A Cross-Sectional Study Comparing Oxidative Stress in Patients with Epilepsy Treated with Old and New Generation Antiseizure Medications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Assessments
2.2. Blood Sampling and Assays
2.3. Statistical Analysis
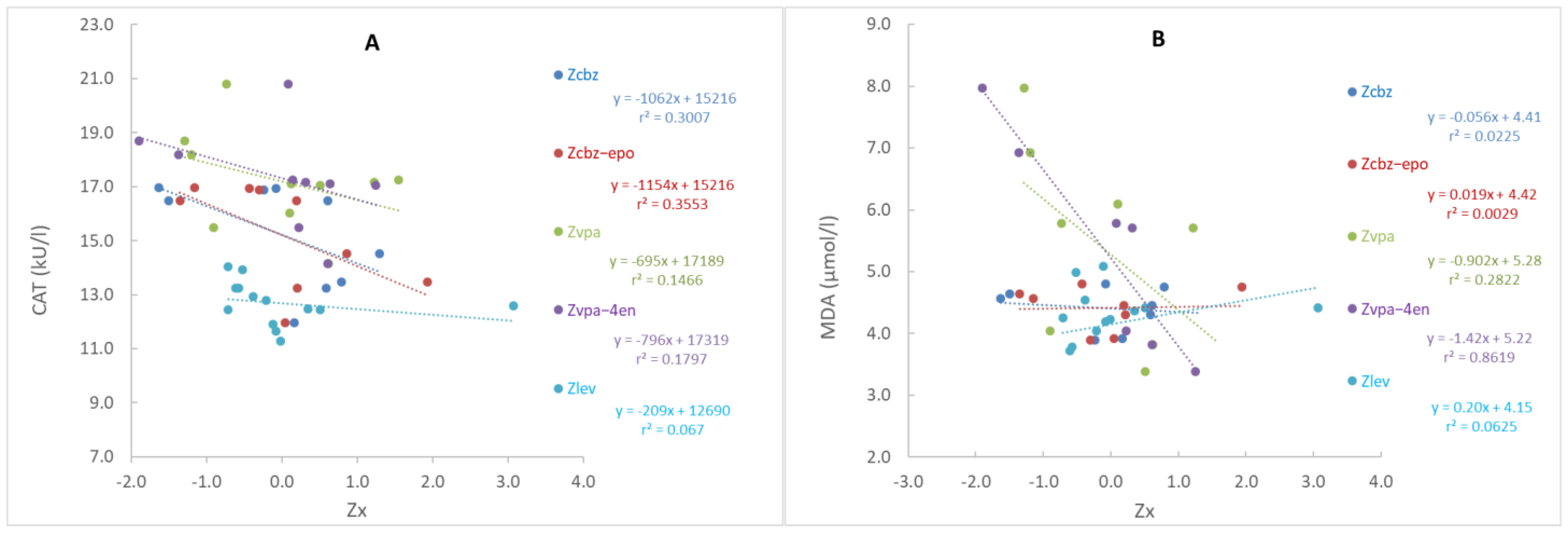
3. Results
3.1. Demographic and Clinical Data
3.2. Biomarkers of Oxidative Stress in Patients Compared to Controls
3.3. Biomarkers of Oxidative Stress in Patients Treated with Different ASMs
4. Discussion
4.1. The Influence of ASMs Treatment on Oxidative Stress
4.2. The Influence of Old and New Generation ASMs on Oxidative Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Yuksel, A.; Cengiz, M.; Seven, M.; Ulutin, T. Changes in the antioxidant system in epileptic children receiving antiepileptic drugs: Two-year prospective studies. J. Child Neurol. 2001, 16, 603–606. [Google Scholar] [CrossRef]
- Cárdenas-Rodríguez, N.; Coballase-Urrutia, E.; Rivera-Espinosa, L.; Romero-Toledo, A.; Sampieri, A.I., 3rd; Ortega-Cuellar, D.; Montesinos-Correa, H.; Floriano-Sánchez, E.; Carmona-Aparicio, L. Modulation of antioxidant enzymatic activities by certain antiepileptic drugs (valproic acid, oxcarbazepine, and topiramate): Evidence in humans and experimental models. Oxid. Med. Cell Longev. 2013, 2013, 598493. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef]
- Ashrafi, M.R.; Shams, S.; Nouri, M.; Mohseni, M.; Shabanian, R.; Yekaninejad, M.S.; Chegini, N.; Khodadad, A.; Safaralizadeh, R. A probable causative factor for an old problem: Selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia 2007, 48, 1750–1755. [Google Scholar] [CrossRef]
- Aycicek, A.; Iscan, A. The effects of carbamazepine, valproic acid and phenobarbital on the oxidative and antioxidative balance in epileptic children. Eur. Neurol. 2007, 57, 65–69. [Google Scholar] [CrossRef]
- Ercegovac, M.; Jović, N.; Simić, T.; Beslać-Bumbaširević, L.; Sokić, D.; Savić-Radojević, A.; Matić, M.; Jovanović, D.; Ristić, A.; Đukić, T.; et al. Antiepileptic drugs affect protein, lipid and DNA oxidative damage and antioxidant defense in patients with epilepsy. J. Med. Biochem. 2013, 32, 121–130. [Google Scholar] [CrossRef]
- Güneş, S.; Dirik, E.; Yiş, U.; Seçkin, E.; Kuralay, F.; Köse, S.; Ünalp, A. Oxidant status in children after febrile seizures. Pediatr. Neurol. 2009, 40, 47–49. [Google Scholar] [CrossRef]
- Mehmet, U.C.; Sefer, V.; Yavuz, Y.; Esref, A.; Tahsin, C.; Adalet, A.; Hatice, Y.; Mehmet, U.A. Serum paroxonase-1 activities and malondialdehyde levels in patients with epilepsy. Dicel Med. J. 2012, 39, 557–560. [Google Scholar]
- Menon, B.; Ramalingam, K.; Kumar, R.V. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure 2012, 21, 780–784. [Google Scholar] [CrossRef]
- Pandey, K.M.; Mittra, P.; Maheshwari, P.K. The Lipid Peroxidation Product as a Marker of Oxidative Stress in Epilepsy. J. Clin. Diagn. Res. 2012, 6, 590–592. [Google Scholar]
