Increased Scan Speed and Pitch on Ultra-Low-Dose Chest CT: Effect on Nodule Volumetry and Image Quality
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. CT Parameters
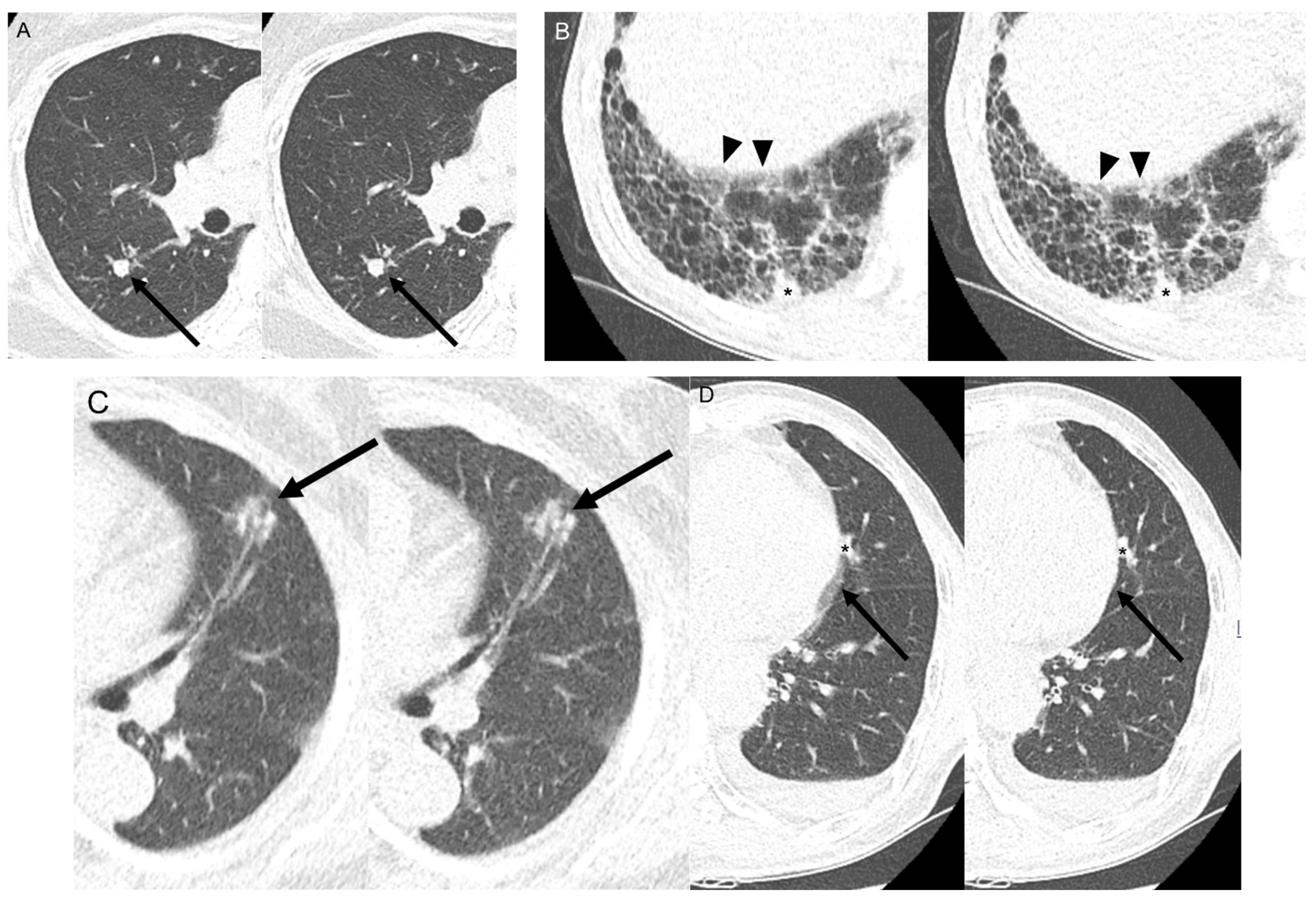
2.3. Objective Image Quality and Nodule Volumetric Analysis
2.4. Subjective Image Quality Analysis
2.5. Statistical Analyses
3. Results
3.1. Patient and Nodule Characteristics
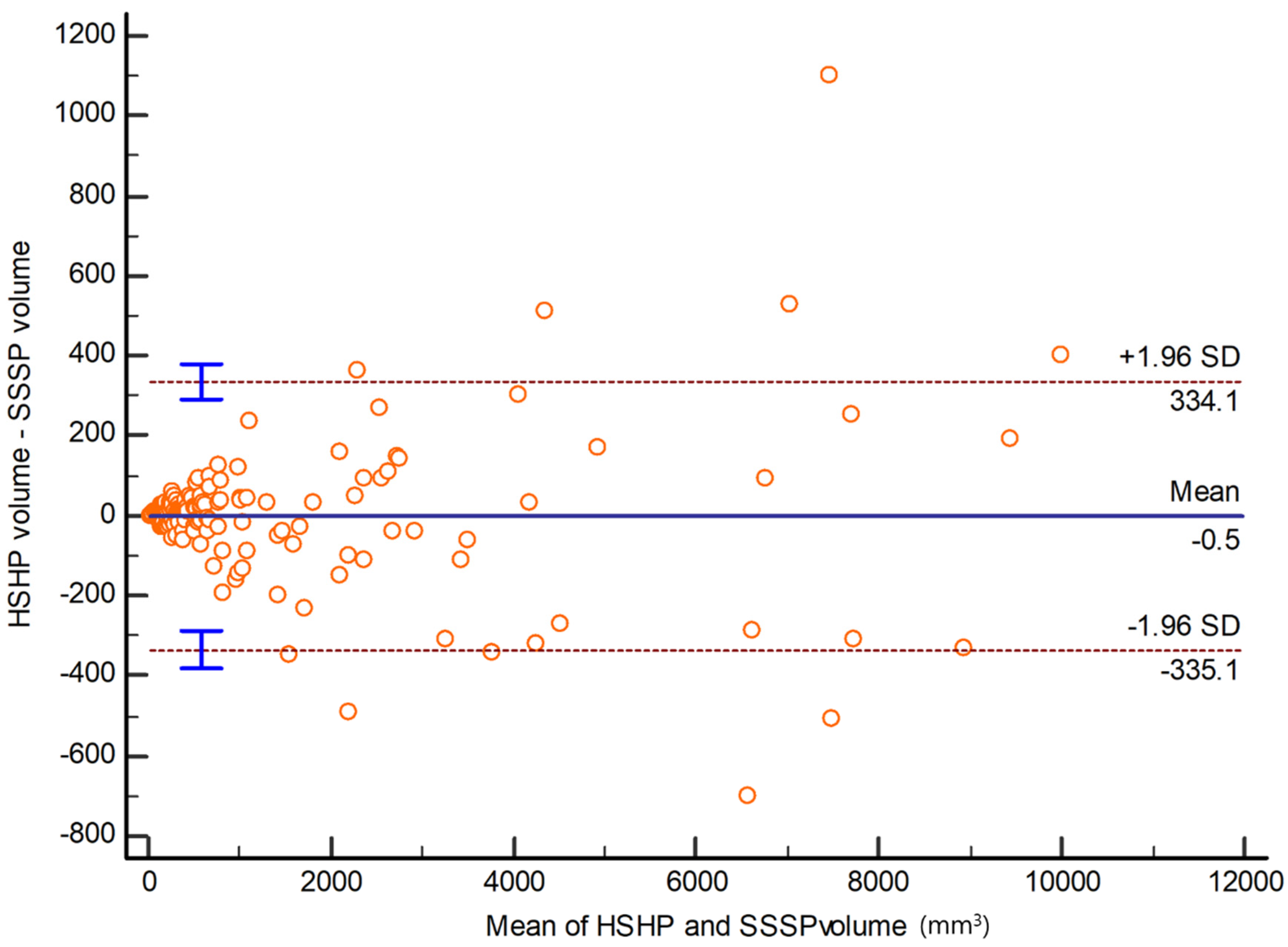
3.2. Objective Image Quality between SSSP and HSHP
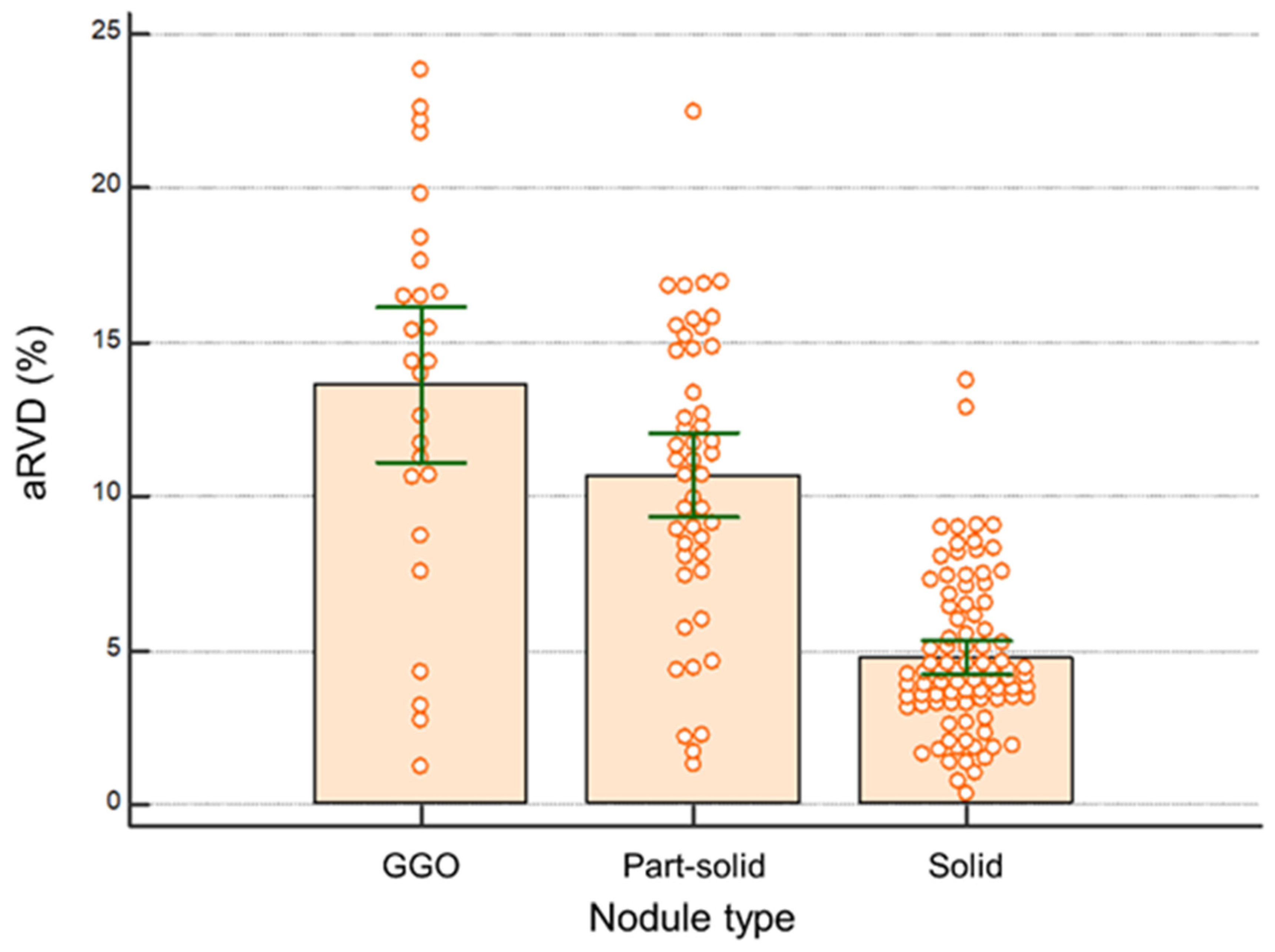
3.3. Volumetry According to Nodule Classification
3.4. Subjective Image Quality Analysis between SSSP and HSHP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Lung Screening Trial Research Team; Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; Gierada, D.S.; et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Gierada, D.S.; Black, W.; Munden, R.; Nath, H.; Aberle, D.; Kazerooni, E. Performance of Lung-RADS in the National Lung Screening Trial: A retrospective assessment. Ann. Intern. Med. 2015, 162, 485–491. [Google Scholar] [CrossRef]
- van Klaveren, R.J.; Oudkerk, M.; Prokop, M.; Scholten, E.T.; Nackaerts, K.; Vernhout, R.; van Iersel, C.A.; van den Bergh, K.A.; van’t Westeinde, S.; van der Aalst, C.; et al. Management of lung nodules detected by volume CT scanning. N. Engl. J. Med. 2009, 361, 2221–2229. [Google Scholar] [CrossRef]
- Field, J.K.; Duffy, S.W.; Baldwin, D.R.; Whynes, D.K.; Devaraj, A.; Brain, K.E.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016, 71, 161–170. [Google Scholar] [CrossRef]
