Low Calcium–High Magnesium Krebs–Henseleit Solution Combined with Adenosine and Lidocaine Improved Rat Aortic Function and Structure Following Cold Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preservation Solution Compositions
- Krebs–Henseleit (KH): 118 mM NaCl, 4.7 mM KCl, 1.2 mM NaH2PO4, 2.5 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 0.03 mM Na-EDTA, and 11 mM glucose.
- Modified KH: 118 mM NaCl, 4.7 mM KCl, 1.2 mM NaH2PO4, 0.22 mM CaCl2, 2.6 mM MgCl2, 25 mM NaHCO3, 0.03 mM Na-EDTA, and 11 mM glucose.
- Modified KH + AL: modified KH, 0.4 mM adenosine, and 1 mM lidocaine.
- Modified KH +ALMI: modified KH, 0.4 mM adenosine, 1 mM lidocaine, 0.1 mM melatonin, and 0.01 IU/mL insulin.
2.3. Animal Preparation
2.4. Aortic Ring Preparation
2.5. Vessel Function Analysis
2.6. Vessel Histopathological Analysis
2.7. Statistical Analysis
3. Results
3.1. NE- and KCl-Induced Aortic Vasoconstrictive Responses with Different Preservation Solutions
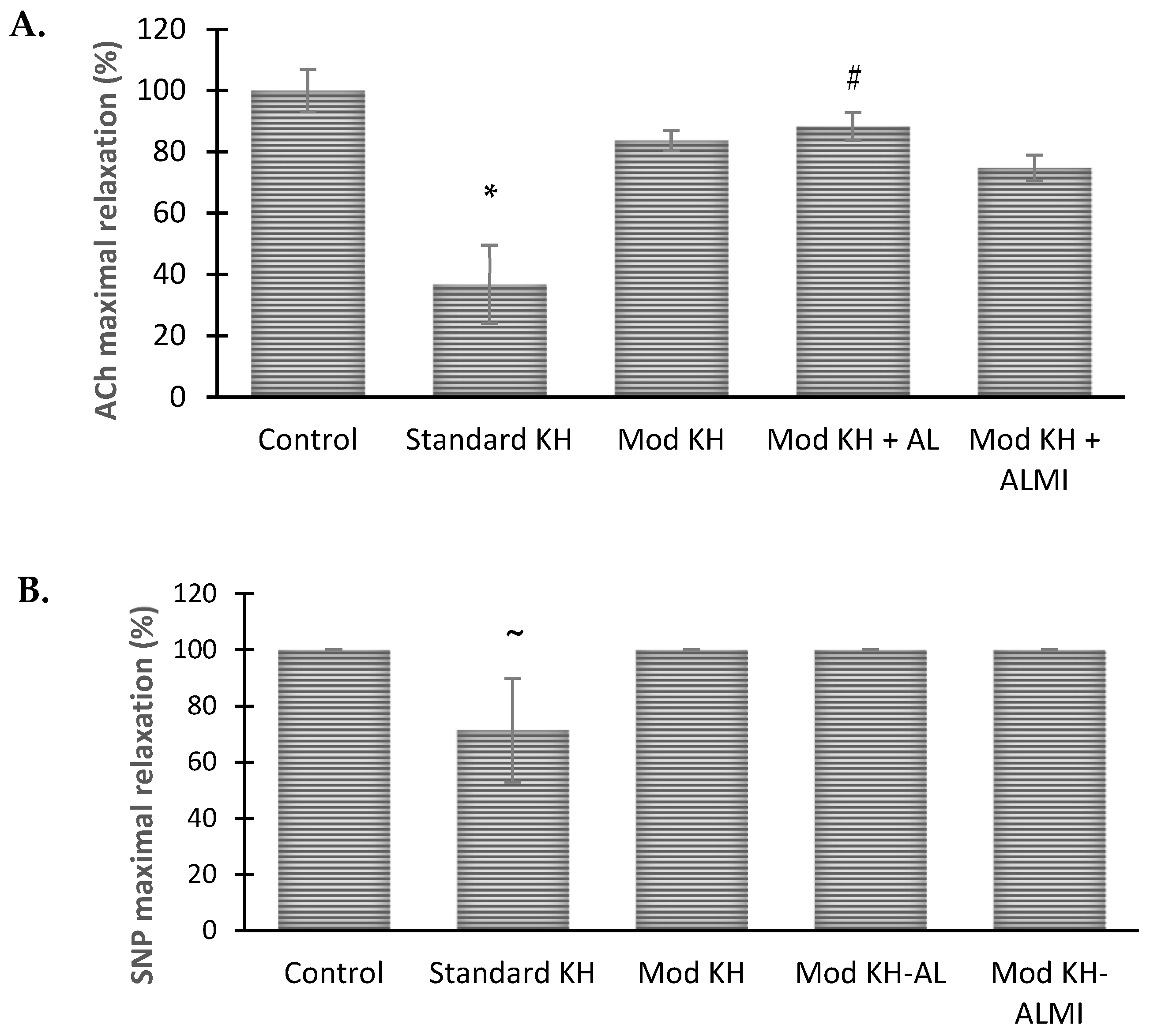
3.2. Ach- and NO-Induced Aortic Vasorelaxation Responses in Different Preservation Solutions
3.3. Maximum Aortic Relaxation Function after 6 Days of Storage in Preservation Solution Compared with Freshly Harvested Aortic Rings
3.4. Vessel Histopathological Analysis after 6 Days of Storage in Preservation Solution
4. Discussion
Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future perspectives in small-diameter vascular graft engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Tatoulis, J. The radial artery: An important component of multiarterial coronary surgery and considerations for its optimal harvest. JTCVS Tech. 2020, 5, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Hisdal, J.; Rosales, A.; Kalvoy, H.; Martinsen, O.G. Evaluating the Effects of Cold Storage on Vascular Grafts Using Bioimpedance Measurement Techniques. In Proceedings of the 17th International Conference on Electrical Bioimpedance, Joinville, Santa Catarina, Brazil, 9–14 June 2019; Springer: Singapore, 2020. [Google Scholar]
- Garbe, S.; Zatschler, B.; Müller, B.; Dieterich, P.; Ebner, A.; Rauen, U.; Matschke, K.; Deussen, A. Preservation of human artery function following prolonged cold storage with a new solution. J. Vasc. Surg. 2011, 53, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Cupitra, N.I.; Calderon, J.C.; Narvaez-Sanchez, R. Increased receptor expression supports vascular reactivity of the rabbit aorta during preservation. Eur. J. Cardiothorac. Surg. 2020, 59, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Ostrozka-Cieslik, A. The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation. Int. J. Mol. Sci. 2022, 23, 3141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019, 28, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, A.; Carnevale, M.; Somov, A.; Osorio, J.; Rodríguez, J.; Guibert, E.; Fuller, B.; Froghi, F. Organ Preservation into the 2020s: The Era of Dynamic Intervention. Transfus. Med. Hemother. 2019, 46, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Wilbring, M.; Tugtekin, S.M.; Zatschler, B.; Ebner, A.; Reichenspurner, H.; Matschke, K.; Deussen, A. Even short-time storage in physiological saline solution impairs endothelial vascular function of saphenous vein grafts. Eur. J. Cardiothorac. Surg. 2011, 40, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs 2019, 19, 449–464. [Google Scholar] [PubMed]
- Dobson, G.P.; Arsyad, A.; Letson, H.L. The adenosine hypothesis revisited: Modulation of coupling between myocardial perfusion and arterial compliance. Front. Physiol. 2017, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Arsyad, A.; Dobson, G.P. Lidocaine relaxation in isolated rat aortic rings is enhanced by endothelial removal: Possible role of Kv, K ATP channels and A2a receptor crosstalk. BMC Anesthesiol. 2016, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- De Klaver, M.J.M.; Buckingham, M.G.; Rich, G.F. Lidocaine attenuates cytokine-induced cell injury in endothelial and vascular smooth muscle cells. Anesth. Analg. 2003, 97, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yamamoto, Y.; Feng, G.G.; Kazaoka, Y.; Fujiwara, Y.; Kinoshita, H. Lidocaine Prevents Oxidative Stress-Induced Endothelial Dysfunction of the Systemic Artery in Rats with Intermittent Periodontal Inflammation. Anesth. Analg. 2017, 124, 2054–2062. [Google Scholar] [CrossRef]
- Unal, Y.; Iriz, E.; Yilmazer, D.; Kavutcu, M.; Alper, M.; Kurtipek, O.; Pekbay, A. Effects of lidocaine on membrane stabilization in harvested vein graft storage. Saudi Med. J. 2009, 30, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Jiang, R.; Dobson, G.P.; Granfeldt, A.; Vinten-Johansen, J. The nondepolarizing, normokalemic cardioplegia formulation adenosine-lidocaine (adenocaine) exerts anti-neutrophil effects by synergistic actions of its components. J. Thorac. Cardiovasc. Surg. 2012, 143, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Arsyad, A.; Sokoya, E.; Dobson, G.P. Adenosine and lidocaine (AL) combination dilates intimally damaged rat thoracic aortic rings and guinea pig mesenteric arteries: Possible significance to cardiac surgery. Am. J. Transl. Res. 2018, 10, 1841. [Google Scholar] [PubMed]