- Sudha, K.; Rao, A.V.; Rao, A. Oxidative stress and antioxidants in epilepsy. Clin. Chim. Acta 2001, 303, 19–24. [Google Scholar] [CrossRef]
- Verrotti, A.; Scardapane, A.; Franzoni, E.; Manco, R.; Chiarelli, F. Increased oxidative stress in epileptic children treated with valproic acid. Epilepsy Res. 2008, 78, 171–177. [Google Scholar] [CrossRef]
- Kösem, A.; Yücel, C.; Titiz, A.P.; Sezer, S.; Neşelioğlu, S.; Erel, Ö.; Turhan, T. Evaluation of serum thiol-disulphide homeostasis parameters as oxidative stress markers in epilepsy patients. Acta Neurol. Belg. 2021, 121, 1555–1559. [Google Scholar] [CrossRef]
- Turkdogan, D.; Toplan, S.; Karakoc, Y. Lipid peroxidation and antioxidative enzyme activities in childhood epilepsy. J. Child Neurol. 2002, 17, 673–676. [Google Scholar] [CrossRef]
- Varoglu, A.O.; Yildirim, A.; Aygul, R.; Gundogdu, O.L.; Sahin, Y.N. Effects of valproate, carbamazepine, and levetiracetam on the antioxidant and oxidant systems in epileptic patients and their clinical importance. Clin. Neuropharmacol. 2010, 33, 155–157. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Lazaropoulou, C.; Regoutas, S.; Karikas, G.A.; Margeli, A.; Tsakiris, S.; Papassotiriou, I. Valproic acid monotherapy induces DNA oxidative damage. Toxicology 2006, 217, 228–232. [Google Scholar] [CrossRef]
- Peker, E.; Oktar, S.; Arı, M.; Kozan, R.; Doğan, M.; Çağan, E.; Söğüt, S. Nitric oxide, lipid peroxidation, and antioxidant enzyme levels in epileptic children using valproic acid. Brain Res. 2009, 1297, 194–197. [Google Scholar] [CrossRef]
- Menon, B.; Ramalingam, K.; Kumar, R.V. Low plasma antioxidant status in patients with epilepsy and the role of antiepileptic drugs on oxidative stress. Ann. Indian. Acad. Neurol. 2014, 17, 398–404. [Google Scholar] [CrossRef]
- Ercegovac, M.; Jovic, N.; Simic, T.; Beslac-Bumbasirevic, L.; Sokic, D.; Djukic, T.; Savic-Radojevic, A.; Matic, M.; Mimic-Oka, J.; Pljesa-Ercegovac, M. Byproducts of protein, lipid and DNA oxidative damage and antioxidant enzyme activities in seizure. Seizure 2010, 19, 205–210. [Google Scholar] [CrossRef]
- Lopez, J.; Gonzalez, M.E.; Lorigados, L.; Morales, L.; Riveron, G.; Bauza, J.Y. Oxidative stress markers in surgically treated patients with refractory epilepsy. Clin. Biochem. 2007, 40, 292–298. [Google Scholar] [CrossRef]
- Nemade, S.T.; Melinkeri, R.R. Oxidative and Antioxidative Status in Epilepsy. Prevara Med. Rev. 2010, 2, 8–10. [Google Scholar]
- Verrotti, A.; Basciani, F.; Trotta, D.; Pomilio, M.P.; Morgese, G.; Chiarelli, F. Serum copper, zinc, selenium, glutathione peroxidase and superoxide dismutase levels in epileptic children before and after 1 year of sodium valproate and carbamazepine therapy. Epilepsy Res. 2002, 48, 71–75. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Zhang, M.; Wang, X.-C.; Yu, Y.-H.; Jin, P.-J.; Wang, Y. Effects of sodium valproate on neutrophils’ oxidative metabolism and oxidant status in children with idiopathic epilepsy. Zhonghua Er Ke Za Zhi 2011, 49, 776–781. [Google Scholar]