- Callister, M.E.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015, 70, ii1–ii54. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Devaraj, A.; van Ginneken, B.; Nair, A.; Baldwin, D. Use of Volumetry for Lung Nodule Management: Theory and Practice. Radiology 2017, 284, 630–644. [Google Scholar] [CrossRef]
- Way, T.W.; Chan, H.P.; Goodsitt, M.M.; Sahiner, B.; Hadjiiski, L.M.; Zhou, C.; Chughtai, A. Effect of CT scanning parameters on volumetric measurements of pulmonary nodules by 3D active contour segmentation: A phantom study. Phys. Med. Biol. 2008, 53, 1295–1312. [Google Scholar] [CrossRef]
- Hwang, S.H.; Oh, Y.W.; Ham, S.Y.; Kang, E.Y.; Lee, K.Y. Effect of the high-pitch mode in dual-source computed tomography on the accuracy of three-dimensional volumetry of solid pulmonary nodules: A phantom study. Korean J. Radiol. 2015, 16, 641–647. [Google Scholar] [CrossRef]
- Baumueller, S.; Alkadhi, H.; Stolzmann, P.; Frauenfelder, T.; Goetti, R.; Schertler, T.; Plass, A.; Falk, V.; Feuchtner, G.; Scheffel, H.; et al. Computed tomography of the lung in the high-pitch mode: Is breath holding still required? Investig. Radiol. 2011, 46, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Jacobi, V.; Beeres, M.; Bodelle, B.; Gruber, T.; Lee, C.; Bauer, R.; Kerl, M.; Vogl, T.; Zangos, S. Quantitative analysis of motion artifacts in high-pitch dual-source computed tomography of the thorax. J. Thorac. Imaging 2012, 27, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.J. Clinical Applications of Wide-Detector CT Scanners for Cardiothoracic Imaging: An Update. Korean J. Radiol. 2019, 20, 1583–1596. [Google Scholar] [CrossRef] [PubMed]
- Menzel, H.; Schibilla, H.; Teunen, D. European Guidelines on Quality Criteria for Computed Tomography; European Commission: Luxembourg, 2000; p. 16262. [Google Scholar]