- Djabir, Y.; Dobson, G.P. Hemodynamic rescue and ECG stability during chest compressions using adenosine and lidocaine after 8-minute asphyxial hypoxia in the rat. Am. J. Emerg. Med. 2013, 31, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Rudd, D.M.; Dobson, G.P. Eight hours of cold static storage with adenosine and lidocaine (Adenocaine) heart preservation solutions: Toward therapeutic suspended animation. J. Thorac. Cardiovasc. Surg. 2011, 142, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Interactions of the pineal hormone melatonin with oxygen-centered free radicals: A brief review. Braz. J. Med. Biol. Res. 1993, 26, 1141–1155. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, R.; Izumi-Nakaseko, H.; Goto, A.; Takei, Y.; Matsumoto, A.; Lorch, U.; Täubel, J.; Sugiyama, A. Both osmolality-dependent and independent mechanisms are associated with acute hyperglycemia-induced cardiovascular adverse reactions: Analysis of the mutual interactions leading to cardiovascular phenotypes in dogs. J. Toxicol. Sci. 2023, 48, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Dreau, D.; Wang, S.; Clemens, M.; Elliott, G.D. Structure and Function of Porcine Arteries Are Preserved for up to 6 Days Using the HypoRP Cold-storage Solution. Transplant 2020, 104, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Arsyad, A.; Dobson, G.P. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle Kv channels, KATP channels and A2a receptors. BMC Pharmacol. Toxicol. 2016, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Olchanheski, L.R., Jr.; Sordi, R.; Oliveira, J.G.; Alves, G.F.; Mendes, R.T.; Santos, F.A.; Fernandes, D. The role of potassium channels in the endothelial dysfunction induced by periodontitis. J. Appl. Oral. Sci. 2018, 26, e20180048. [Google Scholar] [CrossRef] [PubMed]
- Luk, C.; Haywood, N.J.; Bridge, K.I.; Kearney, M.T. Paracrine role of the endothelium in metabolic homeostasis in health and nutrient excess. Front. Cardiovasc. Med. 2022, 9, 882923. [Google Scholar] [CrossRef] [PubMed]
- Kongpol, K.; Yodsheewan, R.; Nernpermpisooth, N.; Kumphune, S. Recombinant human secretory leukocyte protease inhibitor ameliorated vessel preservation in experimentally isolated rat arteries. J. Appl. Pharm. Sci. 2020, 10, 107–114. [Google Scholar]
