013, 118, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Klyubin, I.; Wang, Q.; Reed, M.N.; Irving, E.A.; Upton, N.; Hofmeister, J.; Cleary, J.P.; Anwyl, R.; Rowan, M.J. Protection against Aβ-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol. Aging 2011, 32, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.M.; Ali, S.S.; Dao, D.N.; Lucero, J.; Shekhtman, G.; Quick, K.L.; Dugan, L.L. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 2007, 318, 1645–1647. [Google Scholar] [CrossRef]
- McNally, J.M.; McCarley, R.W.; Brown, R.E. Chronic Ketamine Reduces the Peak Frequency of Gamma Oscillations in Mouse Prefrontal Cortex Ex vivo. Front. Psychiatry 2013, 4, 106. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Olabarria, M.; Noristani, H.N.; Yeh, C.Y.; Rodriguez, J.J. Astrocytes in Alzheimer’s disease. Neurotherapeutics 2010, 7, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Tanzi, R.E. Alzheimer’s disease and post-operative cognitive dysfunction. Exp. Gerontol. 2006, 41, 346–359. [Google Scholar] [CrossRef]
- Sajan, F.D.; Martiniuk, F.; Marcus, D.L.; Frey, W.H., 2nd; Hite, R.; Bordayo, E.Z.; Freedman, M.L. Apoptotic gene expression in Alzheimer’s disease hippocampal tissue. Am. J. Alzheimers Dis. Other Demen. 2007, 22, 319–328. [Google Scholar] [CrossRef]
- Chung, Y.H.; Shin, C.; Kim, M.J.; Lee, B.; Park, K.H.; Cha, C.I. Immunocytochemical study on the distribution of p53 in the hippocampus and cerebellum of the aged rat. Brain Res. 2000, 885, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.R.; Ghafouri, M.; Mukerjee, R.; Bagashev, A.; Chabrashvili, T.; Sawaya, B.E. Role of p53 in neurodegenerative diseases. Neurodegener. Dis. 2012, 9, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Mayes, J.; Tinker-Mill, C.; Kolosov, O.; Zhang, H.; Tabner, B.J.; Allsop, D. β-amyloid fibrils in Alzheimer disease are not inert when bound to copper ions but can degrade hydrogen peroxide and generate reactive oxygen species. J. Biol. Chem. 2014, 289, 12052–12062. [Google Scholar] [CrossRef]
- Yarpuzlu, A.A.; Aydemir, Ç.; Demirtas, S.; Tuncer, E.T.; Canpolat, O.; Genc, Y. Antioxidant Status, Lipid Peroxidation Products and Cystatin C as Potential Clinical Markers of Alzheimer’s Disease in Systemic Circulation. Turk. J. Med. Sci. 2001, 31, 329–335. [Google Scholar]
- Kinumi, T.; Ogawa, Y.; Kimata, J.; Saito, Y.; Yoshida, Y.; Niki, E. Proteomic characterization of oxidative dysfunction in human umbilical vein endothelial cells (HUVEC) induced by exposure to oxidized LDL. Free Radic. Res. 2005, 39, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Sand, P.G. Paraoxonase genes and the susceptibilty to ischemic stroke. Int. J. Stroke 2013, 8, E39. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Kanavouras, K.; Tsatsakis, A.M. Role of paraoxonase 1 (PON1) in organophosphate metabolism: Implications in neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 418–424. [Google Scholar] [CrossRef]
- Saeidi, M.; Shakeri, R.; Marjani, A.; Khajeniazi, S. Alzheimer’s Disease and Paraoxonase 1 (PON1) Gene Polymorphisms. Open Biochem. J. 2017, 11, 47–55. [Google Scholar] [CrossRef]
 ), granulovacuolar degeneration (►), and pycnotic nuclei (
), granulovacuolar degeneration (►), and pycnotic nuclei ( ), which increased in Group A and decreased in Group APK, can be observed (haematoxylin-eosin, ×200 and ×400).
), which increased in Group A and decreased in Group APK, can be observed (haematoxylin-eosin, ×200 and ×400).