- Yeom, J.A.; Kim, K.U.; Hwang, M.; Lee, J.W.; Kim, K.I.; Song, Y.S.; Lee, I.S.; Jeong, Y.J. Emphysema Quantification Using Ultra-Low-Dose Chest CT: Efficacy of Deep Learning-Based Image Reconstruction. Medicina 2022, 58, 939. [Google Scholar] [CrossRef] [PubMed]
- Farshad-Amacker, N.A.; Alkadhi, H.; Leschka, S.; Frauenfelder, T. Effect of high-pitch dual-source CT to compensate motion artifacts: A phantom study. Acad. Radiol. 2013, 20, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bauer, R.W.; Schell, B.; Beeres, M.; Wichmann, J.L.; Bodelle, B.; Vogl, T.J.; Kerl, J.M. High-pitch dual-source computed tomography pulmonary angiography in freely breathing patients. J. Thorac. Imaging 2012, 27, 376–381. [Google Scholar] [CrossRef]
- Lell, M.M.; May, M.; Deak, P.; Alibek, S.; Kuefner, M.; Kuettner, A.; Köhler, H.; Achenbach, S.; Uder, M.; Radkow, T. High-pitch spiral computed tomography: Effect on image quality and radiation dose in pediatric chest computed tomography. Investig. Radiol. 2011, 46, 116–123. [Google Scholar] [CrossRef]
- Dangis, A.; Gieraerts, C.; De Bruecker, Y.; Janssen, L.; Valgaeren, H.; Obbels, D.; Gillis, M.; Van Ranst, M.; Frans, J.; Demeyere, A.; et al. Accuracy and Reproducibility of Low-Dose Submillisievert Chest CT for the Diagnosis of COVID-19. Radiol. Cardiothorac. Imaging 2020, 2, e200196. [Google Scholar] [CrossRef]
- Amacker, N.A.; Mader, C.; Alkadhi, H.; Leschka, S.; Frauenfelder, T. Routine chest and abdominal high-pitch CT: An alternative low dose protocol with preserved image quality. Eur. J. Radiol. 2012, 81, e392–e397. [Google Scholar] [CrossRef]