- Kazemi, K.; Nikeghbalian, Z.; Yaghmaei, S.; Nikeghbalian, S.; Shamsaeifar, A.; Asgharnia, Y.; Dehghankhalili, M.; Golchini, A.; Malekhosseini, S.A. University of Wisconsin vs normal saline solutions for preservation of blood vessels of brain dead donors: A histopathological study. Clin. Transpl. 2018, 32, 13241. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.; Reineke, D.; Heinisch, P.P.; Schönhoff, F.; Huber, C.; Kadner, A.; Englberger, L.; Carrel, T. Graft preservation solutions in cardiovascular surgery. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 300–309. [Google Scholar] [CrossRef]
- Wenceslau, C.F.; McCarthy, C.G.; Earley, S.; England, S.K.; Filosa, J.A.; Goulopoulou, S.; Gutterman, D.D.; Isakson, B.E.; Kanagy, N.L.; Martinez-Lemus, L.A.; et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, 77–111. [Google Scholar] [CrossRef]
- Ingemansson, R.; Sjoberg, T.; Massa, G.; Steen, S. Long-term preservation of vascular endothelium and smooth muscle. Ann. Thorac. Surg. 1995, 59, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Ingemansson, R.; Bolys, R.; Budrikis, A.; Lindgren, A.; Sjoberg, T.; Steen, S. Addition of Calcium to Euro-Collins Solution Is Essential for 24-Hour Preservation of the Vasculature. Ann. Thorac. Surg. 1997, 63, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Ingemansson, R.; Sjoberg, T.; Steen, S. Importance of calcium in long-term preservation of the vasculature. Ann. Thorac. Surg. 1996, 61, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Altura, B.M.; Altura, B.T.; Carella, A.; Gebrewold, A.; Murakawa, T.; Nishio, A. Mg2+–Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can. J. Physiol. Pharmacol. 1987, 65, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Albertini, A.; Curello, S.; Ceconi, C.; Di Lisa, F.; Raddino, R.; Visioli, O. Myocardial recovery during post-ischaemic reperfusion: Effects of nifedipine, calcium and magnesium. J. Mol. Cell Cardiol. 1986, 18, 487–498. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Rivera, C.O.; Davison, C.A. Nitric oxide-dependent and-independent mechanisms account for gender differences in vasodilation to acetylcholine. J. Pharmacol. Exp. Ther. 2000, 292, 375–380. [Google Scholar] [PubMed]