- Arhan, E.; Serdaroglu, A.; Ozturk, B.; Ozturk, H.S.; Ozcelik, A.; Kurt, N.; Kutsal, E.; Sevinc, N. Effects of epilepsy and antiepileptic drugs on nitric oxide, lipid peroxidation and xanthine oxidase system in children with idiopathic epilepsy. Seizure 2011, 20, 138–142. [Google Scholar] [CrossRef]
- Yis, U.; Seckin, E.; Kurul, S.H.; Kuralay, F.; Dirik, E. Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res. 2009, 84, 232–237. [Google Scholar] [CrossRef]
- Martinc, B.; Grabnar, I.; Vovk, T. Antioxidants as a Preventive Treatment for Epileptic Process: A Review of the Current Status. Curr. Neuropharmacol. 2015, 12, 527–550. [Google Scholar] [CrossRef]
- Miziak, B.; Blaszczyk, B.; Chroscinska-Krawczyk, M.; Danilkiewicz, G.; Jagiello-Wojtowicz, E.; Czuczwar, S.J. The problem of osteoporosis in epileptic patients taking antiepileptic drugs. Expert. Opin. Drug Saf. 2014, 13, 935–946. [Google Scholar] [CrossRef]
- Eddy, C.M.; Rickards, H.E.; Cavanna, A.E. The cognitive impact of antiepileptic drugs. Ther. Adv. Neurol. Disord. 2011, 4, 385–407. [Google Scholar] [CrossRef]
- Jakovljević, D.; Nikolić, M.; Jovanović, V.; Uzelac, T.V.; Nikolić-Kokić, A.; Novaković, E.; Miljević, Ć.; Milovanović, M.; Blagojević, D. Influence of Long-Term Anti-Seizure Medications on Redox Parameters in Human Blood. Pharmaceuticals 2024, 17, 130. [Google Scholar] [CrossRef]
- Sobaniec, W.; Solowiej, E.; Kulak, W.; Bockowski, L.; Smigielska-Kuzia, J.; Artemowicz, B. Evaluation of the influence of antiepileptic therapy on antioxidant enzyme activity and lipid peroxidation in erythrocytes of children with epilepsy. J. Child Neurol. 2006, 21, 558–562. [Google Scholar] [CrossRef]
- Yurekli, V.A.; Naziroglu, M. Selenium and topiramate attenuates blood oxidative toxicity in patients with epilepsy: A clinical pilot study. Biol. Trace Elem. Res. 2013, 152, 180–186. [Google Scholar] [CrossRef]
- Yuksel, A.; Cengiz, M.; Seven, M.; Ulutin, T. Erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children with valproate and carbamazepine monotherapy. J. Basic. Clin. Physiol. Pharmacol. 2000, 11, 73–81. [Google Scholar] [CrossRef]
- Liu, C.S.; Wu, H.M.; Kao, S.H.; Wei, Y.H. Serum trace elements, glutathione, copper/zinc superoxide dismutase, and lipid peroxidation in epileptic patients with phenytoin or carbamazepine monotherapy. Clin. Neuropharmacol. 1998, 21, 62–64. [Google Scholar]
- Lu, W.; Uetrecht, J.P. Possible bioactivation pathways of lamotrigine. Drug Metab. Dispos. 2007, 35, 1050–1056. [Google Scholar] [CrossRef]
- Bolayir, E.; Celik, K.; Tas, A.; Topaktas, S.; Bakir, S. The effects of oxcarbazepine on oxidative stress in epileptic patients. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 345–348. [Google Scholar] [CrossRef]