- Bulla, S.; Blanke, P.; Langer, M.; Pache, G. Letter to the editor re: Low-dose computed tomography of the paranasal sinus and facial skull using a high-pitch dual-source system--first clinical results. Eur. Radiol. 2011, 21, 1447–1448, author reply 1451. [Google Scholar] [CrossRef]
- Funama, Y.; Awai, K.; Liu, D.; Oda, S.; Yanaga, Y.; Nakaura, T.; Kawanaka, K.; Shimamura, M.; Yamashita, Y. Detection of nodules showing ground-glass opacity in the lungs at low-dose multidetector computed tomography: Phantom and clinical study. J. Comput. Assist. Tomogr. 2009, 33, 49–53. [Google Scholar] [CrossRef]
- Lassen, B.C.; Jacobs, C.; Kuhnigk, J.M.; van Ginneken, B.; van Rikxoort, E.M. Robust semi-automatic segmentation of pulmonary subsolid nodules in chest computed tomography scans. Phys. Med. Biol. 2015, 60, 1307–1323. [Google Scholar] [CrossRef]
- Jacobs, C.; van Rikxoort, E.M.; Scholten, E.T.; de Jong, P.A.; Prokop, M.; Schaefer-Prokop, C.; van Ginneken, B. Solid, part-solid, or non-solid?: Classification of pulmonary nodules in low-dose chest computed tomography by a computer-aided diagnosis system. Investig. Radiol. 2015, 50, 168–173. [Google Scholar] [CrossRef]
- Doo, K.W.; Kang, E.Y.; Yong, H.S.; Woo, O.H.; Lee, K.Y.; Oh, Y.W. Accuracy of lung nodule volumetry in low-dose CT with iterative reconstruction: An anthropomorphic thoracic phantom study. Br. J. Radiol. 2014, 87, 20130644. [Google Scholar] [CrossRef]
- Horeweg, N.; van der Aalst, C.M.; Vliegenthart, R.; Zhao, Y.; Xie, X.; Scholten, E.T.; Mali, W.; Thunnissen, E.; Weenink, C.; Groen, H.J.; et al. Volumetric computed tomography screening for lung cancer: Three rounds of the NELSON trial. Eur. Respir. J. 2013, 42, 1659–1667. [Google Scholar] [CrossRef]