- Woodward, L.C.; Antoniades, C.; Taggart, D.P. Intraoperative vein graft preservation: What is the solution? Ann. Thorac. Surg. 2016, 102, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Carmona, N.; Wyss, R.K.; Arnold, M.; Segiser, A.; Kalbermatter, N.; Joachimbauer, A.; Carrel, T.P.; Longnus, S.L. Effects of graft preservation conditions on coronary endothelium and cardiac functional recovery in a rat model of donation after circulatory death. J. Heart Lung Transplant. 2021, 40, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Nieman, G.F.; Christie, J.D.; Fisher, A.B. Shear stress-related mechanosignaling with lung ischemia: Lessons from basic research can inform lung transplantation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L668–L680. [Google Scholar] [CrossRef] [PubMed]
- Dobson, G.P.; Morris, J.L.; Letson, H.L. Adenosine, lidocaine and Mg2+ update: Teaching old drugs new tricks. Front. Med. 2023, 10, 1231759. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Yan, S.; Yang, Q.; Zhou, Y.; Zeng, X.; Liu, Z.; An, X.; Toque, H.A.; Dong, Z.; et al. Regulation of endothelial intracellular adenosine via adenosine kinase epigenetically modulates vascular inflammation. Nat. Commun. 2017, 8, 943. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Suh, J.K.; Jeong, J.S.; Cho, S.Y.; Kim, D.W. Antioxidant effect of lidocaine and procaine on reactive oxygen species-induced endothelial dysfunction in the rabbit abdominal aorta. Korean J. Anesthesiol. 2010, 59, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.W.; Belardinelli, L.; Kuo, L. Adenosine A2a receptors mediate coronary microvascular dilation to adenosine: Role of nitric oxide and atp-sensitive potassium channels. J. Pharmacol. Exp. Ther. 1999, 291, 655–664. [Google Scholar] [PubMed]
- del Pozo, B.F.; Pérez-Vizcaíno, F.; Fernández, C.; Zaragozá, F.; Tamargo, J. Effects of several class i antiarrhythmic drugs on isolated rat aortic vascular smooth muscle. Gen. Pharmacol. Vasc. Syst. 1997, 29, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Ostrozka-Cieslik, A.; Dolinska, B. The Role of hormones and trophic factors as components of preservation solutions in protection of renal function before transplantation: A review of the literature. Molecules 2020, 25, 2185. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Zubero, E.; García-Gil, F.A.; López-Pingarrón, L.; Alatorre-Jiménez, M.A.; Iñigo-Gil, P.; Tan, D.X.; García, J.J.; Reiter, R.J. Potential benefits of melatonin in organ transplantation: A review. J. Endocrinol. 2016, 229, R129–R146. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.M.; Erdemli, M.E.; Ozhan, O.; Erdemli, Z.; Gozukara Bag, H.G.; Vardi, N. Effect of melatonin on increasing the effectiveness of liver preservation solution. Turk. J. Gastroenterol. 2023, 34, 943–951. [Google Scholar] [CrossRef]