 ), granulovacuolar degeneration (►), and pycnotic nuclei (
), granulovacuolar degeneration (►), and pycnotic nuclei ( ), which increased in Group A and decreased in Group APK, can be observed (haematoxylin-eosin, ×200 and ×400).
), which increased in Group A and decreased in Group APK, can be observed (haematoxylin-eosin, ×200 and ×400).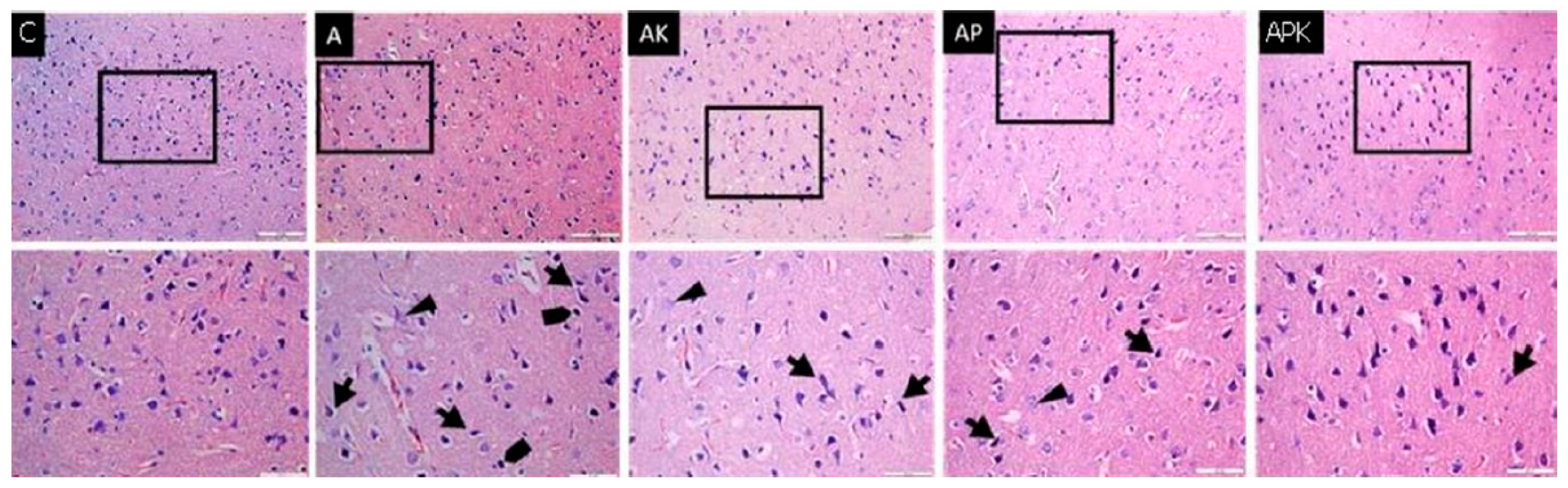

| Rat/Input–Output | Group C (n = 6) | Group A (n = 6) | Group AK (n = 6) | Group AP (n = 6) | Group APK (n = 6) | p ** |
|---|---|---|---|---|---|---|
| Initial | 7.67 ± 1.50 | 8.33 ± 0.52 | 7.83 ± 0.41 | 8.00 ± 0.63 | 7.80 ± 0.82 | 0.653 |
| Week 1 | 7.07 ± 0.82 | 7.17 ± 0.75 | 6.67 ± 0.92 | 6.50 ± 0.55 | 6.67 ± 0.53 | 0.526 |
| Week 2 | 6.50 ± 1.05 | 5.50 ± 0.55 *, ? | 5.00 ± 0.89 *, ? | 5.33 ± 0.52 *, ? | 5.03 ± 0.63 *, ? | 0.012 |
| Week 3 | 7.00 ± 1.26 | 4.23 ± 1.17 *, ? | 3.33 ± 0.52 *, ? | 4.00 ± 0.63 *, ? | 4.33 ± 0.82 *, ? | <0.0001 |
| Week 4 | 6.80 ± 0.89 | 3.83 ± 1.32 *, ? | 3.17 ± 0.41 *, ? | 3.33 ± 0.52 *, ? | 3.67 ± 0.48 *, ? | <0.0001 |
| After 1st anaesthesia | 6.67 ± 0.52 | 3.50 ± 0.84 *, ? | 5.50 ± 1.05 +, ? | 3.83 ± 0.89 *, ? | 5.00 ± 0.63 *, +, ? | <0.0001 |
| After 2nd anaesthesia | 7.33 ± 1.32 | 2.83 ± 0.98 *, ? | 7.17 ± 1.60 + | 5.17 ± 0.67 *, +, ? | 5.67 ± 0.82 *, + | <0.0001 |
| After 3rd anaesthesia | 7.00 ± 0.86 | 3.17 ± 1.04 *, ? | 8.00 ± 1.55 + | 5.67 ± 0.52 +, & | 6.62 ± 0.82 + | <0.0001 |
| Parameters | Group C (n = 6) | Group A (n = 6) | Group AK (n = 6) | Group AP (n = 6) | Group APK (n = 6) | p ** |
|---|---|---|---|---|---|---|
| TBARS (nmol/mg protein) | 0.49 ± 0.13 | 1.01 ± 0.13 * | 0.74 ± 0.13 | 0.62 ± 0.21 + | 0.64 ± 0.24 + | 0.002 |
| PON-1 (IU/mg protein) | 98.57 ± 30.56 | 47.65 ± 10.46 * | 92.20 ± 51.80 + | 89.03 ± 15.60 + | 98.57 ± 47.39 + | 0.011 |