- Xu, D.M.; Gietema, H.; de Koning, H.; Vernhout, R.; Nackaerts, K.; Prokop, M.; Weenink, C.; Lammers, J.W.; Groen, H.; Oudkerk, M.; et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006, 54, 177–184. [Google Scholar] [CrossRef]

| Patients (n = 102) | |
|---|---|
| Age (years) (mean ± SD) | 72.3 ± 12.0 |
| Sex (%) | |
| Male | 68 (66.7%) |
| Female | 34 (33.3%) |
| Nodule characteristics (n = 163) | |
| Location | |
| Upper lobes | 65 (39.9%) |
| Middle lobes * | 16 (9.8%) |
| Lower lobes | 82 (50.3%) |
| Type | |
| Ground glass opacity | 26 (16.0%) |
| Part-solid | 47 (28.8%) |
| Solid | 90 (55.2%) |
| Nodule volume (mm3) | |
| <500 | 81 (49.7%) |
| 500–1000 | 29 (17.8%) |
| >1000 | 53 (32.5%) |
| Location | SSSP (n = 102 Patients) | HSHP (n = 102 Patients) | p-Value |
|---|---|---|---|
| Image noise * (Hounsfield unit) | 59.7 ± 5.8 (49.3–70.2) | 65.6 ± 5.7 (55.3–75.1) | <0.001 |
| Radiation dose (mSv) | 0.42 ± 0.30 (0.37–0.48) | 0.36 ± 0.33 (0.30–0.42) | <0.001 |
| Nodule Classification (n = 163) | aRVD | p-Value |
|---|---|---|
| Nodule location | 0.486 | |
| Upper lobes | 7.3 ± 5.6 (5.9–8.7) | |
| Middle lobes * | 9.0 ± 5.9 (5.8–12.1) | |
| Lower lobes | 8.1 ± 5.1 (7.0–9.2) | |
| Nodule type | <0.001 | |
| Ground glass opacity | 13.62 ± 6.3 (11.1–16.2) | GGO vs. part-solid 0.009 |
| Part-solid | 10.7 ± 4.7 (9.3–12.1) | Part-solid vs. solid <0.001 |
| Solid | 4.8 ± 2.5 (4.2–5.3) | GGO vs. solid <0.001 |
| Nodule volume (mm3) | 0.177 | |
| <500 | 8.3 ± 5.0 (7.2–9.4) | |
| 500–1000 | 8.6 ± 6.1 (6.3–11.0) | |
| >1000 | 6.8 ± 5.5 (5.2–8.3) |