- Ozden, H.; Kabay, S.C.; Toker, A.; Ustüner, M.C.; Ozbayer, C.; Ustüner, D.; Günes, H.V. The effects of levetiracetam on urinary 15f-2t-isoprostane levels in epileptic patients. Seizure 2010, 19, 514–516. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.D.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs—Best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Marounek, M. The simple and sensitive measurement of malondialdehyde in selected specimens of biological origin and some feed by reversed phase high performance liquid chromatography. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2011, 879, 2251–2258. [Google Scholar] [CrossRef]
- Squellerio, I.; Caruso, D.; Porro, B.; Veglia, F.; Tremoli, E.; Cavalca, V. Direct glutathione quantification in human blood by LC-MS/MS: Comparison with HPLC with electrochemical detection. J. Pharm. Biomed. Anal. 2012, 71, 111–118. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Leite, C.E.; Petersen, G.O.; Lunardelli, A.; Thiesen, F.V. A high-performance liquid chromatography method for the determination of carbamazepine and carbamazepine-10,11-epoxide and its comparison with chemiluminescent immunoassay. Clin. Chem. Lab. Med. 2009, 47, 458–463. [Google Scholar] [CrossRef]
- Contin, M.; Mohamed, S.; Albani, F.; Riva, R.; Baruzzi, A. Simple and validated HPLC-UV analysis of levetiracetam in deproteinized plasma of patients with epilepsy. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2008, 873, 129–132. [Google Scholar] [CrossRef]
- Martinc, B.; Roškar, R.; Grabnar, I.; Vovk, T. Simultaneous determination of gabapentin, pregabalin, vigabatrin, and topiramate in plasma by HPLC with fluorescence detection. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2014, 962, 82–88. [Google Scholar] [CrossRef]
- Sasamot, K.; Ushijima, T.; Saito, M.; Ohkura, Y. Precolumn Fluorescence Derivatization of Carboxylic Acids Using 4-Aminomethyl-6,7-dimethoxycoumarin in a Two-Phase Medium. Anal. Sci. 1996, 12, 189–193. [Google Scholar] [CrossRef]
- Arroyo, S.; Anhut, H.; Kugler, A.R.; Lee, C.M.; Knapp, L.E.; Garofalo, E.A.; Messmer, S. Pregabalin Add-on Treatment: A Randomized, Double-blind, Placebo-controlled, Dose-Response Study in Adults with Partial Seizures. Epilepsia 2004, 45, 20–27. [Google Scholar] [CrossRef]
- Berry, D.; Millington, C. Analysis of pregabalin at therapeutic concentrations in human plasma/serum by reversed-phase HPLC. Ther. Drug Monit. 2005, 27, 451–456. [Google Scholar] [CrossRef]
- Martinc, B.; Grabnar, I.; Vovk, T. The Role of Reactive Species in Epileptogenesis and Influence of Antiepileptic Drug Therapy on Oxidative Stress. Curr. Neuropharmacol. 2012, 10, 328–343. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Liang, L.P.; Hellier, J.L.; Staley, K.J.; Patel, M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol. Dis. 2008, 30, 130–138. [Google Scholar] [CrossRef]
- Patel, M.N. Oxidative stress, mitochondrial dysfunction, and epilepsy. Free Radic. Res. 2002, 36, 1139–1146. [Google Scholar] [CrossRef]
- Rowley, S.; Patel, M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic. Biol. Med. 2013, 62, 121–131. [Google Scholar] [CrossRef]
- Baram, T.Z. The brain, seizures and epilepsy throughout life: Understanding a moving target. Epilepsy Curr. 2012, 12, 7–12. [Google Scholar] [CrossRef]