- Korkmaz-Icöz, S.; Ballikaya, B.; Soethoff, J.; Kraft, P.; Sayour, A.A.; Radovits, T.; Loganathan, S.; Karck, M.; Szabó, G.; Veres, G. Graft Preservation Solution DuraGraft® Alleviates Vascular Dysfunction Following In Vitro Ischemia/Reperfusion Injury in Rats. Pharmaceuticals 2021, 14, 1028. [Google Scholar] [CrossRef]
- Ballikaya, B.; Soethoff, J.; Kraft, P.; Sayour, A.; Radovits, T.; Loganathan, S.; Karck, M.; Veres, G.; Szabó, G.; Korkmaz-Icöz, S. Arterial graft preservation with an endothelial damage inhibitor reduces vascular dysfunction in a rat model of in vitro ischemia/reperfusion injury. Arch. Cardiovasc. Dis. Suppl. 2022, 14, 170. [Google Scholar] [CrossRef]
- Haime, M.; McLean, R.R.; Kurgansky, K.E.; Emmert, M.Y.; Kosik, N.; Nelson, C.; Gaziano, M.J.; Cho, K.; Gagnon, D.R. Relationship between intra-operative vein graft treatment with DuraGraft® or saline and clinical outcomes after coronary artery bypass grafting. Expert. Rev. Cardiovasc. Ther. 2018, 16, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Pachuk, C.J.; Rushton-Smith, S.K.; Emmert, M.Y. Intraoperative storage of saphenous vein grafts in coronary artery bypass grafting. Expert. Rev. Med. Devices 2019, 16, 989–997. [Google Scholar] [CrossRef] [PubMed]
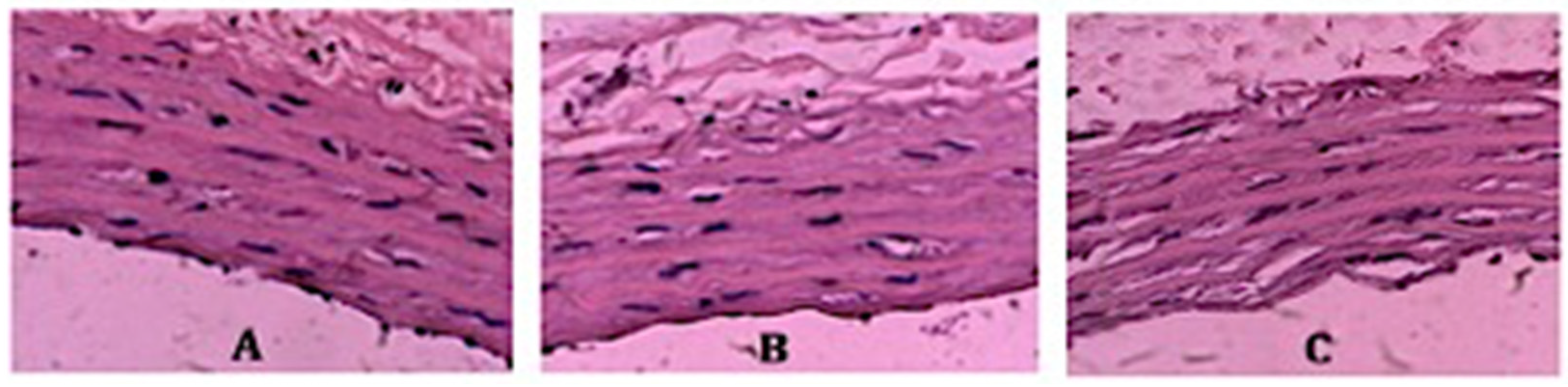


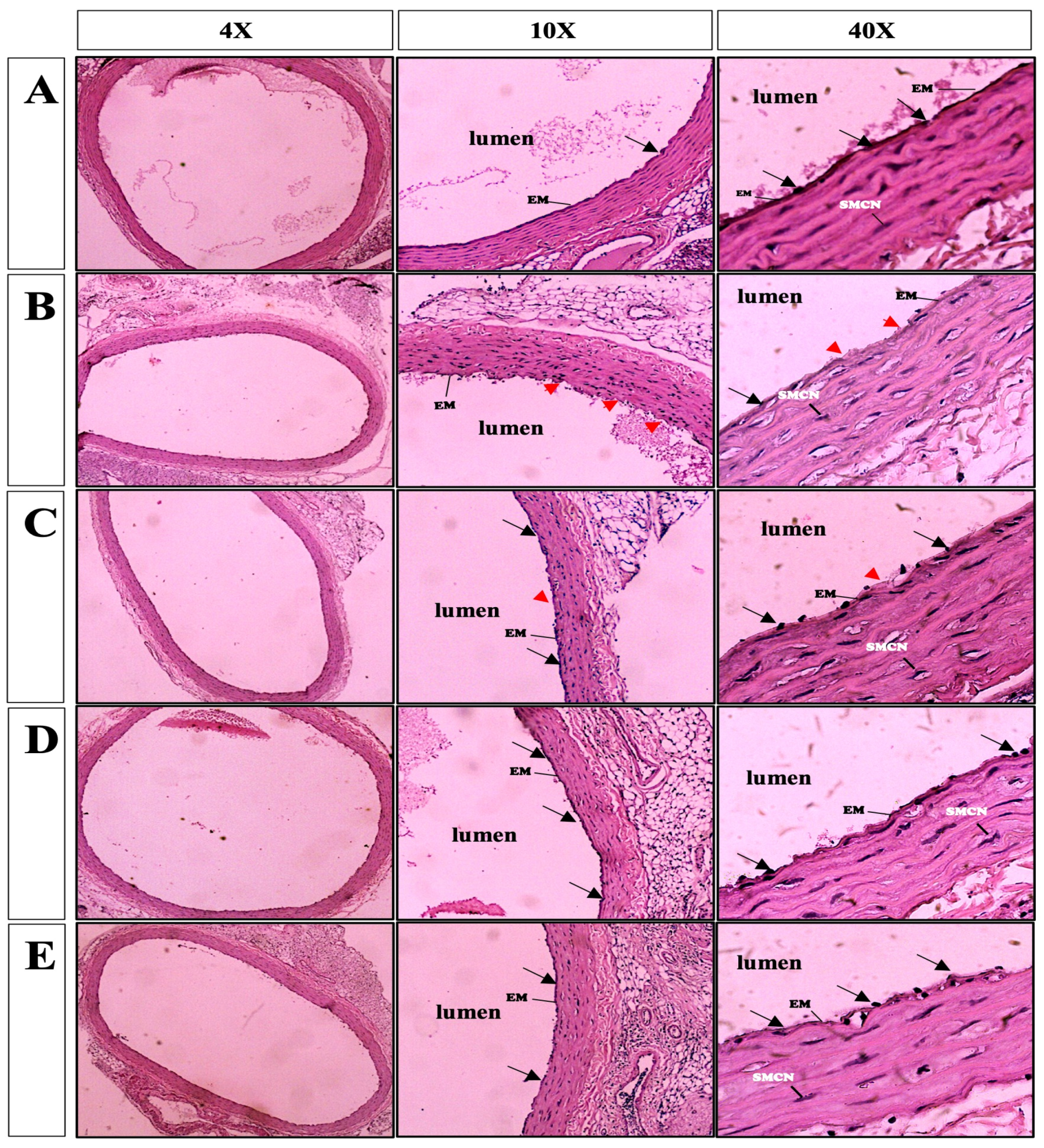
| Groups | Elastic Membrane Deterioration | Denudation of Endothelial Cells |
|---|---|---|
| Control | 0 | 0 |
| Standard KH | 2 | 2 |
| Mod KH | 1 | 1 |
| Mod KH-AL | 0 | 0 |
| Mod KH-ALMI | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsyad, A.; Lembang, G.K.R.; Linda, S.L.; Djabir, Y.Y.; Dobson, G.P. Low Calcium–High Magnesium Krebs–Henseleit Solution Combined with Adenosine and Lidocaine Improved Rat Aortic Function and Structure Following Cold Preservation. Medicina 2024, 60, 1284. https://doi.org/10.3390/medicina60081284
Arsyad A, Lembang GKR, Linda SL, Djabir YY, Dobson GP. Low Calcium–High Magnesium Krebs–Henseleit Solution Combined with Adenosine and Lidocaine Improved Rat Aortic Function and Structure Following Cold Preservation. Medicina. 2024; 60(8):1284. https://doi.org/10.3390/medicina60081284
Chicago/Turabian StyleArsyad, Aryadi, Geni K. R. Lembang, Sesilia L. Linda, Yulia Y. Djabir, and Geoffrey P. Dobson. 2024. "Low Calcium–High Magnesium Krebs–Henseleit Solution Combined with Adenosine and Lidocaine Improved Rat Aortic Function and Structure Following Cold Preservation" Medicina 60, no. 8: 1284. https://doi.org/10.3390/medicina60081284