| Location/Score | SSSP (n = 102) | HSHP (n = 102) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Right middle lobe | 79 | 21 | 2 | 0 | 86 | 15 | 1 | 0 | 0.203 |
| Lingular division | 45 | 54 | 3 | 0 | 66 | 34 | 2 | 0 | 0.005 |
| Right lung base | 82 | 17 | 3 | 0 | 85 | 15 | 2 | 0 | 0.545 |
| Left lung base | 44 | 54 | 4 | 0 | 64 | 34 | 4 | 0 | 0.015 |
| Subjective Analysis | SSSP | HSHP | p-Value | |
|---|---|---|---|---|
| Nodule (n = 163 nodules) | Detection | 1.07 ± 0.30 | 1.06 ± 0.27 | 0.989 |
| Contour | 1.31 ± 0.67 | 1.22 ± 0.58 | 0.162 | |
| Image | Streak artifact | 1.26 ± 0.58 | 1.31 ± 0.64 | 0.674 |
| (n = 102 patients) | Diagnostic acceptability | 1.24 ± 0.47 | 1.17 ± 0.42 | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.; Lee, J.W.; Jeong, Y.J.; Hwang, M.-H.; Lee, G. Increased Scan Speed and Pitch on Ultra-Low-Dose Chest CT: Effect on Nodule Volumetry and Image Quality. Medicina 2024, 60, 1301. https://doi.org/10.3390/medicina60081301
Bae H, Lee JW, Jeong YJ, Hwang M-H, Lee G. Increased Scan Speed and Pitch on Ultra-Low-Dose Chest CT: Effect on Nodule Volumetry and Image Quality. Medicina. 2024; 60(8):1301. https://doi.org/10.3390/medicina60081301
Chicago/Turabian StyleBae, Heejoo, Ji Won Lee, Yeon Joo Jeong, Min-Hee Hwang, and Geewon Lee. 2024. "Increased Scan Speed and Pitch on Ultra-Low-Dose Chest CT: Effect on Nodule Volumetry and Image Quality" Medicina 60, no. 8: 1301. https://doi.org/10.3390/medicina60081301
APA StyleBae, H., Lee, J. W., Jeong, Y. J., Hwang, M.-H., & Lee, G. (2024). Increased Scan Speed and Pitch on Ultra-Low-Dose Chest CT: Effect on Nodule Volumetry and Image Quality. Medicina, 60(8), 1301. https://doi.org/10.3390/medicina60081301