- Hamed, S.A.; Abdellah, M.M.; El-Melegy, N. Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J. Pharmacol. Sci. 2004, 96, 465–473. [Google Scholar] [CrossRef]
- Solowiej, E.; Sobaniec, W. The effect of antiepileptic drug therapy on antioxidant enzyme activity and serum lipid peroxidation in young patients with epilepsy. Neurol. Neurochir. Pol. 2003, 37, 991–1003. [Google Scholar]
- Cengiz, M.; Yuksel, A.; Seven, M. The effects of carbamazepine and valproic acid on the erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children. Pharmacol. Res. 2000, 41, 423–425. [Google Scholar] [CrossRef]
- Martínez-Ballesteros, C.; Pita-Calandre, E.; Sánchez-González, Y.; Rodríguez-López, C.M.; Agil, A. Lipid peroxidation in adult epileptic patients treated with valproic acid. Rev. Neurol. 2004, 38, 101–106. [Google Scholar]
- Santos, N.A.; Medina, W.S.; Martins, N.M.; Rodrigues, M.A.; Curti, C.; Santos, A.C. Involvement of oxidative stress in the hepatotoxicity induced by aromatic antiepileptic drugs. Toxicol. Vitr. 2008, 22, 1820–1824. [Google Scholar] [CrossRef]
- Bjornsson, E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol. Scand. 2008, 118, 281–290. [Google Scholar] [CrossRef]
- Lu, W.; Uetrecht, J.P. Peroxidase-mediated bioactivation of hydroxylated metabolites of carbamazepine and phenytoin. Drug Metab. Dispos. 2008, 36, 1624–1636. [Google Scholar] [CrossRef]
- Michoulas, A.; Tong, V.; Teng, X.W.; Chang, T.K.; Abbott, F.S.; Farrell, K. Oxidative stress in children receiving valproic acid. J. Pediatr. 2006, 149, 692–696. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Combination therapy in epilepsy: When and what to use. Drugs 2006, 66, 1817–1829. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Reactive species can pose special problems needing special solutions: Some examples. In Free Radicals in Biology and Medicine, 4th ed.; Chapter 6; Oxford University Press: New York, NY, USA, 2008; pp. 341–394. [Google Scholar]
- Rumià, J.; Marmol, F.; Sanchez, J.; Giménez-Crouseilles, J.; Carreño, M.; Bargalló, N.; Boget, T.; Pintor, L.; Setoain, X.; Donaire, A.; et al. Oxidative stress markers in the neocortex of drug-resistant epilepsy patients submitted to epilepsy surgery. Epilepsy Res. 2013, 107, 75–81. [Google Scholar] [CrossRef]
- Erakovic, V.; Zupan, G.; Varljen, J.; Laginja, J.; Simonic, A. Lithium plus pilocarpine induced status epilepticus—Biochemical changes. Neurosci. Res. 2000, 36, 157–166. [Google Scholar] [CrossRef]
- Walford, G.A.; Moussignac, R.L.; Scribner, A.W.; Loscalzo, J.; Leopold, J.A. Hypoxia potentiates nitric oxide-mediated apoptosis in endothelial cells via peroxynitrite-induced activation of mitochondria-dependent and -independent pathways. J. Biol. Chem. 2004, 279, 4425–4432. [Google Scholar] [CrossRef]
- Jones, S.P.; Bolli, R. The ubiquitous role of nitric oxide in cardioprotection. J. Mol. Cell Cardiol. 2006, 40, 16–23. [Google Scholar] [CrossRef]