| CAT (IU/mg protein) | 82.98 ± 31.23 | 51.30 ± 6.57 * | 62.28 ± 11.96 | 73.25 ± 11.53 + | 73.22 ± 19.61 + | 0.025 |
| Protein | Group C (n = 6) | Group A (n = 6) | Group AK (n = 6) | Group AP (n = 6) | Group APK (n = 6) | p ** |
|---|---|---|---|---|---|---|
| Bax | 1.56 ± 0.25 | 10.21 ± 0.79 * | 9.85 ± 0.56 * | 11.64 ± 0.66 * | 9.73 ± 0.85 * | <0.0001 |
| Bcl-2 | 2.60 ± 0.44 | 0.07 ± 0.05 * | 1.38 ± 0.38 + | 2.08 ± 0.33 + | 1.43 ± 0.30 + | <0.0001 |
| Caspase-3 | 0.92 ± 0.09 | 14.91 ± 0.55 * | 12.18 ± 0.69 * | 14.50 ± 0.79 * | 9.36 ± 0.65 *, + | <0.0001 |
| p53 | 2.76 ± 0.31 | 11.04 ± 1.23 * | 7.96 ± 0.64 * | 7.75 ± 0.65 * | 7.36 ± 0.50 * | <0.0001 |
| GFAP | 1.93 ± 0.29 | 11.63 ± 1.14 * | 7.69 ± 0.93 * | 10.64 ± 0.96 * | 8.29 ± 0.66 * | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eryilmaz, N.C.; Arslan, M.; Kucuk, A.; Tuna, A.T.; Guney, S.; Kaplanoglu, G.T.; Kavutcu, M. Evaluation of the Effects of Repetitive Anaesthesia Administration on the Brain Tissues and Cognitive Functions of Rats with Experimental Alzheimer’s Disease. Medicina 2024, 60, 1266. https://doi.org/10.3390/medicina60081266
Eryilmaz NC, Arslan M, Kucuk A, Tuna AT, Guney S, Kaplanoglu GT, Kavutcu M. Evaluation of the Effects of Repetitive Anaesthesia Administration on the Brain Tissues and Cognitive Functions of Rats with Experimental Alzheimer’s Disease. Medicina. 2024; 60(8):1266. https://doi.org/10.3390/medicina60081266
Chicago/Turabian StyleEryilmaz, Nuray Camgoz, Mustafa Arslan, Aysegul Kucuk, Ayca Tas Tuna, Sevin Guney, Gulnur Take Kaplanoglu, and Mustafa Kavutcu. 2024. "Evaluation of the Effects of Repetitive Anaesthesia Administration on the Brain Tissues and Cognitive Functions of Rats with Experimental Alzheimer’s Disease" Medicina 60, no. 8: 1266. https://doi.org/10.3390/medicina60081266