- Dezfulian, C.; Raat, N.; Shiva, S.; Gladwin, M.T. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc. Res. 2007, 75, 327–338. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef]
- Webb, A.; Bond, R.; McLean, P.; Uppal, R.; Benjamin, N.; Ahluwalia, A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA 2004, 101, 13683–13688. [Google Scholar] [CrossRef]
- Ono, H.; Sakamoto, A.; Sakura, N. Plasma total glutathione concentrations in epileptic patients taking anticonvulsants. Clin. Chim. Acta 2000, 298, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Yip, V.L.M.; Meng, X.; Maggs, J.L.; Jenkins, R.E.; Marlot, P.T.; Marson, A.G.; Park, B.K.; Pirmohamed, M. Mass Spectrometric Characterization of Circulating Covalent Protein Adducts Derived from Epoxide Metabolites of Carbamazepine in Patients. Chem. Res. Toxicol. 2017, 30, 1419–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yeo, H.C.; Doniger, S.J.; Ames, B.N. Assay of aldehydes from lipid peroxidation: Gas chromatography-mass spectrometry compared to thiobarbituric acid. Anal. Biochem. 1997, 245, 161–166. [Google Scholar] [CrossRef] [PubMed]
| Patients with Epilepsy | Monotherapy | Poly-Therapy | Controls | ||
|---|---|---|---|---|---|
| Old ASMs | New ASMs | ||||
| Subjects, n | 49 | 9 | 12 | 28 | 14 |
| a Age [years], median (Q1–Q3) | 40 (31–51) | 42 (35–50.5) | 43 (28–57.5) | 37 (29.3–48.3) | 40.5 (30–53.8) |
| b Gender | |||||
| Male, n (%) | 29 (59) | 5 (56) | 7 (58) | 17 (61) | 7 (50) |
| Female, n (%) | 20 (41) | 4 (44) | 5 (42) | 11 (39) | 7 (50) |
| a Body weight (kg), median (Q1–Q3) | 68 (63–82) | 75 (63–86) | 64 (60.3–79.3) | 69 (62.0–82.8) | 72 (62.3–81.3) |
| a BMI (kg/m2), median (Q1–Q3) | 24.4 (21.9–26.6) | 25.9 (22.2–28.5) | 23.7 (20.0–25.9) | 24.5 (21.8–26.4) | 25.0 (21.9–27.1) |
| a Duration of illness [years], median (Q1–Q3) | 18 (10.5–24.5) | 20 (17–32) | 11 (7–18.3) | 19 (14–26) | / |
| a Seizure frequency, [seizure number/month], median (Q1–Q3) | 0.7 (0–2) | 0.1 (0–1) | 0.4 (0–1.5) | 2.5 (0–10) | / |
| ASM dose [mg/day] | |||||
| CBZ median, (Q1–Q3) | 800 (400–1600) | 800 (600–1600) | / | 800 (800–1300) | / |
| VPA median, (Q1–Q3) | 1250 (875–1625) | 1000 (625–1375) | / | 1500 (875–2250) | / |
| LEV median, (Q1–Q3) | 1500 (1000–3000) | / | 1000 (625–1000) | 2000 (1500–3000) | / |
| PGB median, (Q1–Q3) | 338 (300–600) | / | 600 (/) | 300 (300–356) | / |
| TPM median, (Q1–Q3) | 225 (150–263) | / | 175 (/) | 250 (175–288) | / |
| Parameter | Patients with Epilepsy (n = 49) | Controls (n = 14) | p Value |
|---|---|---|---|
| SOD (U/mL) | 202 (173–227) | 142 (138–150) | <0.001 |
| CAT (U/L) | 14.1 (12.5–16.8) | 11.7 (11.0–12.3) | <0.001 |
| GR (U/L) | 545 (452–595) | 669 (651–702) | <0.001 |
| GPx (U/L) | 6003 (3925–9038) | 8834 (7940–11,712) | 0.003 |
| GSH (µmol/L) | 898 (574–1282) | 1558 (1441–1826) | <0.001 |
| GSSG (µmol/L) | 199 (163–249) | 264 (240–301) | 0.014 |
| GSH/GSSG | 4.20 (3.33–5.96) | 6.36 (5.39–7.07) | 0.032 |
| MDA (µmol/L) | 4.39 (3.90–4.83) | 4.35 (3.78–4.99) | 0.757 |
| PC (nmol/L) | 42.5 (36.6–49.2) | 21.0 (16.3–24.3) | <0.001 |
| NO3− + NO2− (µmol/L) | 54.3 (42.2–68.5) | 23.3 (19.9–25.0) | <0.001 |
| NO2− (µmol/L) | 1.96 (1.05–2.81) | 3.08 (2.35–3.62) | 0.005 |
| SOD | CAT | GR | GPx | GSH | GSSG | GSH/ GSSG | MDA | PC | NO2− + NO3− | NO2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SOD | - | ||||||||||
| CAT | 0.695 (<0.001) | - | |||||||||
| GR | −0.730 (<0.001) | −0.586 (<0.001) | - | ||||||||
| GPx | −0.822 (<0.001) | −0.739 (<0.001) | 0.659 (<0.001) | - | |||||||
| GSH | ns | ns | ns | ns | - | ||||||
| GSSG | ns | ns | ns | ns | 0.376 (0.010) | - | |||||
| GSH/ GSSG | ns | ns | ns | ns | 0.860 (<0.001) | ns | - | ||||
| MDA | 0.367 (0.014) | 0.391 (0.009) | −0.337 (0.025) | −0.360 (0.018) | ns | ns | ns | - | |||
| PC | 0.688 (<0.001) | 0.543 (<0.001) | −0.501 (<0.001) | −0.711 (<0.001) | ns | ns | ns | 0.374 (0.012) | - | ||
| NO2− + NO3− | 0.622 (<0.001) | 0.539 (<0.001) | −0.431 (0.002) | −0.522 (<0.001) | ns | ns | ns | ns | 0.440 (0.002) | - | |
| NO2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | - |
| Parameter | Old ASMs (n = 9) | New ASMs (n = 12) | Polytherapy (n = 28) | pG/G |
|---|---|---|---|---|
| SOD (U/mL) | 292 (255–308) | 173 (164–180) | 206 (180–220) | O/N, O/P, N/P |
| CAT (U/L) | 17.0 (16.5–18.4) | 12.9 (11.4–14.0) | 14.2 (12.4–16.9) | O/N, O/P |
| GR (U/L) | 411 (388–469) | 597 (583–655) | 524 (452–591) | O/N, O/P, N/P |
| GPx (U/L) | 2049 (1526–3414) | 8976 (7697–12,396) | 5572 (4161–8553) | O/N, O/P, N/P |
| GSH (µmol/L) | 870 (790–1486) | 961 (583–1320) | 899 (566–1222) | ns |
| GSSG (µmol/L) | 222 (182–244) | 192 (150–237) | 199 (159–268) | ns |
| GSH/GSSG | 4.00 (3.30–8.00) | 4.32 (3.59–6.25) | 4.23 (3.01–5.96) | ns |
| MDA (µmol/L) | 4.64 (4.17–6.35) | 4.11 (3.67–4.41) | 4.39 (3.95–5.01) | ns |
| PC (nmol/L) | 53.8 (48.4–58.6) | 34.4 (31.1–42.9) | 39.0 (37.2–47.3) | O/N, O/P, N/P |
| NO3− + NO2− (µmol/L) | 97.3 (73.6–106) | 51.8 (40.6–55.5) | 51.1 (42.1–64.0) | O/N, O/P |
| NO2− (µmol/L) | 1.66 (1.41–3.62) | 1.96 (0.70–2.79) | 2.02 (1.21–2.80) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinc, B.; Grabnar, I.; Milosheska, D.; Lorber, B.; Vovk, T. A Cross-Sectional Study Comparing Oxidative Stress in Patients with Epilepsy Treated with Old and New Generation Antiseizure Medications. Medicina 2024, 60, 1299. https://doi.org/10.3390/medicina60081299
Martinc B, Grabnar I, Milosheska D, Lorber B, Vovk T. A Cross-Sectional Study Comparing Oxidative Stress in Patients with Epilepsy Treated with Old and New Generation Antiseizure Medications. Medicina. 2024; 60(8):1299. https://doi.org/10.3390/medicina60081299
Chicago/Turabian StyleMartinc, Boštjan, Iztok Grabnar, Daniela Milosheska, Bogdan Lorber, and Tomaž Vovk. 2024. "A Cross-Sectional Study Comparing Oxidative Stress in Patients with Epilepsy Treated with Old and New Generation Antiseizure Medications" Medicina 60, no. 8: 1299. https://doi.org/10.3390/medicina60081299







